Abstract
Purpose
To characterize the outcomes of initial and repeated office-based probing as a primary treatment for congenital nasolacrimal duct obstruction (CNLDO) in children.
Methods
The medical records of patients who underwent nasolacrimal duct office-based probing for CNLDO between March 2004 and January 2008 were reviewed retrospectively. Nasolacrimal duct probing was performed on 244 eyes from 229 consecutive patients with CNLDO. Patients who were refractory to the first probing underwent a second probing 4 to 8 weeks later.
Results
Based on exclusion criteria, 244 eyes from 229 patients (117 males and 112 females), aged 6 to 71 months (mean, 12.4 ± 8.36) were included. The success rate of the initial probing was 80% (196 of 244) for all patients, 82% (111 of 136) in the 6 to 12 month age group, 79% (64 of 81) in the 13 to 18 months age group, and 78% (21 of 27) among individuals older than 19 months (p = 0.868, Pearson chi-square test). The success rate of the second probing was 61% (25 of 41) for all patients, 74% (17 of 23) in the 6 to 12 months age group, 58% (7 of 12) in the 13 to 18 months age group, and 17% (1 of 6) among individuals older than 19 months (p = 0.043, Fisher's exact test).
Conclusions
While the success rate of initial nasolacrimal duct probing is not affected by age, the rate of success rate with a second probing was significantly lower in patients older than 19 months. Based on the results, authors recommend further surgical interventions, such as silicone tube intubation or balloon dacryocystoplasty, instead of repeated office probing for patients older than 19 months, if an initial office probing has failed.
Keywords: Congenital nasolacrimal duct obstruction, Nasolacrimal duct probing, Office probing, Repeated probing
Congenital nasolacrimal duct obstruction (CNLDO) is a fairly common condition in early childhood, with a reported prevalance of 1.25% to 12.5% [1-3].
In infants with CNLDO, hydrostatic massage of the lacrimal sac and administration of topical antibiotics is considered the standard, firs-line treatment, and results in a cure rate greater than 90% [4,5]. In patients less than 13 months of age where CNLDO persists for longer than several months, nasolacrimal probing is often recommended and yields a high success rate [6,7]. However, as some reports have suggested that the success rate of this intervention drops substantially in older children [6,8-10], more complicated and invasive procedures - such as silastic intubation or dacryocystorhinostomy - have been attempted in these individuals [8-12]. Yet, these more aggressive interventions are still controversial, as several reports have recently been published that demonstrate high cure rates with nasolacrimal probing in children older than 13 months [13-16]. Moreover, numerous studies also indicate that a second round of nasolacrimal probing is often successful in patients unresponsive to the initial intervention, with cure rates ranging from 11.1 to 100% [6,13,15-20]. In one recent study, Older [21] further specified that repeat probings had the highest rates of success in cases where the probe was able to be passed into the nose, or where irrigation fluid was recovered from the nose.
Few studies exist concerning the effect of age on the success rate of repeat nasolacrimal probing [6,15,20], leaving clinicians little guidance for further treatment options in cases where the initial probing has failed. Here, we report the outcomes of initial and repeated office probings in the primary treatment of CNLDO among children in three distinct age groups: 6 to 12 months, 13 to 18 months, and older than 19 months. Our goal was to characterize the efficacy initial and repeated probings by age, and ultimately identify optimal age-appropriate interventions and treatment modalities for CNLDO.
Materials and Methods
The current study is a retrospective comparative case series of consecutive patients on whom the author performed nasolacrimal duct probing as the primary treatment method for CNLDO. The medical records of patients who underwent nasolacrimal duct probing at Korea University Hospital for CNLDO between March 2004 and January 2008 were reviewed retrospectively. In total, nasolacrimal duct probing was performed on 259 eyes of 242 consecutive patients with CNLDO. After enrollment, fifteen eyes from 13 patients were excluded, leaving 244 eyes of 229 patients. Exclusion criteria included epiblepharon (4 eyes from 2 patients), acute dacryocystitis (2 eyes), canalicular obstruction (2 eyes), and loss to follow-up (7 eyes). Patients with previous history of probing were also excluded, as were patients with dacryocistitis (diagnosed by visualizing pus after digital sac compression), in whom silicone tube intubation was performed directly.
Several positive findings during the ocular examination were used to screen for CNLDO: increased tear lake size or tear pooling, enlarged lacrimal sacs, or the regurgitation of mucous during lacrimal sac pressure. This diagnosis was confirmed by dye disappearance test, performed by instilling 0.5% alcaine and either one drop of 2% fluorescein or a moistened fluorescein filter paper strip into the conjunctival fornix of each eye. After the excess dye was wiped away, the child was then examined using light from a cobalt blue filter lamp at a distance of 1 meter in a semi-darkened room. In this study, dye retention test values greater than 1+ were interpreted as positive result. Additionally, the regurgitation of purulent material during lacrimal sac massage also confirmed the diagnosis.
Prior to probing, enrolled subjects did not receive any therapy besides lacrimal sac massage and/or topical antibiotics. In all cases, nasolacrimal duct probing was performed in an outpatient setting under topical anesthesia. Specifically, the infant was first immobilized on a bed, and topical anesthetic eyedrops were applied to the affected eye(s). Probing was then performed via the lower punctum using standardized Bowman probes. In infants younger than 36 months, an initial attempt was made using the size 00 probe (0.90-mm diameter), while a size 0 probe (1.00-mm diameter) was used in patients 37 to 48 months and a size 1 (1.10-mm diameter) in patients older than 49 months. After the initial dilation of the lower punctum by fine punctual dilator, the Bowman probe was inserted perpendicular to the lower eyelid margin reaching the ampulla. The probe was then rotated horizontally to the lower canaliculus and inserted toward the lacrimal sac at a slightly upward angle, while lateral traction was applied to the eyelid. When a hard stop was felt, the probe was rotated 90 degrees and advanced toward the nasolacrimal duct until a "popping" sensation is felt. The probe was then removed, and the patency of the lacrimal drainage system was confirmed by fluorescein irrigation, using a cotton swab in the nasal cavity to check for direct dye drainage. For all enrolled patients, probing was performed by one oculoplastic surgeon (SHB).
After probing, patients were given two different antibiotic drops - Tobra® (0.3% tobramycin; Daewoong, Seoul, Korea) and Flucon® (0.1% fluorometholone; Alcon, Fort Worth, TX, USA) - to be applied four times daily for one week. Patients additionally underwent postoperatively reevaluations at 1 week, 1 month, and 2 months. Here, success of the nasolacrimal duct probing was defined as a complete remission of symptoms and signs (tearing, crusting, discharge, regurgitation on pressure over the lacrimal sac) within 1 month of the procedure with no evidence of recurrence during the study period. If the first probing failed, a second probing was performed using the same technique 4 to 8 weeks after the initial attempt.
Results
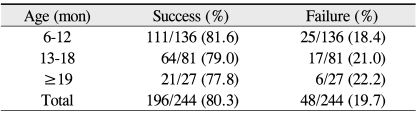
In total, 244 eyes from 229 patients were evaluated: 117 males and 112 females, 118 right eyes and 126 left eyes, and 15 individuals with bilateral CNLDO. At the time of initial probing, patients ranged in age from 6 to 71 months (mean 12.4 ± 8.36). In patients undergoing repeated probings, ages ranged from 6 to 26 months (mean 13.3 ± 6.22). The success rate of the initial probing was 80% (196 of 244) among all subjects, 82% (111 of 136) in the 6 to 12 month age group, 79% (64 of 81) in the 13 to 18 months age group, and 78% (21 of 27) in subjects greater than 19 months of age (p = 0.868, Pearson chi-square test) (Table 1).
Table 1.
Success rate of initial probing for congenital nasolacrimal duct obstruction in 244 eyes
Of the 48 eyes refractory to the initial probing, seven eyes did not undergo a second round, instead receiving direct silicone tube intubation due to parental preference for a more definite surgical treatment. Thus, a second probing was only performed in 41 eyes.
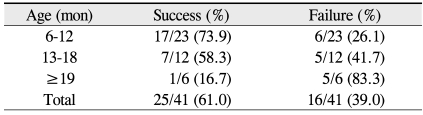
The success rate of the second probing was 61% (25 of 41) among all patients, 74% (17 of 23) in the 6 to 12 month age group, 58% (7 of 12) in the 13 to 18 months age group, and 17% (1 of 6) in the 19 to 26 months age group. Notably, the cure rate among individuals older than 19 months was significantly different from the other two groups (p = 0.043, Fisher's exact test) (Table 2). Accordingly, while the success rate of the initial nasolacrimal duct probing was not found to decline with increased age, a second round of probing was less likely to be effective in older individuals.
Table 2.
Success rate of a second probing for congenital nasolacrimal duct obstruction in 41 eyes
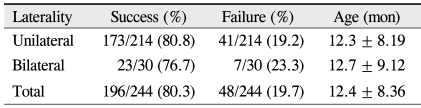
The success rate of the initial probing was 81% (173 of 214) in unilaterally affected patients, and 77% (23 of 30) in bilaterally cases (p = 0.625, chi-square test) (Table 3). Among sixteen eyes that were refractory to both probings, only 1 was from a bilaterally affected patient.
Table 3.
Success rate of initial probing for congenital nasolacrimal duct obstruction in 244 eyes by laterality
Discussion
Although nasolacrimal duct probing is a standard therapeutic procedure in the management of CNLDO, some controversy exists regarding the optimal timing of probing, outcomes in older children, and treatment of choice after a failed attempt.
Here, the success rates were 82% (111 of 136) for the 6 to 12 months age group, 79% (64 of 81) for the 13 to 18 months age group, and 78% (21 of 27) for the individuals greater than 19 months in age. Although the total number of patients in the oldest age group was relatively small, the success rate of initial probing did not seem to be affected by patient age.
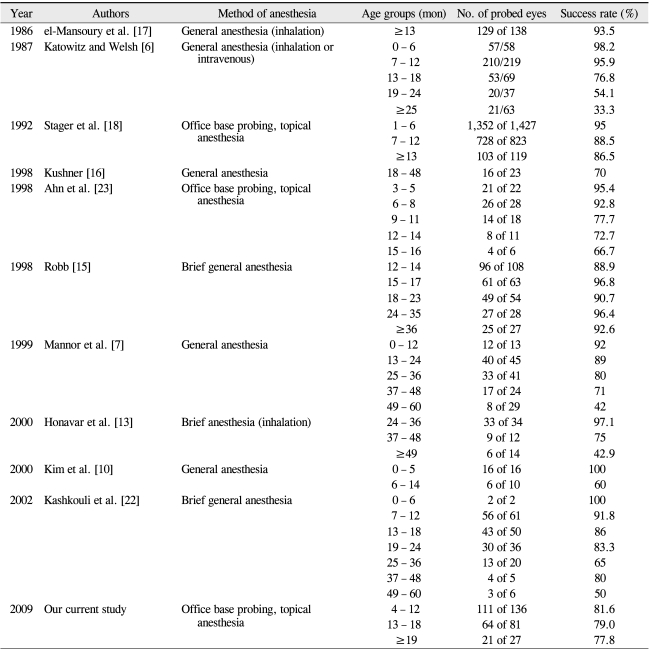
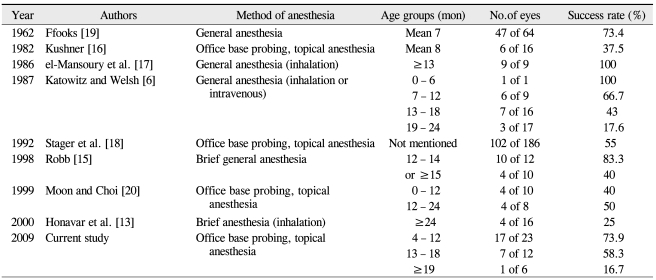
Conversely, age and efficacy of repeat probings were found to be inversely correlated. In a similar study, Katowitz and Welsh [6] reported a probing success rate of 98.2% in subjects aged 0 to 6 months, 95.9% in subjects aged 7 to 12 months, 76.8% in subjects aged 13 to 18 months, and 54.1% in subjects aged 19 to 24 months. Likewise, the success rates in a study from Mannor et al. [7] were 92% for subjects aged 0 to 12 months, 89% for subjects aged 13 to 24 months, 80% for subjects aged 25 to 36 months, and 71% for subjects aged 37 to 48 months. In a study from Kashkouli et al. [22], the reported success rate with probing were 92% for subjects aged 0 to 12 months, 84.4% for subjects aged 13 to 24 months, 65% for subjects aged 25 to 36 months, and 63.5% for subjects aged 37 to 60 months. Lastly, the data from Ahn et al. [23] showed probing success rates of 95.4% for subjects aged 3 to 5 months, 92.8% for subjects aged 6 to 8 months, 77.7% for subjects aged 9 to 11 months, 72.7% for subjects aged 12 to 14 months, and 66.7% for subjects aged 15 to 16 months.
However, in contrast to these studies, el-Mansoury et al. [17] reported a significantly higher probing success rate at 93.5% among individuals older than 13 months (range, 13-84; mean, 22). In a study from Stager et al. [18], the reported probing success rates were 95% among subjects aged 1 to 6 months, 88.5% in subjects aged 7 to 12 months, and 86.5% in subjects older than 13 months. The data from Robb [15] also followed this trend, with probing success rates of 88.9% in subjects aged 12 to 14 months, 96.8% in subjects aged 15 to 17 months, 90.7% in subjects aged 18 to 23 months, 96.4% in subjects aged 24 to 35 months, and 92.6% in subjects aged 36 to 111 months (Table 4).
Table 4.
Comparison of success rates of initial nasolacrimal duct probing for congenital nasolacrimal duct obstruction
Several case series have reviewed the outcomes of repeated probings in the treatment of persistent CNLDO, with success ranging from 11.1% to 100% (Table 5) [6,13,15-19]. Here, the success rate with repeated probing was significantly higher in the younger age groups (74% in the 6 to 12 months age group, 58% in the 13 to 18 months age group) than in older individuals (17% in subjects at least 19 months) (p = 0.043). As such, our results mirror those of Katowitz and Welsh [6], who reported repeated nasolacrimal probing success rates of 67% in children between the ages of 6 to 13 months and 18% in children between the ages of 18 to 24 months. The data from the Ghuman et al. [24] study was also similar to ours, as they reported repeated probing success rate of 69% in subjects younger than 13 months, whereas the comparable group in our study has a success rate of 74%. However, Moon and Choi [20] found that the success rates of repeat probings did not decrease with age: 40% for subjects aged 0 to 12 months and 50% for subjects aged 12 to 48 months.
Table 5.
Comparison of success rates of repeated nasolacrimal duct probing for congenital nasolacrimal duct obstruction
Many factors are believed to affect the success rate of nasolacrimal probing: age, bilaterality, prior failed probing attempts, prior failed conservative treatments, dilated sacs, and non-membranous CNLDO have all been shown to significantly impact probing success (p < 0.05) [13]. Similarly, new evidence also suggests that concurrent fistulae also reduce the probing success rates [25], and a study from Kashkouli et al. [22] indicates that increasing age, non-membranous obstruction, and canalicular stenosis all independently predict probing failure. While controversy regarding these associations still persist, in this study series, neither age at the time of the initial intervention nor bilaterality were found not to affect the probing success rate, whereas increased age was associated with significantly lower probing success rates in cases requiring repeated probings. Although the reason for the discrepant effect of age on probing success is unclear, two hypotheses exist regarding the lower cure rates of probing in older children with CNLDO. First, prolonged inflammation in lacrimal drainage system may result in a fibrosis that increases with age [6,20]. Similarly, some speculate that, while less severe obstructions may spontaneously clear, more complex cases present later [3,16,17], and thus the success of probing is not affected by age at the time of probing, but rather the increased frequency of severe and mixed obstructions in the older age group [13]. However, to definitively characterize this associations additional studies addressing complex CNLDO, the impact of probing, and the distribution of this condition in older children are needed.
Additionally, some controversy exists regarding whether bilaterality is also a poor prognostic indicator of probing success. While Kashkouli et al. [22] reported that bilaterality had no significant impact on cure rate, Honavar et al. [13] found that patients with bilateral CNLDO had increased rates of probe failure (p = 0.012). Here, the success rate of initial probing was 81% in unilaterally affected patients and 77% in bilaterally affected patients (p = 0.619). The correlation between success rate and bilaterality also did not reach significance in individuals undergoing repeated probing: among the sixteen eyes that failed the second probing, only one was from a bilaterally affected patient.
In comparing the different probing success rates, both method of anesthesia and probing location could represent factors that affect probing efficacy. One study showed that office-based procedures performed under topical anesthesia with physical restraints were associated with significantly lower rates of success (72%) when compared with procedures performed in a medical facility under brief general anesthesia (80%) [26]. In their analysis of these data, the authors of that study concluded that the lower rate of success associated with office-based interventions may result from less aggressive probing (e.g., probe only being passed once). However, other studies contradict these findings: Stager et al. [18] reported a cure rate of 89% among 119 children older than 12 months in a retrospective review of 2,369 office-based procedures, while another retrospective review from Baker [27] demonstrated a success rate of 94% among 860 office probing.
Notably, office probing has several significant advantages. Such procedures are more likely to be performed early, when infants can be more easily restrained, hypothetically resulting in higher success rates. In contrast, surgeons who perform all probings in a medical facility under general anesthesia often wait until patients reach a target age, possibly allowing for the spontaneous resolution of the less severe cases [17]. Office-based probing may also be less psychologically traumatic to infants than general anesthesia, which involves blood draws, restraint of the child during the use of the anesthesia mask, and the disorientation and separation anxiety associated with the recovery room [6]. In our experience, infants undergoing probing stopped crying and relaxed within one or two minutes after the procedure was complete, suggesting that the associated discomfort is likely quickly forgotten. Furthermore, the probing site varies between the two procedures. Under general anesthesia, probing occurs via the upper punctum rather than inferior punctum, since the risk of canalicular damage is lower. However, in office-based procedures, the lower punctum is probed perpendicularly to the lower eyelid margin, as this technique is technically easier. Additionally, unsuccessful probings most commonly result from incomplete mucosal perforation and false passage formation, both of which are difficult to identify in office-based probings.
Nevertheless, it is difficult to confirm the passage of the probe into the nasal cavity, since an endoscope is not used, and a "popping" sensation is used to certify a good passage of the probe. Unsuccessful probings mainly result from intraoperative problems, such as incomplete mucosal perforation, submucosal passage, and false passage formation [28], all of which could be regarded as substantial disadvantages to office-based procedures.
One limitation of our study was that probing was performed under an outpatient based topical anesthesia. Moreover, the obstruction type was difficult to determine in many cases. In cases of mixed-type obstruction, treatment can fail for multiple reasons, including structural blockages and functional blockages of the nasolacrimal duct. Thus, a prospective, randomized trial is needed to definitively evaluate the efficacy of both initial and repeated probing. As with all retrospective studies, imperfect standardization of probing techniques and postoperative examination intervals may have acted as confounding factors here. Additionally, too few children from the 19 to 26 months age group were enrolled to accurately reach a conclusion regarding probing success in this age group. In office-based probings, infants are immobilized on a bed and probing is performed after the application of topical anesthetic eyedrops. Office probing is markedly more difficult to perform in older children, given the possibility of canalicular injury.
The limited scope of the present study is another major limitation. Specifically, only the patients' age and laterality were considered, and no data regarding obstruction type an probing impression were collected, even though both variables may affect the clinical course. Complex-type obstructions (non-membranous, firm, or complicated obstruction) and submucosal passages are more resistant to probing and result in worse success rates [29,30]. Accordingly, further studies that identify the effect of such factors on the success of probing for nasolacrimal duct obstruction are needed.
In conclusion, our results indicate that while the success rate for initial nasolacrimal duct probing is not affected by age, the cure rate of a second round of probing declines in individuals older than 19 months. Based on these results, we contend that although probing should be the initial treatment of choice for children older than 19 months, if this fails, surgical interventions (e.g. as silicone tube intubation or balloon dacryocystoplasty) should be employed rather than a second probing.
Footnotes
Presented in part at 102nd the Korean Ophthalmological Society Meeting, November 6-8, 2009, Goyang, Korea
No potential conflict of interest relevant to this article was reported.
References
- 1.Guerry D, 3rd, Kendig EL., Jr Congenital impatency of the nasolacrimal duct. Arch Ophthal. 1948;39:193–204. doi: 10.1001/archopht.1948.00900020198006. [DOI] [PubMed] [Google Scholar]
- 2.Noda S, Hayasaka S, Setogawa T. Congenital nasolacrimal duct obstruction in Japanese infants: its incidence and treatment with massage. J Pediatr Ophthalmol Strabismus. 1991;28:20–22. [PubMed] [Google Scholar]
- 3.Paul TO, Shepherd R. Congenital nasolacrimal duct obstruction: natural history and the timing of optimal intervention. J Pediatr Ophthalmol Strabismus. 1994;31:362–367. doi: 10.3928/0191-3913-19941101-04. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RA, Robb RM. The natural course of congenital obstruction of the nasolacrimal duct. J Pediatr Ophthalmol Strabismus. 1978;15:246–250. doi: 10.3928/0191-3913-19780701-14. [DOI] [PubMed] [Google Scholar]
- 5.Nelson LB, Calhoun JH, Menduke H. Medical management of congenital nasolacrimal duct obstruction. Pediatrics. 1985;76:172–175. [PubMed] [Google Scholar]
- 6.Katowitz JA, Welsh MG. Timing of initial probing and irrigation in congenital nasolacrimal duct obstruction. Ophthalmology. 1987;94:698–705. doi: 10.1016/s0161-6420(87)33392-5. [DOI] [PubMed] [Google Scholar]
- 7.Mannor GE, Rose GE, Frimpong-Ansah K, Ezra E. Factors affecting the success of nasolacrimal duct probing for congenital nasolacrimal duct obstruction. Am J Ophthalmol. 1999;127:616–617. doi: 10.1016/s0002-9394(98)00432-2. [DOI] [PubMed] [Google Scholar]
- 8.Havins WE, Wilkins RB. A useful alternative to silicone intubation in congenital nasolacrimal duct obstructions. Ophthalmic Surg. 1983;14:666–670. [PubMed] [Google Scholar]
- 9.Ogawa GS, Gonnering RS. Congenital nasolacrimal duct obstruction. J Pediatr. 1991;119(1 Pt 1):12–17. doi: 10.1016/s0022-3476(05)81031-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim YS, Moon SC, Yoo KW. Congenital nasolacrimal duct obstruction: irrigation or probing? Korean J Ophthalmol. 2000;14:90–96. doi: 10.3341/kjo.2000.14.2.90. [DOI] [PubMed] [Google Scholar]
- 11.Becker BB, Berry FD, Koller H. Balloon catheter dilatation for treatment of congenital nasolacrimal duct obstruction. Am J Ophthalmol. 1996;121:304–309. doi: 10.1016/s0002-9394(14)70279-x. [DOI] [PubMed] [Google Scholar]
- 12.Repka MX, Chandler DL, Holmes JM, et al. Balloon catheter dilation and nasolacrimal duct intubation for treatment of nasolacrimal duct obstruction after failed probing. Arch Ophthalmol. 2009;127:633–639. doi: 10.1001/archophthalmol.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honavar SG, Prakash VE, Rao GN. Outcome of probing for congenital nasolacrimal duct obstruction in older children. Am J Ophthalmol. 2000;130:42–48. doi: 10.1016/s0002-9394(00)00388-3. [DOI] [PubMed] [Google Scholar]
- 14.Gunton KB, Chung CW, Schnall BM, et al. Comparison of balloon dacryocystoplasty to probing as the primary treatment of congenital nasolacrimal duct obstruction. J AAPOS. 2001;5:139–142. doi: 10.1067/mpa.2001.115218. [DOI] [PubMed] [Google Scholar]
- 15.Robb RM. Success rates of nasolacrimal duct probing at time intervals after 1 year of age. Ophthalmology. 1998;105:1307–1309. doi: 10.1016/S0161-6420(98)97038-5. [DOI] [PubMed] [Google Scholar]
- 16.Kushner BJ. The management of nasolacrimal duct obstruction in children between 18 months and 4 years old. J AAPOS. 1998;2:57–60. doi: 10.1016/s1091-8531(98)90112-4. [DOI] [PubMed] [Google Scholar]
- 17.el-Mansoury J, Calhoun JH, Nelson LB, Harley RD. Results of late probing for congenital nasolacrimal duct obstruction. Ophthalmology. 1986;93:1052–1054. doi: 10.1016/s0161-6420(86)33621-2. [DOI] [PubMed] [Google Scholar]
- 18.Stager D, Baker JD, Frey T, et al. Office probing of congenital nasolacrimal duct obstruction. Ophthalmic Surg. 1992;23:482–484. [PubMed] [Google Scholar]
- 19.Ffooks OO. Dacryocystitis in infancy. Br J Ophthalmol. 1962;46:422–434. doi: 10.1136/bjo.46.7.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon JS, Choi WC. Office probing of congenital nasolacrimal duct obstruction. J Korean Ophthalmol Soc. 1999;40:2357–2361. [Google Scholar]
- 21.Older J. Congenital lacrimal disorders and management. London: Churchill Livingstone; 1988. [Google Scholar]
- 22.Kashkouli MB, Kassaee A, Tabatabaee Z. Initial nasolacrimal duct probing in children under age 5: cure rate and factors affecting success. J AAPOS. 2002;6:360–363. doi: 10.1067/mpa.2002.129041. [DOI] [PubMed] [Google Scholar]
- 23.Ahn DH, Lew H, Kim HY, Lee SY. The effect of probing for congenital nasolacrimal duct obstruction. J Korean Ophthalmol Soc. 1998;39:836–840. [Google Scholar]
- 24.Ghuman T, Gonzales C, Mazow ML. Treatment of congenital nasolacrimal duct obstruction. Am Orthopt J. 1999;49:163–168. [Google Scholar]
- 25.Welham RA, Bergin DJ. Congenital lacrimal fistulas. Arch Ophthalmol. 1985;103:545–548. doi: 10.1001/archopht.1985.01050040087025. [DOI] [PubMed] [Google Scholar]
- 26.Repka MX, Chandler DL, Beck RW, et al. Primary treatment of nasolacrimal duct obstruction with probing in children younger than 4 years. Ophthalmology. 2008;115:577–584. doi: 10.1016/j.ophtha.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JD. Treatment of congenital nasolacrimal system obstruction. J Pediatr Ophthalmol Strabismus. 1985;22:34–36. doi: 10.3928/0191-3913-19850101-12. [DOI] [PubMed] [Google Scholar]
- 28.Steindler P, Mantovani E, Incorvaia C, Parmeggiani F. Efficacy of probing for children with congenital nasolacrimal duct obstruction: a retrospective study using fluorescein dye disappearance test and lacrimal sac echography. Graefes Arch Clin Exp Ophthalmol. 2009;247:837–846. doi: 10.1007/s00417-008-1022-1. [DOI] [PubMed] [Google Scholar]
- 29.Limbu B, Akin M, Saiju R. Age-based comparison of successful probing in Nepalese children with nasolacrimal duct obstruction. Orbit. 2010;29:16–20. doi: 10.3109/01676830903207844. [DOI] [PubMed] [Google Scholar]
- 30.Choi WC, Kim KS, Park TK, Chung CS. Intranasal endoscopic diagnosis and treatment in congenital nasolacrimal duct obstruction. Ophthalmic Surg Lasers. 2002;33:288–292. [PubMed] [Google Scholar]