Abstract
AIM: To analyze the association between Helicobacter spp. and some common gut bacteria in patients with cholecystitis.
METHODS: A nested-polymerase chain reaction (PCR), specific to 16S rRNA of Helicobacter spp. was performed on paraffin-embedded gallbladder samples of 100 cholecystitis and 102 control cases. The samples were also analyzed for some common gut bacteria by PCR. Positive samples were sequenced for species identification.
RESULTS: Helicobacter DNA was found in seven out of 100 cases of acute and chronic cholecystitis. Sequence analysis displayed Helicobacter pullorum (H. pullorum) in six cases and Helicobacter pylori in one; H. pullorum was only found in cases with metaplasia. Control samples were negative for Helicobacter spp. and some common gut bacteria. There was a significant difference (P = 0.007) between cholecystitis and control samples for Helicobacter DNA.
CONCLUSION: A possible relationship was detected between Helicobacter DNA and cholecystitis. Further serological and immunohistochemical studies are needed to support these data.
Keywords: Helicobacter, Gallbladder, Cholecystitis, 16S rRNA, Polymerase chain reaction
INTRODUCTION
The most well-known member of the Helicobacter genus, Helicobacter pylori (H. pylori), is classified as a type 1 carcinogen[1], and infects the human stomach and causes gastritis, peptic ulcer disease and gastric cancer. Besides H. pylori, the genus Helicobacter contains more than 25 species[2], many of which cause extragastric diseases in humans and animals[3-10]. These are named enterohepatic Helicobacter species (EHS) or EHS and colonize the hepatobiliary tract of humans, and include Helicobacter hepaticus (H. hepaticus), Helicobacter bilis (H. bilis), Helicobacter rappini (H. rappini), Helicobacter ganmani (H. ganmani) and Helicobacter pullorum (H. pullorum). Several of these EHS are associated with the pathogenesis of chronic biliary disorders, such as cholecystitis, cholelithiasis, gallbladder carcinoma and bile tract carcinoma and some liver diseases, such as primary sclerosing cholangitis, primary biliary cirrhosis and hepatocellular carcinoma[11-14]. Moreover, chronic pancreatitis and pancreatic cancer, as well as inflammatory bowel diseases in humans have also been reported to be positive for EHS in various polymerase chain reaction (PCR)-based studies[15-17].
Chronic cholecystitis is the most prevalent disease in various populations in industrialized countries[18]. During the 20 years from 1965-1969 to 1985-1989, the mortality from gallbladder cancer increased by 30% in Sweden. However, not all high-risk European countries showed such an increase and the mortality decreased in some countries[19]. Chronic cholecystitis is commonly associated with gallstone disease[20] and some studies have shown that cholecystitis and gallstones can cause epithelial hyperplasia of the gallbladder mucosa or cancer, and various bacterial genomes have been detected in gallbladder carcinoma tissue[21]. Moreover, a recent study has shown that H. pylori can damage human gallbladder epithelial cells in vitro, and could be the key factor that leads to clinical cholecystitis[22]. Some studies have revealed the presence of bile-resistant EHS in the gallbladder mucosa and in gallstones. It has been shown that the presence of H. pylori and EHS in bile might represent a risk factor for bile stone formation[4,23-26]. One study has clearly demonstrated the presence of a mixed bacterial population in gallstones[4]. Salmonella typhi is another bacterial pathogen of the biliary tree in human gallstones and gallbladder cancer[27,28]. Salmonella biofilm has been shown on human gallstones[29]. Moreover, Campylobacter spp. have also been detected in bile and epithelial samples in cholecystolithiasis[30].
H. pylori, H. pullorum and H. bilis have been isolated from humans with gallbladder disease such as cholecystitis, cholelithiasis[9,31,32], gallbladder carcinoma and bile tract carcinoma[33]. A possible relationship between chronic cholecystitis and Helicobacter DNA has been shown by some investigators[9,31,32,34] but, as far as we are aware, there has been no study published on Scandinavian patients with cholecystitis. Therefore, we examined the relationship between Helicobacter spp. and some common gut bacteria in Swedish patients with cholecystitis.
MATERIALS AND METHODS
Patients and histological methods
We re-examined the gallbladders from 100 cholecystitis patients from 2006-2007 (mean age: 48 years; range: 20-84 years; 35 male, 65 female) and 102 control patients (mean age: 58 years; range: 11-85 years; 54 male, 48 female) from 1999 to 2009, taken from the files of the Department of Pathology, Lund University Hospital. Of the 100 cholecystitis samples, 50 were acute (mean age: 55 years; range: 23-81 years; 22 male, 28 female), and 50 were chronic (mean age: 44 years; range: 20-84 years; 13 male, 37 female). Among the 50 patients with acute cholecystitis, 34 cases (median age: 56 years; range: 23-79 years; 15 male, 19 female) were without metaplasia and 16 (median age: 54 years; range: 36-81 years; 7 male, 9 female) had metaplasia. Among the 50 patients with chronic cholecystitis, 27 cases (median age: 45 years; range 20-84 years; 8 male, 19 female) were without metaplasia and 23 (median age: 42 years; range: 20-71 years; 5 male, 18 female) had metaplasia. As control samples, we used 18 normal gallbladders from patients with pancreatic malignancies reported elsewhere[17], and 84 consecutive patients with normal gallbladders from 1999 to 2009 (median age: 61 years; range: 11-85 years; 44 male, 40 female). There was no metaplasia in these gallbladders. The diagnosis was: six hepatocellular carcinoma, 40 liver metastases (mainly colorectal), four intestinal carcinoids, three liver carcinoid metastases, seven focal nodal hyperplasias, three bile duct cysts, one gallbladder adenoma, three splenomegalies, two pancreatic neuroendocrine malignancies, one benign pancreatic cyst, one adrenal carcinoma, and 13 normal gallbladders with no other diagnosis.
Two to five sections were taken from each case, and one section from the ductus cysticus. Sections that showed mucosal metaplasia were stained with Alcian blue-periodic acid Schiff (AB-PAS), pH 2.5, and Warthin-Starry silver stain for Helicobacter spp. One section was immunostained with anti-H. pylori antibody (DAKO, Glostrup, Denmark; diluted 1:300) according to Apostolov et al[9]. Mucosa was cut from the paraffin blocks with the tip of a scalpel by careful comparison with the slides. Areas with gastric metaplasia, if present, were included in the samples. The Research Ethics Committee at Lund University approved this study (permit number 588/2006).
DNA extraction
DNA was extracted from approximately 5 mg of each paraffin-embedded gallbladder tissue sample. To ascertain that epithelium was included, two pieces, each of 2-3 mg, were taken from each case. Paraffin-embedded gallbladder samples were de-embedded as previously described[10]. Gallbladder tissue samples were de-embedded by heating at 60°C for 10 min, followed by washing in xylene for 2 × 5 min. The specimens were rehydrated through graded ethanol (99% and 95% for 2 × 5 min and 70% for 5 min), and finally washed for 5 min in double-distilled water. DNA was extracted by a QIAamp DNA Mini Kit tissue protocol (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The extracts (200 μL total volume) were combined, and 5 μL of the mixtures was analyzed by PCR.
Helicobacter-specific PCR
DNA extracts were amplified in a GeneAmp 2700 Thermocycler (Applied Biosystems, Foster City, CA, USA) using a semi-nested PCR assay specific for Helicobacter 16S rDNA, as previously described[11], using primers 1F (5'CTATGACGGGTATCCGGC3'), 1R (5'CTCACGACACGAGCTGAC3') and 2R (5'TCGCCTTCGCAATGAGTATT3'). Primers 1F and 1R were used in the first step, whereas primers 1F and 2R were used in the second step. The reaction mixture of the first step (25 μL) contained 0.5 μmol/L each primer (1F and 1R), 0.8 mmol/L each dNTP (Amersham Biosciences, Uppsala, Sweden), 1 × chelating buffer, 2.5 mmol/L MgCl2, 0.05% casein, 0.05% formamid, 1.25 U rTth DNA polymerase (Applied Biosystems), and 5 μL extracted DNA. H. pylori (CCUG 17 874) was used as a positive control in all PCR reactions. The amplification conditions for the first step were 94°C for 2 min; 30 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and finally 72°C for 5 min. The reaction mixture of the second step (25 μL) contained 0.5 μmol/L each primer (1F and 2R), 0.2 mmol/L each dNTP, 1 × buffer II, 2.5 mmol/L MgCl2, 1.0 U AmpliTaq Gold DNA polymerase (Applied Biosystems), and 2 μL 10 × diluted PCR product from the first step. The 416-bp PCR products were visualized by 1.3% agarose gel electrophoresis.
Amplification of non-Helicobacter bacteria
Enterobacteriaceae-, Bacterioides-Prevotella group- and Enterococcus-specific PCRs were performed. The reaction mixture and amplification conditions, except for annealing temperatures, for non-Helicobacter PCR assays were the same as in the first step of the semi-nested Helicobacter PCR. The annealing temperatures and primers used for detection of Enterobacteriaceae, Bacterioides-Prevotella group and Enterococcus were as described before[11]. Primers Eco1457F (5'CATTGACGTTACCCGCAGAAGAAGC3') and Eco1652R (5'CTCTACGAGACTCAAGCTTGC3') were used to amplify Enterobacteriaceae and primers Ent1F (5'TACTGACAAACCATTCATGATG3') and Ent2R (5'AACTTCGTCACCAACGCGAAC3') were used to amplify Enterococcus, whereas primers Bac303F (5'GAAGGTCCCCCACATTG3') and Bac708R (5'CAATCGGAGTTCTTCGTG3') were used to amplify the Bacteroides-Prevotella group. As positive controls, Escherichia coli (CCUG 17620), Bacteroides fragilis (CCUG 4856), and Enterococcus faecalis (CCUG 9997) were used in all PCR reactions. The 112-bp PCR product of Enterococcus, 418-bp product of Bacteroides and 195-bp product of Enterobacteriaceae were visualized by 1.3% agarose gel electrophoresis.
DNA sequence analysis
Helicobacter-specific PCR products were purified from agarose gels using the Montage DNA Gel Extraction Kit (Millipore, Bedford, MA, USA) according to the manufacturer’s instructions. DNA sequence reactions were performed using the ABI PRISM™ dRhodamine Terminator Cycle Sequencing Ready Reaction Kit version 3.0 (Applied Biosystems), as described by Tolia et al[10]. Products of the sequence reaction were aligned and the closest homologous DNA was identified by BLASTn-analysis.
Statistical analysis
Statistical analyses were done by χ2 and Fisher’s exact tests. P < 0.05 was considered to be significant.
RESULTS
Histology
Little metaplasia was detected in the sections and only a few glands or a few cells displayed gastric (antrum) metaplasia and/or acid mucin. Acid or neutral mucins were often seen only in parts of the epithelial cell cytoplasm. The AB-PAS staining method for metaplasia revealed among the chronic cases three with only gastric metaplasia (neutral mucosubstances), five with only non-gastric metaplasia (acid mucosubstances), and 15 with both types. For acute cholecystitis, these figures were two, five and nine, respectively (Table 1). The two types of metaplasia are displayed in Figure 1. Whartin-Starry staining and immunohistochemistry for H. pylori were negative in all studied specimens. The H. pylori-positive specimen was from a case of acute cholecystitis with extensive necrosis, but with a small area of preserved epithelium without metaplasia, from which the sample was taken.
Table 1.
Number of cases with metaplasia in patients with cholecystitis
| Acute | Chronic | |
| Gastric | 2 | 3 |
| Non-gastric | 5 | 5 |
| Both | 9 | 15 |
| None | 34 | 27 |
| Total | 50 | 50 |
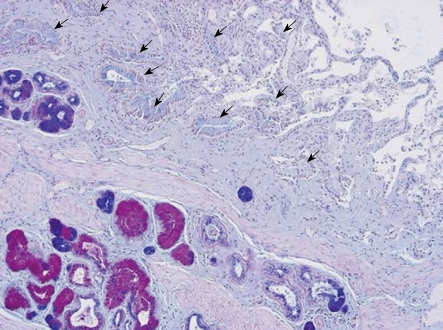
Figure 1.
Histological section of ductus cysticus from a patient with chronic cholecystitis. Low-power view displaying antrum-type (red) and intestinal-type (blue) mucous metaplasia in glands. The ductus lumen is seen in the opposite corner of the photo with folds of the mucosa layer covered by epithelium without metaplasia, arrows (no intense color). Alcian blue-periodic acid Schiff staining.
Helicobacter-specific PCR assay and sequencing results
Using the Helicobacter-specific PCR assay and agarose electrophoresis, Helicobacter DNA was detected in 7/100 of gallbladder specimens of patients with cholecystitis. There were 4/50 (8%) and 3/50 (6%) samples positive for Helicobacter spp. among acute and chronic cholecystitis patients, respectively. Six samples showed 98-99% sequence similarity to H. pullorum and one to H. pylori (Table 2). H. pullorum was only found in cases with metaplasia, in six out of 39, as compared to none out of 61 without metaplasia. The difference was statistically significant (P = 0.002). All control samples were negative for Helicobacter spp. The difference between Helicobacter DNA prevalence in gallbladder of cholecystitis patients and controls was also significant (P = 0.007).
Table 2.
Prevalence of Helicobacter spp. and some common gut bacteria n (%)
| Patient group | Helicobacter PCR | Gut bacteria PCR | Sequencing results (No. of samples) |
| Acute cholecystitis | 4/50 (8) | 0/50 (0) | H. pylori (1) |
| H. pullorum (3) | |||
| Chronic cholecystitis | 3/50 (6) | 0/50 (0) | H. pullorum (3) |
| Controls | 0/102 (0) | 0/102 (0) | - |
Results are shown as the number of positive patients and the number of all patients in the group followed by the percentage in parenthesis. PCR: Polymerase chain reaction; H. pylori: Helicobacter pylori; H. pullorum: Helicobacter pullorum.
PCR and sequence detection of bacterial DNA other than Helicobacter
None of the tested patients’ samples with acute and chronic cholecystitis and control samples was positive using the Bacteroides-, Enterobacteriaceae- and Enterococcus-specific PCR assays.
DISCUSSION
Helicobacter DNA was found in 7% of cholecystitis mucosa (8% acute, 6% chronic cholecystitis); none of the control samples was positive for Helicobacter. There are several reports on the presence of Helicobacter DNA in cholecystitis mucosa (Table 3). The studies in Germany, China and Japan with a prevalence of 2%-27% were more similar to our study[34-37] than was the study in Chile (39% prevalence)[31]. However, in a study from Ukraine (73%) the prevalence was much higher than in our study[9].
Table 3.
Prevalence of Helicobacter DNA in cholecystitis mucosa in different studies from various geographical regions
Six samples (three from acute and three from chronic cholecystitis) were positive for H. pullorum. Fox et al[31] have reported a link between EHS infections and chronic cholecystitis. H. bilis was the most common but H. pullorum was also reported[31]. Apostolov et al[9] have developed a first generation of enzyme immunoassays and immunoblotting to serodiagnose EHS infections in mice and humans. H. pullorum was found in 18% of patients with hepatitis C virus by immunohistochemistry in one of our previous studies[38]. However, H. pullorum is most commonly seen in poultry[39]. There is most likely a zoonotic transmission between humans and chickens by undercooked chicken.
One sample with a similar sequence to H. pylori was detected. Other studies on gallbladders or gallstones from patients with cholecystitis and cholelithiasis have shown the presence of H. pylori[9,32,40]. Other Helicobacter species have also been detected in different studies such as, H. rappini, H. ganmani[35] and H. hepaticus[41].
Kawaguchi et al[42] were the first to demonstrate Helicobacter spp. in cholecystits mucosa that displayed gastric metaplasia. Metaplasia was seen in all cases of cholecystitis in a Chilean study[31], in 15% of cases in a British study[43], and in 14% of cases in a Ukrainian study[9]. In the British study, no Helicobacter was found by immunostaining. Our results confirm the importance of gastric metaplasia for detection of Helicobacter DNA. Misra et al[40] have detected Helicobacter only in areas with gastric metaplasia, with a prevalence of 45%, but could not detect Helicobacter DNA in paraffin blocks or formalin-fixed mucosal tissue.
None of the gallbladder samples was positive for Bacteroides and Enterococcus spp. in our study. Enteric bacteria have been detected from gallstones and bile samples by culturing and PCR methods in some studies[44-47], but not by fluorescence in situ hybridization[48].
Apart from geographical differences, the variation in H. pylori, EHS and some gut pathogens between countries could be due to the use of different PCR methods. Our PCR technique was evaluated as a highly reliable method for genus level identification of Helicobacter spp.[49], and inhibitors that might influence the PCR results have been discussed in our other studies[50]. Moreover, some of the studies have used inappropriate control groups. We selected 102 normal control gallbladders from patients diagnosed with diseases other than cholecystitis.
In cholecystitis, Helicobacter DNA might preferentially or only be found in epithelial cells or on their surface, thus, much care has to be taken when selection the samples from the paraffin blocks.
In conclusion, several Helicobacter spp. infect a range of hosts (most probably, certain species are pathogens in some animals and humans). Divergent results might be due to different geographical areas, different PCR methods, using different control groups or lack of control groups, and sampling from different areas of the biopsy. The present study shows the possible relationship between Helicobacter spp. and cholecystitis in Swedish patients. Further studies are needed to determine the possible role of EHS and other pathogens in biliary tract infections and the possible relationship to various hepatobiliary malignancies such as cholangiocarcinoma.
COMMENTS
Background
Helicobacter genus has nearly 25 species and many of them cause extragastric diseases in humans and animals.
Research frontiers
Helicobacter DNA in gallbladder mucosa has been reported with different prevalence and is associated with several biliary tract diseases, but there are still doubts about the relationship between enterohepatic Helicobacter species (EHS), Helicobacter pylori and hepatobiliary diseases.
Innovations and breakthroughs
Recent reports have highlighted the presence of Helicobacter in the biliary tract in different regions. However, this is believed to be the first study to report the possible relationship between chronic cholecystitis in Scandinavian patients.
Applications
By understanding the relationship between Helicobacter and cholecystitis, this study could represent a future strategy for further pathological studies of patients with cholecystitis.
Terminology
EHS are species in the genus Helicobacter that colonize the hepatobiliary tract and can cause extragastric diseases in humans or in animals.
Peer review
The authors have tackled a newly developing area of interest to many researchers. The work is a contribution to the study of the association between Helicobacter spp. and some common gut bacteria in patients with cholecystitis. They concluded that there is a possible relationship between Helicobacter DNA and cholecystitis, and recommended further serological and immunohistochemical studies to support their data.
Acknowledgments
Hans-Olof Nilsson’s expert knowledge transfer on PCR and specimen handling and PCR inhibitor removal, and Ingrid Nilsson’s valuable expertise on Helicobacter spp. are most appreciated. We also thank Jonas Ranstam for help with the statistical analysis.
Footnotes
Supported by ALF and John Forssmans grants from the Lund University Hospital, Sweden
Peer reviewer: Zeinab Nabil Ahmed, Professor of Microbiology, Microbiology and Immunology Department, Faculty of Medicine (for girls), Al-Azhar University, Nasr City, 1047, Cairo, Egypt
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
References
- 1.NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 2.Fox JG. The non-H pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut. 2002;50:273–283. doi: 10.1136/gut.50.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsukura N, Yokomuro S, Yamada S, Tajiri T, Sundo T, Hadama T, Kamiya S, Naito Z, Fox JG. Association between Helicobacter bilis in bile and biliary tract malignancies: H. bilis in bile from Japanese and Thai patients with benign and malignant diseases in the biliary tract. Jpn J Cancer Res. 2002;93:842–847. doi: 10.1111/j.1349-7006.2002.tb01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monstein HJ, Jonsson Y, Zdolsek J, Svanvik J. Identification of Helicobacter pylori DNA in human cholesterol gallstones. Scand J Gastroenterol. 2002;37:112–119. doi: 10.1080/003655202753387455. [DOI] [PubMed] [Google Scholar]
- 5.Myung SJ, Kim MH, Shim KN, Kim YS, Kim EO, Kim HJ, Park ET, Yoo KS, Lim BC, Seo DW, et al. Detection of Helicobacter pylori DNA in human biliary tree and its association with hepatolithiasis. Dig Dis Sci. 2000;45:1405–1412. doi: 10.1023/a:1005572507572. [DOI] [PubMed] [Google Scholar]
- 6.Fox J. Enterohepatic Helicobacters: natural and experimental models. Ital J Gastroenterol Hepatol. 1998;30 Suppl 3:S264–S269. [PubMed] [Google Scholar]
- 7.Fox JG, Yan LL, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorlin P, Vandamme P, Nortier J, Hoste B, Rossi C, Pavlof S, Struelens MJ. Recurrent "Flexispira rappini" bacteremia in an adult patient undergoing hemodialysis: case report. J Clin Microbiol. 1999;37:1319–1323. doi: 10.1128/jcm.37.5.1319-1323.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Apostolov E, Al-Soud WA, Nilsson I, Kornilovska I, Usenko V, Lyzogubov V, Gaydar Y, Wadström T, Ljungh A. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol. 2005;40:96–102. doi: 10.1080/00365520410009546. [DOI] [PubMed] [Google Scholar]
- 10.Tolia V, Nilsson HO, Boyer K, Wuerth A, Al-Soud WA, Rabah R, Wadström T. Detection of Helicobacter ganmani-like 16S rDNA in pediatric liver tissue. Helicobacter. 2004;9:460–468. doi: 10.1111/j.1083-4389.2004.00266.x. [DOI] [PubMed] [Google Scholar]
- 11.Abu Al-Soud W, Stenram U, Ljungh A, Tranberg KG, Nilsson HO, Wadström T. DNA of Helicobacter spp. and common gut bacteria in primary liver carcinoma. Dig Liver Dis. 2008;40:126–131. doi: 10.1016/j.dld.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson HO, Taneera J, Castedal M, Glatz E, Olsson R, Wadström T. Identification of Helicobacter pylori and other Helicobacter species by PCR, hybridization, and partial DNA sequencing in human liver samples from patients with primary sclerosing cholangitis or primary biliary cirrhosis. J Clin Microbiol. 2000;38:1072–1076. doi: 10.1128/jcm.38.3.1072-1076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nilsson HO, Mulchandani R, Tranberg KG, Stenram U, Wadström T. Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323–324. doi: 10.1053/gast.2001.21382. [DOI] [PubMed] [Google Scholar]
- 14.Ponzetto A, Pellicano R, Leone N, Berrutti M, Turrini F, Rizzetto M. Helicobacter pylori seroprevalence in cirrhotic patients with hepatitis B virus infection. Neth J Med. 2000;56:206–210. doi: 10.1016/s0300-2977(00)00033-4. [DOI] [PubMed] [Google Scholar]
- 15.Laharie D, Asencio C, Asselineau J, Bulois P, Bourreille A, Moreau J, Bonjean P, Lamarque D, Pariente A, Soulé JC, et al. Association between entero-hepatic Helicobacter species and Crohn's disease: a prospective cross-sectional study. Aliment Pharmacol Ther. 2009;30:283–293. doi: 10.1111/j.1365-2036.2009.04034.x. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson HO, Pietroiusti A, Gabrielli M, Zocco MA, Gasbarrini G, Gasbarrini A. Helicobacter pylori and extragastric diseases--other Helicobacters. Helicobacter. 2005;10 Suppl 1:54–65. doi: 10.1111/j.1523-5378.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson HO, Stenram U, Ihse I, Wadstrom T. Helicobacter species ribosomal DNA in the pancreas, stomach and duodenum of pancreatic cancer patients. World J Gastroenterol. 2006;12:3038–3043. doi: 10.3748/wjg.v12.i19.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elwood DR. Cholecystitis. Surg Clin North Am. 2008;88:1241–1252, viii. doi: 10.1016/j.suc.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Caygill CPJ, Hill MJ. Salmonella typhilparatyphi and gallbladder cancer. In: James J, Goedert MD, editors. Infectious causes of cancer. Totowa: Humana Press; 2000. p. 424. [Google Scholar]
- 20.Maurer KJ, Carey MC, Fox JG. Roles of infection, inflammation, and the immune system in cholesterol gallstone formation. Gastroenterology. 2009;136:425–440. doi: 10.1053/j.gastro.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, Zhang BY, Shi JS, Wu LQ. Expression of the bacterial gene in gallbladder carcinoma tissue and bile. Hepatobiliary Pancreat Dis Int. 2004;3:133–135. [PubMed] [Google Scholar]
- 22.Chen DF, Hu L, Yi P, Liu WW, Fang DC, Cao H. Helicobacter pylori damages human gallbladder epithelial cells in vitro. World J Gastroenterol. 2008;14:6924–6928. doi: 10.3748/wjg.14.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farshad Sh, Alborzi A, Malek Hosseini SA, Oboodi B, Rasouli M, Japoni A, Nasiri J. Identification of Helicobacter pylori DNA in Iranian patients with gallstones. Epidemiol Infect. 2004;132:1185–1189. doi: 10.1017/s0950268804002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson I, Shabo I, Svanvik J, Monstein HJ. Multiple displacement amplification of isolated DNA from human gallstones: molecular identification of Helicobacter DNA by means of 16S rDNA-based pyrosequencing analysis. Helicobacter. 2005;10:592–600. doi: 10.1111/j.1523-5378.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 25.Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, Carey MC, Fox JG. Identification of cholelithogenic enterohepatic helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128:1023–1033. doi: 10.1053/j.gastro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Maurer KJ, Rogers AB, Ge Z, Wiese AJ, Carey MC, Fox JG. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol. 2006;290:G175–G182. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dutta U, Garg PK, Kumar R, Tandon RK. Typhoid carriers among patients with gallstones are at increased risk for carcinoma of the gallbladder. Am J Gastroenterol. 2000;95:784–787. doi: 10.1111/j.1572-0241.2000.01860.x. [DOI] [PubMed] [Google Scholar]
- 28.Lai CW, Chan RC, Cheng AF, Sung JY, Leung JW. Common bile duct stones: a cause of chronic salmonellosis. Am J Gastroenterol. 1992;87:1198–1199. [PubMed] [Google Scholar]
- 29.Crawford RW, Gibson DL, Kay WW, Gunn JS. Identification of a bile-induced exopolysaccharide required for Salmonella biofilm formation on gallstone surfaces. Infect Immun. 2008;76:5341–5349. doi: 10.1128/IAI.00786-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harada K, Ozaki S, Kono N, Tsuneyama K, Katayanagi K, Hiramatsu K, Nakanuma Y. Frequent molecular identification of Campylobacter but not Helicobacter genus in bile and biliary epithelium in hepatolithiasis. J Pathol. 2001;193:218–223. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH776>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 31.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 32.Silva CP, Pereira-Lima JC, Oliveira AG, Guerra JB, Marques DL, Sarmanho L, Cabral MM, Queiroz DM. Association of the presence of Helicobacter in gallbladder tissue with cholelithiasis and cholecystitis. J Clin Microbiol. 2003;41:5615–5618. doi: 10.1128/JCM.41.12.5615-5618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulajic M, Maisonneuve P, Schneider-Brachert W, Müller P, Reischl U, Stimec B, Lehn N, Lowenfels AB, Löhr M. Helicobacter pylori and the risk of benign and malignant biliary tract disease. Cancer. 2002;95:1946–1953. doi: 10.1002/cncr.10893. [DOI] [PubMed] [Google Scholar]
- 34.Chen DF, Hu L, Yi P, Liu WW, Fang DC, Cao H. H pylori exist in the gallbladder mucosa of patients with chronic cholecystitis. World J Gastroenterol. 2007;13:1608–1611. doi: 10.3748/wjg.v13.i10.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bohr UR, Kuester D, Meyer F, Wex T, Stillert M, Csepregi A, Lippert H, Roessner A, Malfertheiner P. Low prevalence of Helicobacteraceae in gall-stone disease and gall-bladder carcinoma in the German population. Clin Microbiol Infect. 2007;13:525–531. doi: 10.1111/j.1469-0691.2007.01690.x. [DOI] [PubMed] [Google Scholar]
- 36.Murata H, Tsuji S, Tsujii M, Fu HY, Tanimura H, Tsujimoto M, Matsuura N, Kawano S, Hori M. Helicobacter bilis infection in biliary tract cancer. Aliment Pharmacol Ther. 2004;20 Suppl 1:90–94. doi: 10.1111/j.1365-2036.2004.01972.x. [DOI] [PubMed] [Google Scholar]
- 37.Fukuda K, Kuroki T, Tajima Y, Tsuneoka N, Kitajima T, Matsuzaki S, Furui J, Kanematsu T. Comparative analysis of Helicobacter DNAs and biliary pathology in patients with and without hepatobiliary cancer. Carcinogenesis. 2002;23:1927–1931. doi: 10.1093/carcin/23.11.1927. [DOI] [PubMed] [Google Scholar]
- 38.Lönngren V, Nilsson I, Verbaan H, Wadström T, Ljungh A. High levels of serum antibodies to cell surface proteins of Helicobacter pullorum and Helicobacter pylori in hepatitis C virus-infected patients. Scand J Gastroenterol. 2009;44:505–506. doi: 10.1080/00365520802449294. [DOI] [PubMed] [Google Scholar]
- 39.Ceelen L, Decostere A, Martel A, Pasmans F, Haesebrouck F. First report of Helicobacter pullorum in the faeces of a diarrhoeic psittacine bird (Psephotus haematogaster) Vet Rec. 2006;159:389–390. doi: 10.1136/vr.159.12.389. [DOI] [PubMed] [Google Scholar]
- 40.Misra V, Misra SP, Dwivedi M, Shouche Y, Dharne M, Singh PA. Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology. 2007;39:419–424. doi: 10.1080/00313020701444473. [DOI] [PubMed] [Google Scholar]
- 41.Hamada T, Yokota K, Ayada K, Hirai K, Kamada T, Haruma K, Chayama K, Oguma K. Detection of Helicobacter hepaticus in human bile samples of patients with biliary disease. Helicobacter. 2009;14:545–551. doi: 10.1111/j.1523-5378.2009.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawaguchi M, Saito T, Ohno H, Midorikawa S, Sanji T, Handa Y, Morita S, Yoshida H, Tsurui M, Misaka R, et al. Bacteria closely resembling Helicobacter pylori detected immunohistologically and genetically in resected gallbladder mucosa. J Gastroenterol. 1996;31:294–298. doi: 10.1007/BF02389534. [DOI] [PubMed] [Google Scholar]
- 43.Arnaout AH, Abbas SH, Shousha S. Helicobacter pylori is not identified in areas of gastric metaplasia of gall bladder. J Pathol. 1990;160:333–334. doi: 10.1002/path.1711600410. [DOI] [PubMed] [Google Scholar]
- 44.Hazrah P, Oahn KT, Tewari M, Pandey AK, Kumar K, Mohapatra TM, Shukla HS. The frequency of live bacteria in gallstones. HPB (Oxford) 2004;6:28–32. doi: 10.1080/13651820310025192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abeysuriya V, Deen KI, Wijesuriya T, Salgado SS. Microbiology of gallbladder bile in uncomplicated symptomatic cholelithiasis. Hepatobiliary Pancreat Dis Int. 2008;7:633–637. [PubMed] [Google Scholar]
- 46.Darko R, Archampong EQ, Qureshi Y, Muphy GM, Dowling RH. How often are Ghananian gallbladder stones cholesterol-rich. West Afr J Med. 2000;19:64–70. [PubMed] [Google Scholar]
- 47.Abayli B, Colakoglu S, Serin M, Erdogan S, Isiksal YF, Tuncer I, Koksal F, Demiryurek H. Helicobacter pylori in the etiology of cholesterol gallstones. J Clin Gastroenterol. 2005;39:134–137. [PubMed] [Google Scholar]
- 48.Swidsinski A, Schlien P, Pernthaler A, Gottschalk U, Bärlehner E, Decker G, Swidsinski S, Strassburg J, Loening-Baucke V, Hoffmann U, et al. Bacterial biofilm within diseased pancreatic and biliary tracts. Gut. 2005;54:388–395. doi: 10.1136/gut.2004.043059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moyaert H, Pasmans F, Ducatelle R, Haesebrouck F, Baele M. Evaluation of 16S rRNA gene-based PCR assays for genus-level identification of Helicobacter species. J Clin Microbiol. 2008;46:1867–1869. doi: 10.1128/JCM.00139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Soud WA, Ouis IS, Li DQ, Ljungh S, Wadström T. Characterization of the PCR inhibitory effect of bile to optimize real-time PCR detection of Helicobacter species. FEMS Immunol Med Microbiol. 2005;44:177–182. doi: 10.1016/j.femsim.2004.12.004. [DOI] [PubMed] [Google Scholar]