Abstract
AIM: To investigate hepatitis virus, genetic and environmental factors, and their interactions in predisposing patients to liver diseases in Northeast India.
METHODS: A total of 104 jaundice patients and 124 community controls were included. Serological analysis was performed by routine enzyme-linked immunosorbent assay, and nucleic acid testing for hepatitis viruses was done by polymerase chain reaction (PCR), followed by PCR direct sequencing for viral genotyping. Cytochrome P450 2E1 (CYP2E1) polymorphism was studied by PCR-restriction fragment length polymorphism. Nitrite and volatile nitrosamines in indigenous foods consumed routinely by the Northeast Indian ethnic population were estimated by Griess’s reagent and GC-MS, respectively.
RESULTS: Hepatitis A virus (HAV) infection was predominantly prevalent (36.5%) in our cohort, followed by hepatitis B virus (HBV), hepatitis E virus (HEV) and hepatitis C virus. HBV genotype D and HEV genotype 1 were the most dominant. CYP2E1 c1/c2 genotype frequency was comparatively higher in alcoholic (P < 0.0001, OR = 30.5) and cryptogenic (P = 0.014, OR = 8.714) patients, and was associated with significantly higher hepatitis risk (P = 0.0.007, OR = 6.489). Mutant C allele of Cyp2E1 DraI frequency was comparatively higher in HAV (P = 0.006), alcoholic (P = 0.003) and cryptogenic (P = 0.014) cases, and was associated with overall hepatitis risk (P = 0.026, OR = 5.083). Indigenous foods, Gundruk, Kharoli, betel leaf and nuts were found to have the highest nitrite content.
CONCLUSION: Apart from viral factors, CYP2E1 polymorphism might be associated with increased risk of liver diseases in Northeast India. Indigenous foods that contain nitrite and nitrosamine might be an associated risk factor.
Keywords: Viral hepatitis, Cytochrome P450 2E1, Gene polymorphism, Nitrites, Nitrosamines
INTRODUCTION
Application of molecular genetic techniques in human cancer risk assessment will likely emerge as a method of identifying subpopulations with different sensitivities to carcinogen exposure[1]. Liver diseases and cancer show a marked worldwide geographic and ethnic distribution[2]. Etiological factors that have been associated with liver diseases and cancer include hepatitis virus infection[3], liver flukes[4], aflatoxins[5], alcohol[6], smoking[6] and dietary nitroso compounds[7]. Differences in hepatitis B virus (HBV)[8] and hepatitis C virus (HCV)[9] genotypes are linked to various degrees of liver disease severity and rate of disease progression towards hepatocarcinogenesis. Unfortunately, there are no data available on these important aspects in liver disease patients from Northeast India, who are ethnically distinct from those in other parts of India, and have the incidence of cancer of various etiologies in the country, according to a survey done by the National Cancer Registry Program of the Indian Council for Medical Research (ICMR).
According to epidemiological studies, 90% of cancers are associated with environmental factors, including nitrosamines, which are acquired through tobacco smoke, vehicle exhaust and foodstuffs[10]. It has been shown that nitrite alone can cause cancer; however, an even more serious cause of concern is the well-documented potential of nitrites/nitrates to cause cancer through the formation of nitrosamines[11].
Cytochrome P450 2E1 (CYP2E1) is an N-nitrosodimethyl-amine demethylase that is expressed primarily in the liver. It takes part in the metabolism of drugs, but also activates many pre-carcinogens and pre-toxins[12]. Cyp2E1 activates N-nitrosamines, which are contained in tobacco smoke and foodstuffs[13] and several industrial[14] and endogenous carcinogens[15]. Cyp2E1 activity is mediated by various determinants, such as obesity, fasting and liver dysfunction, and by a number of environmental factors[16]. Cyp2E1 activity is accompanied by generation of a significant amount of an active oxygen form, which damages cell membranes and macromolecules and leads to formation of DNA adducts. Polymorphism in the Cyp2E1 gene is associated with malignancies of different cellular origins, including the liver[17]. CYP2E1 polymorphism in the 5’regulatory region with C→T replacement at position -1019 and RsaIrestriction site loss (CYP2E1*5B) is one of the most important polymorphisms identified. Homozygous c2/c2 genotype is associated with a 10-fold increase in CYP2E1 gene transcription[18]. Another important CYP2E1 polymorphism is located in intron 6, revealed by DraIand identified as C (minor) and D (common) alleles[19].
Here, we present the results from Guwahati (the capital of Assam, and hub and gateway of Northeast India) of a case-control study designed to explore the viral, environmental and genetic risk factors for liver diseases. The study was approved by the Institutional Biosafety and Ethics Committee.
MATERIALS AND METHODS
Blood samples were obtained from 104 acute hepatitis patients with clinical jaundice and liver disease during the non-rainy season, who were receiving care at the Central Hospital, NF Railway, Maligaon, Guwahati. One hundred and twenty-four sex, age and residence pair-matched community controls, with similar ethnicity and food habits were also recruited for this study. Cases and controls were evaluated on the basis of history (including their food, drinking, smoking and tobacco chewing habits), clinical examination, liver function profile, and serological test of hepatitis A, B, C and E using commercially available IgM enzyme-linked immunosorbent assay (ELISA) kits (therefore including acute cases).
Viral DNA isolation and genotyping
Viral DNA isolation of HBV for hepatitis B surface antigen (HBsAg)-positive cases was performed using the standard phenol-chloroform method using 150 μL of patient plasma, followed by ethanol precipitation. The viral DNA thus isolated was resuspended in an adequate amount of nuclease-free water. HBV genotyping was performed by multiplex polymerase chain reaction (PCR) using specific primers for each genotype (A-F) of HBV[20], and validated by sequencing of representative cases for the basal core promoter, precore and core region of HBV genome.
RNA extraction and HCV and hepatitis E virus genotyping
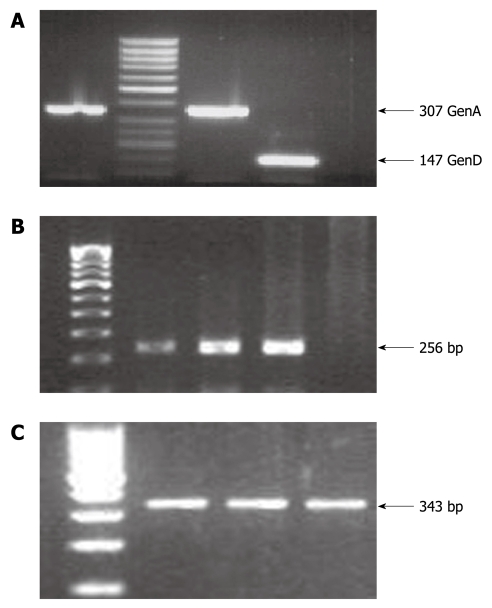
Viral RNA was extracted from 140 μL of serum with QIAamp@Viral RNA Kit (Qiagen, Germany) according to the manufacturer’s instructions. RNA pellets were reconstituted in 60 μL elution buffer and stored at -20°C until use. One-tube nested reverse transcription PCR (RT-PCR) amplification was performed using specific primers for the conserved 5’UTR region as described earlier for genotyping of HCV[21]. Amplification of the specific 256-bp product was achieved for the anti-HCV-positive cases. Briefly, 10 μL RNA was mixed with 0.2 μL (20 pmol) of antisense primer, incubated for 1 min at 94°C and 1 min at 56°C, and then stored on ice. Fifty microliters of the reaction mixture that contained 10 × reaction buffer, dNTPs (10 mmol/L), MgCl2 (2.5 mmol/L), 20 pmol primers AS1 and S1, 0.5 U Taq Polymerase (New England Biolabs, Ipswich, MA, USA) and 2.5 μL (20 U/μL) MMuLV Reverse Transcriptase (New England Biolabs) was added to the pre-cooled RNA mix for one-step RT-PCR. The conditions were 60 min at 42°C for reverse transcription, 2 min at 95°C for denaturation of the RT, followed by 35 cycles of 30 s at 95°C, annealing for 30 s at 54°C, and extension for 30 s at 72°C. After the last cycle, a final extension was made at 72°C for 7 min. The second round of PCR was performed with the same master mix that contained AS2 and S2 primers using 5 μL of the first product as a template under the same reaction conditions. Positive and negative controls were included in every PCR amplification experiment. This was followed by direct sequencing and comparison with the standard NCBI Genbank database. Hepatitis E virus (HEV) genotyping was performed by RT-PCR amplification using the primers for the HEV ORF1 region reported by Jilani et al[22], which gave a PCR-amplified product of 343 bp; followed by direct sequencing and comparison with the available genotype database for HEV from the NCBI Genbank database.
PCR-restriction fragment length polymorphism analysis of CYP2E1 gene polymorphism
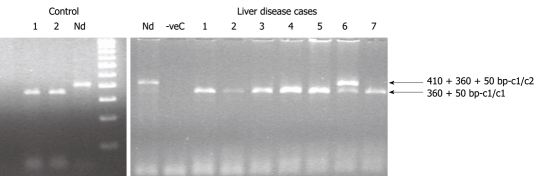
CYP2E1*5B (5’ flanking region, -1019 bp site) genotyping was performed by PCR-fragment length polymorphism (RFLP) analysis with the primers reported by Hayashi et al[23]; and RsaIrestriction enzyme. An allele with an RsaIsite (characterized by two bands of 360 and 50 bp on agarose gel electrophoresis) was defined as wild-type and designated c1, and an allele without this site, as a variant or rare type and designated c2 (characterized by presence of a single band at 410 bp)[24].
DraI digestion detects a polymorphism in intron 6 of the CYP2E1 gene. Genomic DNA was PCR-amplified with primers reported by Kato et al[25], which yielded a 995-bp fragment that was subjected to DraI restriction enzyme digestion. Two DraI restriction enzyme recognition sites exist in this amplified DNA sequence but only one is known to be polymorphic. The presence of the polymorphic DraI restriction site yielded three fragments of 572, 302 and 121 bp (type D, major allele), whereas the absence of the polymorphic site was determined by the presence of 874-bp and 121-bp fragments (type C, minor allele)[26].
To improve the genotyping quality and validation, 20% of samples were re-genotyped by other laboratory personnel and results were reproducible with no discrepancy in genotyping. Genotyping of 10% of samples was confirmed by DNA sequencing.
Nitrite determination and analysis of volatile nitrosamines
Several indigenously prepared fermented food products and the raw material used to prepare them were short-listed and collected, based on a questionnaire of the food habits of jaundice patients and community controls enrolled in the present study. Nitrite estimation in fresh and fermented foods from Northeast India was done using the standard Griess’s reagent method followed by spectrophotometric detection at 540 nm. The presence of volatile N-nitrosamines in fresh and fermented foods was done by GC-MS analysis following the protocol of Mitacek et al[27], followed by detection using a Hewlett-Packard Model 5890 GC coupled to a model 610 Thermal Energy Analyzer (TEA; Thermo Electron, Waltham, MA, USA).
Statistical analysis
Results were expressed as mean ± SD. Serum aspartate aminotransferase and alanine aminotransferase levels in each group were analyzed by student’s t test. ORs were calculated using logistic regression. Statistical analysis was carried out for CYP2E1 genotypes in liver disease and hepatitis subgroups (specific for viral hepatitis groups, and alcoholic and cryptogenic cases) and compared to community controls using SPSS version 13.0 software. An adjusted two-tailed P value (corrected) less than 0.05 at 95% CI was considered statistically significant.
RESULTS
Blood samples were obtained from patients with liver disease who were receiving care in a regional referral hospital in Guwahati. These patients had a median age of 41 ± 16 years and showed a male to female ratio of 2.15:1. The majority of the liver disease patients were male (71/104, 68.27%). The hepatitis virus infection spectrum analyzed based on IgM ELISA results was, hepatitis A virus (HAV, 38/104, 36.5%), HBV (22/104, 21.15%), HCV (4/104, 3.8%), HEV (10/104, 9.6%), and HAV-HBV co-infection (2/104, 1.92%). Others had alcoholic (12/104, 11.53%) and cryptogenic (16/104, 15.38%) liver disease etiology (Table 1).
Table 1.
Demographical, biochemical and serological profiles of liver disease patients
| Parameter | HAV | HBV | HCV | HEV | HAV+HBV | Alcoholic | Cryptogenic |
| n | 38 | 22 | 4 | 10 | 2 | 12 | 16 |
| Male:female | 22:16 | 16:6 | 2:2 | 6:4 | 2:0 | 11:1 | 12:4 |
| Mean age (yr) | 23 ± 16 | 40 ± 28 | 45 ± 8 | 38 ± 15 | 55 | 44 ± 5 | 43 ± 21 |
| Mean SGOT | 241 ± 168 | 333 ± 108 | 86 ± 46 | 778 ± 336 | 40 ± 5 | 323 ± 212 | 109 ± 66 |
| Mean SGPT | 265 ± 198 | 243 ± 148 | 73 ± 28 | 513 ± 366 | 39 ± 8 | 283 ± 155 | 85 ± 43 |
HAV: Hepatitis A virus; HBV: Hepatitis B virus; HEV: Hepatitis E virus; HCV: Hepatitis C virus; SGOT: Serum glutamic oxaloacetic transaminase; SGPT: Serum glutamic pyruvic transaminase.
Viral genotyping
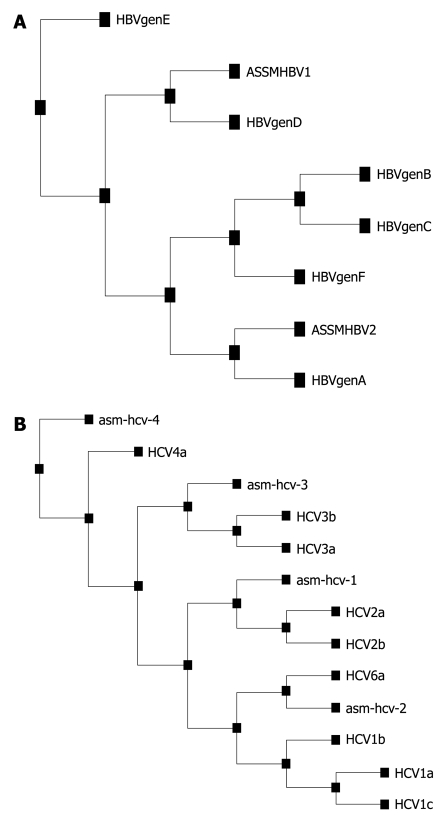
Viral genotyping was performed for HBV, HCV and HEV samples. HBV genotyping was performed by multiplex PCR. HBV genotype D (13/22, 59.1%) was the most prevalent in the HBV-positive cases, followed by HBV genotype A (4/22, 18.2%), mixed genotype A + D (4/12, 18.2%) and genotype C (1/22, 4.5%) (Figures 1A and 2A). A few of the randomly selected genotyped samples were cross-checked and validated by direct sequencing of the core region of HBV followed by phylogenetic analysis.
Figure 1.
Polymerase chain reaction amplification results. A: Hepatitis B virus (HBV) genotyping results, where an amplicon of 307 bp represents HBV genotype A whereas an amplicon of 147 bp represents HBV genotype D; B: Hepatitis C virus (HCV) amplicon of 256 bp for the 5’UTR region; C: Hepatitis E virus (HEV) amplicon of 343 bp for the ORF1 region. The HCV and HEV amplicons were purified by gel extraction and subjected to direct sequencing for genotype determination on comparison with the standard NCBI representative sequences for HCV and HEV.
Figure 2.
Phylogenetic analysis using the Expasy software tool. A: Randomly sequenced hepatitis B virus (HBV) cases representing HBV genotype D (ASSMHBV1) and A (ASSMHBV2) by multiplex polymerase chain reaction genotyping, therefore confirming and validating our genotyping results; B: Hepatitis C virus (HCV) genotype base on direct sequencing of 5’UTR for isolate from Guwahati.
HCV genotype was determined by direct sequencing of the PCR amplicon generated from the conserved 5’UTR region of the HCV genome. The nucleotides that were sequenced by direct sequencing were aligned using ClustalW, and version 1.6 of the tree view program from Expasy (POWER) was then used to construct an uprooted phylogenetic tree (Figures 1B and 2B). After comparison with known sequences from the NCBI Genbank database, the distribution of the genotypes based on four isolated HCVs were found to be one each from genotypes 4 (asm-hcv-4), 3 (asm-hcv-3), 2 (asm-hcv-1) and 6 (asm-hcv-2).
HEV genotype was determined by amplification of the ORF1 region and subjecting the amplified product to direct sequencing, and then comparing the sequences with the standard NCBI Genbank sequences for HEV. HEV genotype 1 was the only genotype found in our cohort.
PCR-RFLP analysis of CYP2E1 gene polymorphism
The distribution of CYP2E1*5B c1c1, c1c2 and c2/c2 genotypes in liver disease cases were 90.38%, 9.62% and 0%, respectively compared to 98.39%, 1.61% and 0% in healthy controls (Figure 3). The CYP2E1*6 DD, DC and CC genotype frequencies in liver disease cases were 92.3%, 3.85% and 3.85%, respectively, compared to 98.39%, 1.61% and 0% in healthy controls (Tables 2 and 3). Cyp2E1 RsaIgenotype distributions were consistent with Hardy-Weinberg equilibrium, but Cyp2E1 DraIgenotype was only consistent for the control population. The c1/c2 variant genotype was significantly more prevalent in alcoholic [P < 0.0001, OR = 30.5 (4.835-192.418)] and cryptogenic hepatitis [P = 0.014, OR = 8.714 (1.137-66.784)] cases. The prevalence of mutant C allele of DraI was also predominant in alcoholic [P = 0.003, OR = 12.220 (1.550-96.035)] and cryptogenic hepatitis [P = 0.014, OR = 8.714 (1.137-66.784)] cases.
Figure 3.
Polymerase chain reaction-restriction fragment length polymorphism results for Cytochrome P450 2E1 rsaI genotyping for the control and liver disease cases. Lane 1 and 2 in the control section and lane 1-5 and 7 of the liver disease section represents c1/c1 genotype (360 + 50 bp); whereas lane 6 of the liver disease section repre-sents c1/c2 genotype (410 + 360 + 50 bp). Nd: Non-digested samples of 410 bp.
Table 2.
Distribution of Cytochrome P450 2E1 genotype in hepatitis cases compared to controls
|
Number of individuals (% of group) |
Less common allele frequency | χ2value (P value) | OR (95% CI) | |||
| Homozygous-more common allele | Heterozygous | Homozygous-less common allele | ||||
| RsaI polymorphism | c1/c1 | c1/c2 | c2/c2 | |||
| Controls (n = 124) | 122 (98.39) | 2 (1.61) | 0 | 1.61 | ref. | |
| Hepatitis (n = 104) | 94 (90.39) | 10 (9.71) | 0 | 9.61 | 0.007 | 6.489 (1.389-30.326) |
| DraI polymorphism | DD | DC | CC | |||
| Controls (n = 124) | 122 (98.39) | 2 (1.61) | 0 | 1.61 | ref. | |
| Hepatitis (n = 104) | 96 (92.31) | 4 (3.84) | 4 (3.84) | 7.69 | 0.026 | 5.083 (1.055-24.492) |
ata represented as number of subjects showing respective genotype (%); P < 0.05 was considered to be statistically significant, control group was considered as reference group.
Table 3.
Detail distribution of Cytochrome P450 2E1 genotypes in different underlying etiology of hepatitis
|
Number of individuals (% of group) |
Less common allelefrequency | χ2value (P value) | OR (95% CI) | |||
| Homozygous-more common allele | Heterozygous | Homozygous-less common allele | ||||
| RsaI polymorphism | c1/c1 | c1/c2 | c2/c2 | |||
| Controls (n = 124) | 122 (98.4) | 2 (1.6) | 0 | 1.6 | ref. | |
| HAV (n = 38) | 36 (94.7) | 2 (5.3) | 0 | 5.3 | 0.160 | 5.229 (0.840-32.537) |
| HBV (n = 22) | 20 (90.9) | 2 (9.1) | 0 | 9.1 | 0.080 | 2.905 (0.252-33.484) |
| HCV (n = 4) | 4 (100) | 0 (0) | 0 | 0 | 0.798 | 0.968 (0.938-1.0) |
| HEV (n = 10) | 10 (100) | 0 (0) | 0 | 0 | 0.686 | 0.924 (0.880-0.971) |
| HAV + HBV (n = 2) | 2 (100) | 0 (0) | 0 | 0 | 0.856 | 0.984 (0.962-1.006) |
| Alcoholic (n = 12) | 8 (66.66) | 4 (33.33) | 0 | 33.33 | < 0.0001 | 30.500 (4.835-192.418) |
| Cryptogenic (n = 16) | 14 (87.5) | 2 (12.5) | 0 | 12.5 | 0.014 | 8.714 (1.137-66.784) |
| DraI polymorphism | DD | DC | CC | |||
| Controls (n = 124) | 122 (98.4) | 2 (1.6) | 0 | 1.6 | ref. | |
| HAV (n = 38) | 34 (89.5) | 2 (5.25) | 2 (5.25) | 10.5 | 0.006 | 7.176 (1.260-40.863) |
| HBV (n = 22) | 22 (100) | 0 | 0 | 0 | 0.514 | 0.847 (0.790-0.908) |
| HCV (n = 4) | 4 (100) | 0 | 0 | 0 | 0.798 | 0.968 (0.938-1.0) |
| HEV (n = 10) | 10 (100) | 0 | 0 | 0 | 0.164 | 0.924 (0.880-0.971) |
| HAV + HBV (n = 2) | 2 (100) | 0 | 0 | 0 | 0.856 | 0.984 (0.962-1.006) |
| Alcoholic (n = 12) | 10 (83.3) | 2 (16.7) | 0 | 16.7 | 0.003 | 12.220 (1.550-96.035) |
| Cryptogenic (n = 16) | 14 (87.5) | 0 | 2 (12.5) | 12.5 | 0.014 | 8.714 (1.137-66.784) |
Data represented as number of subjects showing respective genotype (%); P < 0.05 was considered to be statistically significant, control group was considered as reference group. HAV: Hepatitis A virus; HEV: Hepatitis E virus; HBV: Hepatitis B virus; HCV: Hepatitis C virus.
Nitrite determination and analysis of volatile nitrosamines
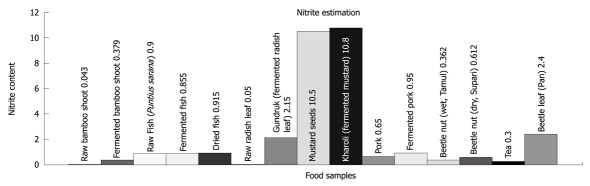
To study the correlation of environmental factors with severity of liver disease, nitrite concentration in various fermented food products widely used in the households of Assam and Northeast India were estimated using Griess reagent. The concentration of nitrites in raw and fermented food products produced by indigenously developed fermentation techniques, as well as a few common supplementary food products consumed routinely, are shown in Figure 4 and Table 4. The highest amount of nitrite was found in mustard (fresh and fermented), followed by another fermented food product, Gundruk (fermented radish leaf) and betel leaf (commonly known as Pan, and taken in combination with betel nut).
Figure 4.
Presence of nitrite in (μg/mL) in different food products routinely consumed in Northeast India.
Table 4.
Nitrite estimation in food products routinely consumed in Northeast India
| Sl. No. | Name of foodstuff | Amount of nitrite present (μg/mL) |
| 1 | Raw bamboo shoot | 0.043 |
| 2 | Fermented bamboo shoot | 0.379 |
| 3 | Raw fish (Puntius sarana) | 0.900 |
| 4 | Fermented fish | 0.855 |
| 5 | Dried fish | 0.915 |
| 6 | Raw radish leaf | 0.050 |
| 7 | Gundruk (fermented radish leaf) | 2.150 |
| 8 | Mustard seeds | 10.500 |
| 9 | Kharoli (fermented mustard) | 10.800 |
| 10 | Pork | 0.650 |
| 11 | Fermented pork | 0.950 |
| 12 | Beetle nut (wet, Tamul) | 0.362 |
| 13 | Beetle nut (dry, Supari) | 0.612 |
| 14 | Tea | 0.300 |
| 15 | Betel leaf (Pan) | 2.400 |
Based on the case histories of the liver disease patients, which included their food habits, as well as considering the food habits of the general Northeast Indian population, we analyzed raw and fermented bamboo shoots and fish (including dried fish) for the presence of volatile N-nitrosamines by GC-MS. The GC-MS/TEA analysis revealed the presence of detectable amounts of N-nitrosamines in raw fish (data not shown), whereas there was no detectable amount of N-nitrosamines in fermented and dry fish or raw and fermented bamboo shoots.
DISCUSSION
Along with age, sex and viral factors, alterations in host genetic factors and environmental factors are also considered to be important in the development of liver disease, but unfortunately, there are very limited data available on these important aspects in liver disease patients from Northeast India, who are ethnically distinct from those in other parts of the country. More importantly, the population in the northeast region is vulnerable to cancer of different etiology, as shown by the ICMR National Cancer Registry Program of. In this study, we determined the prevalence of systemic hepatotropic viruses and their genotypes, Cyp2E1 polymorphism, and the presence of dietary toxic environmental carcinogens such as nitrites and nitrosamines in fresh and fermented food from Northeast India, and evaluated the role that they might play in the severity of the liver disease or its predisposition.
The highest prevalence of fecal-oral infection occurs in regions where low standards of sanitation promote virus transmission[28]. In most industrialized nations, where hepatitis A is no longer considered a childhood disease, infections with HAV are increasingly contracted by adults[29]. Despite the high prevalence of antibody in highly endemic populations, the virus perpetuates in the region due to its high physical stability. In our study, HAV infection was found in 36.5% of the population and most of the infected individuals were children and young people. This was followed by HBV infection in 21.2% of the study population, which is relatively on the higher side compared to other reports from different parts of India[30-33]. This is a major concern because HBV infection is associated with a high rate of hepatocellular carcinoma[34]. HBV genotype D was the most predominant genotype in our cohort, which is contrary to a report from the sister state in Northeast India, Arunachal Pradesh[35].
HEV and HCV infection was found in 9.6% and 3.8% of the cases, respectively. Viral hepatitis is a major public health problem in India, which is hyperendemic for HAV and HEV. HEV is also the major cause of sporadic adult acute viral hepatitis and acute liver failure, and many epidemics of HEV have already been reported in India. HCV infection in India has a population prevalence of around 1%, and occurs predominantly through transfusion and the use of unsterile glass syringes. HCV genotypes 2 and 3 are found in 60%-80% of the population[36]. Our results showed the prevalence of different HCV genotypes, namely, 2, 3, 4 and 6, which warrants further study, including large cohort populations from all Northeast Indian states. Here, to the best of our knowledge, we reported the presence of HEV genotype 1 in Northeast India for the first time, which is similar to reports published from other parts of India.
CYP2E1 enzymes belong to the phase I group of drug-metabolizing enzymes that are involved in the metabolic activation and detoxification of various potential genotoxic compounds. CYP2E1 is involved in metabolism of more than 80 low-molecular-weight, hydrophobic, toxicologically dangerous compounds and contributes to activation of many pro-carcinogens and several drugs to highly reactive metabolites[14]. Hepatic CYP2E1 has been shown to activate various carcinogens, therefore, there has been interest in whether certain CYP2E1 polymorphisms might predispose to liver diseases and cancer[37]. The functional polymorphism in these genes exhibits inter-individual variations in susceptibility towards various diseases and differences in therapeutic response. The variant sequences of these genes differ considerably between ethnic groups. Therefore, the objective of the study was to assess the prevalence of CYP2E1 gene variants in healthy volunteers and compare them with the liver disease patients from Guwahati.
The most important polymorphisms identified in 5’regulatory region with C→T replacement in position -1019 and rsaIrestriction site loss (CYP2E1*5B) (77, 25). Variant c2 allele is expressed in vitro at a higher rate compared to wild-type, and homozygous c2/c2 genotype is associated with a 10-fold increase in CYP2E1 gene transcription. The functional significance of CYP2E1*5B polymorphism might be due to its localization in presumed binding sites for hepatic transcription factor, hepatocyte nuclear factor-1[18,23]. Rare c2 allele frequency constitutes 24%-30% for Asian populations[25], 2%-3% for Caucasians[23], 0.3%-7% for Afro-Americans[23,38], 15% for Mexican Americans[39], and 18% for Taiwanese[40]. The DraIpolymorphism is also associated with altered activity of CYP2E1, although DraIis located in intron 6 and is not thought to affect transcription of the gene[19].
Our study showed that the prevalence of mutant C1/C2 Cyp2E1 RsaIallele and the mutant C allele of DraI was significantly higher in liver disease patients. The presence of mutant Cyp2E1 RsaI allele (P = 0.007, OR = 6.489 at 95% CI: 1.389-30.326) and mutant C allele of DraI(P = 0.026, OR = 5.083 at 95% CI: 1.055-24.492) was significantly associated with hepatitis risk in Northeast Indian patients.
The prevalence of the c1/c2 genotypes was lower than that reported in other Asian countries, but amongst the highest reported in the Indian population[41]. The serum glutamic oxaloacetic transaminase (SGOT) levels were also significantly higher in HAV cases that contained wild-type Cyp2E1 DraIallele (P = 0.019). The prevalence of mutant DraIallele among patients with viral hepatitis was found to be significantly more only in cases of HAV infection (P = 0.006). The presence of underlying mutant DraIallele might play a role in liver damage caused by acute HAV infection, but this also augments more indebt studies to conclude on the molecular interactions influenced by HAV on CYP2E1 genes functionality or activity.
Induction of cytochrome P450 2E1 by ethanol is believed to be one of the central pathways by which ethanol generates a state of oxidative stress and causes hepatotoxicity. Hepatic CYP2E1 enzyme activity is significantly higher in alcoholic patients with liver disease than in those without signs of liver disease[42]. In our study, Cyp2E1 c1/c2 (P < 0.0001) and mutant DraI(P = 0.003) allele was significantly associated with alcoholic liver disease. Mutant c1/c2 and DraI(P = 0.014) was also found to be associated with cryptogenic hepatitis in liver disease patients from Northeast India.
There is a concern to maintain the levels of nitrite as low as possible because of suspected adverse effects on oxygenation of the blood, and/or indirect carcinogenic effects, through formation of nitrosamines. Nitrites have been known to cause cancer directly. Although there is little correlation between nitrate/nitrite and nitrosamine content of food, nitrates and nitrites are agents in endogenous nitrosamine formation in the gastrointestinal tract[11]. Therefore, the presence of high nitrite concentration in raw and fermented mustard, radish and betel leaf and nut is also a high risk factor for adverse health effects, along with genetic and viral factors. Addition of nitrite-containing salts for storage of some dried fish products and fermented pork also adversely affects the quality of the food. The European Commission Scientific Committee for Food (document 111/5611/95) has recommended that nitrate and nitrite should be limited to an acceptable daily intake of 0.06 mg/kg. Therefore, ingestion of the above food products that contain high nitrite concentrations is a high risk factor, especially for children.
Case-control studies have suggested that exposure to exogenous and possibly endogenous nitrosamines in food or tobacco in betel nut and cigarettes plays a role in the development of liver disease and cancer. There is evidence that endogenous nitrosation of areca nut alkaloids can occur in animals and humans, and areca-nut-derived nitrosamines, including 3-(methylnitrosamino) propionitrile, have been detected in the saliva of betel quid chewers which is a common practice in Guwahati and throughout Northeast India. Epidemiological data have linked the use of areca nut with other cancers such as liver cancer[43]. The presence of volatile nitrosamines (N-diethylnitrosamine and N-dimethylnitrosamine) in raw fish has been detected using the protocol followed by Mitacek et al[27]. The presence of volatile nitrosamines could be an indication of increasing pollution of the River Brahmaputra, which is one of the life lines of Northeast India, and its tributaries, from where the fish Puntius sarana is caught and fermented and dried. Our results is of grave importance as case-control studies conducted in Thailand have implicated traditional lifestyle and especially consumption of fermented-style fish and fermented vegetables[44]. Fortunately, the nitrosamine levels were undetectable in fermented and dried fish, contrary to what has been reported in other countries[27]. The non-detection of nitrosamines in fermented fish, irrespective of its presence in raw fish, could be attributed to the activities of lactic acid bacteria during fermentation.
To conclude, the diversity of etiological factors associated with liver disease burden in Northeast India is enormous with respect to the high prevalence of certain hepatitis viruses, such as HBV, as well as the various HBV and HCV genotypes found in our study cohort. Moreover, strict vigilance and upgrading of overall hygiene standards is mandatory to investigate epidemics of HAV or HEV, which are also prevalent in Northeast India. CYP2E1 polymorphism is supposedly associated with the risk of liver disease, especially in non-viral hepatitis patients, and the presence of higher nitrite concentration in fermented dietary products in Northeast India, and nitrosamines in Areca catechu (betel nut) and raw fish, have clinical significance, because these environmental factors can act as additional risk factors in liver disease susceptibility, by virtue of the gene-environment interaction.
COMMENTS
Background
Molecular epidemiology of risk factors such as viral (e.g. hepatitis A, B, C and E viruses), host genetic [e.g. Cytochrome P450 2E1 (CYP2E1) gene polymorphism] and environmental factors such as toxic components in food and alcohol, is important for liver disease assessment, which might help to identify subpopulations with different sensitivities and predisposition to different grades or severity of liver disease. Scanty or no data are available on the above aspects from Northeast India, which has an ethnically distinct and different population from the rest of the country. The authors present the results of a case-control study from Guwahati (the capital of Assam, and hub and gateway of Northeast India), which was designed to explore the viral, environmental and genetic risk factors for liver diseases.
Research frontiers
The authors identified patient subgroups by serological profiling based on standard enzyme-linked immunosorbent assay techniques, followed by a molecular-genotyping-based approach using polymerase chain reaction-restriction fragment length polymorphism direct sequencing for identifying hepatitis B, C and E genotypes, as well as CYP2E1 polymorphisms. Biochemical assays (Griess’s method) and GC-MS were used for analyzing and quantifying nitrites and nitrosoamines.
Innovations and breakthroughs
For the first time, all three critical parameters, that is, viral, host genetic and environmental risk factors were evaluated in a case-control study from Northeast India. The study analyzed hepatitis virus genotypes, the role of CYP2E1 polymorphisms, and the presence of toxic carcinogenic components in routinely consumed indigenous food products of Northeast India.
Applications
The molecular genotyping data on viral hepatitis could be useful for clinicians because viral genotypes have been shown to influence disease progression and antiviral therapies, and therefore, will helpful for clinical interventions and patient care. CYP2E1 genotyping data are useful as a prognostic marker for assessing the predisposition of patients towards liver disease. The safety aspects of the food products exclusively consumed in Northeast India were elucidated. Information about this is important for the public in Northeastern states of India.
Peer review
The results of this paper are interesting and well presented.
Acknowledgments
The authors would like to thank the staff of the Central Railway Hospital, Maligaon, Guwahati; Professor MC Kalita (Department of Biotechnology, GU) and his research scholars, Professor P Kar (Department of Medicine, MAMC, New Delhi); and Sofia Banu Ahmed for their kind co-operation and help in completing the work.
Footnotes
Peer reviewers: Juan-Ramón Larrubia, PhD, Gastroenterology Unit and Liver Research Unit, Guadalajara University Hospital, Donante de Sangre s/n, 19002 Guadalajara, Spain; Francesco Feo, Professor, Department of Biomedical Sciences, Section of Experimental Pathology and Oncology, University of Sassari, Via P, Manzella 4, 07100 Sassari, Italy
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
References
- 1.Shields PG. Inherited factors and environmental exposures in cancer risk. J Occup Med. 1993;35:34–41. [PubMed] [Google Scholar]
- 2.Shields PG, Harris CC. Molecular epidemiology and the genetics of environmental cancer. JAMA. 1991;266:681–687. [PubMed] [Google Scholar]
- 3.Bortolotti F. Chronic hepatitis B acquired in childhood: unanswered questions and evolving issues. J Hepatol. 1994;21:904–909. doi: 10.1016/s0168-8278(94)80257-2. [DOI] [PubMed] [Google Scholar]
- 4.Bosch FX, Muñoz N. Prospects for epidemiological studies on hepatocellular cancer as a model for assessing viral and chemical interactions. IARC Sci Publ. 1988;89:427–438. [PubMed] [Google Scholar]
- 5.Shank RC, Bhamarapravati N, Gordon JE, Wogan GN. Dietary aflatoxins and human liver cancer. IV. Incidence of primary liver cancer in two municipal populations of Thailand. Food Cosmet Toxicol. 1972;10:171–179. doi: 10.1016/s0015-6264(72)80195-0. [DOI] [PubMed] [Google Scholar]
- 6.Austin H, Delzell E, Grufferman S, Levine R, Morrison AS, Stolley PD, Cole P. A case-control study of hepatocellular carcinoma and the hepatitis B virus, cigarette smoking, and alcohol consumption. Cancer Res. 1986;46:962–966. [PubMed] [Google Scholar]
- 7.Thamavit W, Bhamarapravati N, Sahaphong S, Vajrasthira S, Angsubhakorn S. Effects of dimethylnitrosamine on induction of cholangiocarcinoma in Opisthorchis viverrini-infected Syrian golden hamsters. Cancer Res. 1978;38:4634–4639. [PubMed] [Google Scholar]
- 8.Thakur V, Guptan RC, Kazim SN, Malhotra V, Sarin SK. Profile, spectrum and significance of HBV genotypes in chronic liver disease patients in the Indian subcontinent. J Gastroenterol Hepatol. 2002;17:165–170. doi: 10.1046/j.1440-1746.2002.02605.x. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Ikematsu H, Hirohata T, Kashiwagi S. Hepatitis C virus infection and risk of hepatocellular carcinoma among Japanese: possible role of type 1b (II) infection. J Natl Cancer Inst. 1996;88:742–746. doi: 10.1093/jnci/88.11.742. [DOI] [PubMed] [Google Scholar]
- 10.Guslitser LN. Epidemiology of tumors: the main results of the studies carried in R.E. Kavetsky Institute of Experimental Pathology, Oncology and Radiobiology NAS of Ukraine. Exp Oncol. 2001;23:229–235. [Google Scholar]
- 11.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 12.Ramaiah SK, Apte U, Mehendale HM. Cytochrome P4502E1 induction increases thioacetamide liver injury in diet-restricted rats. Drug Metab Dispos. 2001;29:1088–1095. [PubMed] [Google Scholar]
- 13.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima T, Aoyama T. Polymorphism of drug-metabolizing enzymes in relation to individual susceptibility to industrial chemicals. Ind Health. 2000;38:143–152. doi: 10.2486/indhealth.38.143. [DOI] [PubMed] [Google Scholar]
- 15.Bartsch H, Nair U, Risch A, Rojas M, Wikman H, Alexandrov K. Genetic polymorphism of CYP genes, alone or in combination, as a risk modifier of tobacco-related cancers. Cancer Epidemiol Biomarkers Prev. 2000;9:3–28. [PubMed] [Google Scholar]
- 16.Camus AM, Geneste O, Honkakoski P, Bereziat JC, Henderson CJ, Wolf CR, Bartsch H, Lang MA. High variability of nitrosamine metabolism among individuals: role of cytochromes P450 2A6 and 2E1 in the dealkylation of N-nitrosodimethylamine and N-nitrosodiethylamine in mice and humans. Mol Carcinog. 1993;7:268–275. doi: 10.1002/mc.2940070410. [DOI] [PubMed] [Google Scholar]
- 17.Kang JS, Wanibuchi H, Morimura K, Gonzalez FJ, Fukushima S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res. 2007;67:11141–11146. doi: 10.1158/0008-5472.CAN-07-1369. [DOI] [PubMed] [Google Scholar]
- 18.Nomura F, Itoga S, Uchimoto T, Tomonaga T, Nezu M, Shimada H, Ochiai T. Transcriptional activity of the tandem repeat polymorphism in the 5’-flanking region of the human CYP2E1 gene. Alcohol Clin Exp Res. 2003;27:42S–46S. doi: 10.1097/01.ALC.0000078612.01626.96. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu F, Kikuchi H, Motomiya M, Abe T, Sagami I, Ohmachi T, Wakui A, Kanamaru R, Watanabe M. Association between restriction fragment length polymorphism of the human cytochrome P450IIE1 gene and susceptibility to lung cancer. Jpn J Cancer Res. 1991;82:254–256. doi: 10.1111/j.1349-7006.1991.tb01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschberg O, Schuttler C, Repp R, Schaefer S. A multiplex-PCR to identify hepatitis B virus--enotypes A-F. J Clin Virol. 2004;29:39–43. doi: 10.1016/s1386-6532(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 21.Das U, Kar P, Gopalkrishna V, Sharma JK, Madan K, Das BC. Comparative evaluation of hepatitis C virus infection in serum and liver tissue of patients with chronic liver disease by reverse transcription-polymerase chain reaction. Clin Microbiol Infect. 1999;5:256–261. doi: 10.1111/j.1469-0691.1999.tb00138.x. [DOI] [PubMed] [Google Scholar]
- 22.Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676–682. doi: 10.1111/j.1440-1746.2007.04913.x. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi S, Watanabe J, Kawajiri K. Genetic polymorphisms in the 5’-flanking region change transcriptional regulation of the human cytochrome P450IIE1 gene. J Biochem. 1991;110:559–565. doi: 10.1093/oxfordjournals.jbchem.a123619. [DOI] [PubMed] [Google Scholar]
- 24.Lu XM, Zhang YM, Lin RY, Arzi G, Wang X, Zhang YL, Zhang Y, Wang Y, Wen H. Relationship between genetic polymorphisms of metabolizing enzymes CYP2E1, GSTM1 and Kazakh’s esophageal squamous cell cancer in Xinjiang, China. World J Gastroenterol. 2005;11:3651–3654. doi: 10.3748/wjg.v11.i24.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato S, Shields PG, Caporaso NE, Hoover RN, Trump BF, Sugimura H, Weston A, Harris CC. Cytochrome P450IIE1 genetic polymorphisms, racial variation, and lung cancer risk. Cancer Res. 1992;52:6712–6715. [PubMed] [Google Scholar]
- 26.Kato S, Shields PG, Caporaso NE, Sugimura H, Trivers GE, Tucker MA, Trump BF, Weston A, Harris CC. Analysis of cytochrome P450 2E1 genetic polymorphisms in relation to human lung cancer. Cancer Epidemiol Biomarkers Prev. 1994;3:515–518. [PubMed] [Google Scholar]
- 27.Mitacek EJ, Brunnemann KD, Suttajit M, Martin N, Limsila T, Ohshima H, Caplan LS. Exposure to N-nitroso compounds in a population of high liver cancer regions in Thailand: volatile nitrosamine (VNA) levels in Thai food. Food Chem Toxicol. 1999;37:297–305. doi: 10.1016/s0278-6915(99)00017-4. [DOI] [PubMed] [Google Scholar]
- 28.Ceyhan M, Yildirim I, Kurt N, Uysal G, Dikici B, Ecevit C, Aydogan A, Koc A, Yasa O, Koseoglu M, et al. Differences in hepatitis A seroprevalence among geographical regions in Turkey: a need for regional vaccination recommendations. J Viral Hepat. 2008;15 Suppl 2:69–72. doi: 10.1111/j.1365-2893.2008.01034.x. [DOI] [PubMed] [Google Scholar]
- 29.Mathur P, Arora NK. Epidemiological transition of hepatitis A in India: issues for vaccination in developing countries. Indian J Med Res. 2008;128:699–704. [PubMed] [Google Scholar]
- 30.Nayak NC, Panda SK, Zuckerman AJ, Bhan MK, Guha DK. Dynamics and impact of perinatal transmission of hepatitis B virus in North India. J Med Virol. 1987;21:137–145. doi: 10.1002/jmv.1890210205. [DOI] [PubMed] [Google Scholar]
- 31.Roychoudhury A, Bhattacharyya DK. Incidence of hepatitis B carriers in Calcutta, West Bengal. J Assoc Physicians India. 1989;37:160–161. [PubMed] [Google Scholar]
- 32.Tandon BN, Gandhi BM, Joshi YK. Etiological spectrum of viral hepatitis and prevalence of markers of hepatitis A and B virus infection in north India. Bull World Health Organ. 1984;62:67–73. [PMC free article] [PubMed] [Google Scholar]
- 33.Verma J, Joshi PL, Raj B, Bhattacharaya M, Sebastian M, Kumari S. An epidemiological study of hepatitis B virus amongst blood donors. J Commun Dis. 1989;21:52–58. [PubMed] [Google Scholar]
- 34.Sarin SK, Thakur V, Guptan RC, Saigal S, Malhotra V, Thyagarajan SP, Das BC. Profile of hepatocellular carcinoma in India: an insight into the possible etiologic associations. J Gastroenterol Hepatol. 2001;16:666–673. doi: 10.1046/j.1440-1746.2001.02476.x. [DOI] [PubMed] [Google Scholar]
- 35.Borkakoty BJ, Mahanta J, Biswas D. Circulating genotypes of hepatitis B virus in Arunachal Pradesh. Indian J Med Res. 2008;127:65–70. [PubMed] [Google Scholar]
- 36.Acharya SK, Madan K, Dattagupta S, Panda SK. Viral hepatitis in India. Natl Med J India. 2006;19:203–217. [PubMed] [Google Scholar]
- 37.Ladero JM, Agúndez JA, Rodríguez-Lescure A, Diaz-Rubio M, Benítez J. RsaI polymorphism at the cytochrome P4502E1 locus and risk of hepatocellular carcinoma. Gut. 1996;39:330–333. doi: 10.1136/gut.39.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.London SJ, Daly AK, Cooper J, Carpenter CL, Navidi WC, Ding L, Idle JR. Lung cancer risk in relation to the CYP2E1 Rsa I genetic polymorphism among African-Americans and Caucasians in Los Angeles County. Pharmacogenetics. 1996;6:151–158. doi: 10.1097/00008571-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Shi H, Jiang H, Kemp B, Hong WK, Delclos GL, Spitz MR. Associations between cytochrome P4502E1 genotype, mutagen sensitivity, cigarette smoking and susceptibility to lung cancer. Carcinogenesis. 1997;18:967–973. doi: 10.1093/carcin/18.5.967. [DOI] [PubMed] [Google Scholar]
- 40.Hildesheim A, Chen CJ, Caporaso NE, Cheng YJ, Hoover RN, Hsu MM, Levine PH, Chen IH, Chen JY, Yang CS. Cytochrome P4502E1 genetic polymorphisms and risk of nasopharyngeal carcinoma: results from a case-control study conducted in Taiwan. Cancer Epidemiol Biomarkers Prev. 1995;4:607–610. [PubMed] [Google Scholar]
- 41.Soya SS, Padmaja N, Adithan C. Genetic polymorphisms of CYP2E1 and GSTP1 in a South Indian population--comparison with North Indians, Caucasians and Chinese. Asian Pac J Cancer Prev. 2005;6:315–319. [PubMed] [Google Scholar]
- 42.Dupont I, Lucas D, Clot P, Mânez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol. 1998;28:564–571. doi: 10.1016/s0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- 43.Tsai JF, Jeng JE, Chuang LY, Ho MS, Ko YC, Lin ZY, Hsieh MY, Chen SC, Chuang WL, Wang LY, et al. Habitual betel quid chewing and risk for hepatocellular carcinoma complicating cirrhosis. Medicine (Baltimore) 2004;83:176–187. doi: 10.1097/01.md.0000126971.80227.a4. [DOI] [PubMed] [Google Scholar]
- 44.Srivatanakul P, Parkin DM, Khlat M, Chenvidhya D, Chotiwan P, Insiripong S, L'Abbe KA, Wild CP. Liver cancer in Thailand. II. A case-control study of hepatocellular carcinoma. Int J Cancer. 1991;48:329–332. doi: 10.1002/ijc.2910480303. [DOI] [PubMed] [Google Scholar]