Abstract
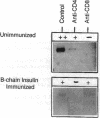
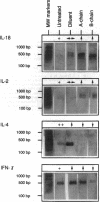
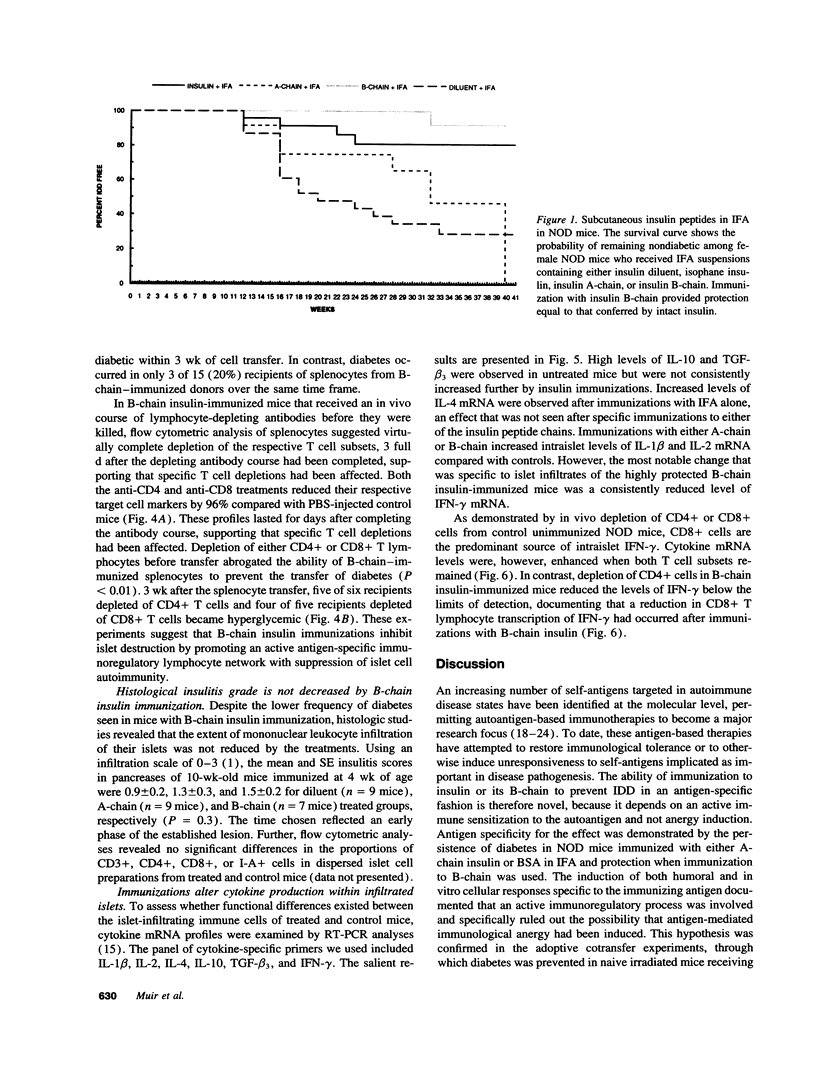
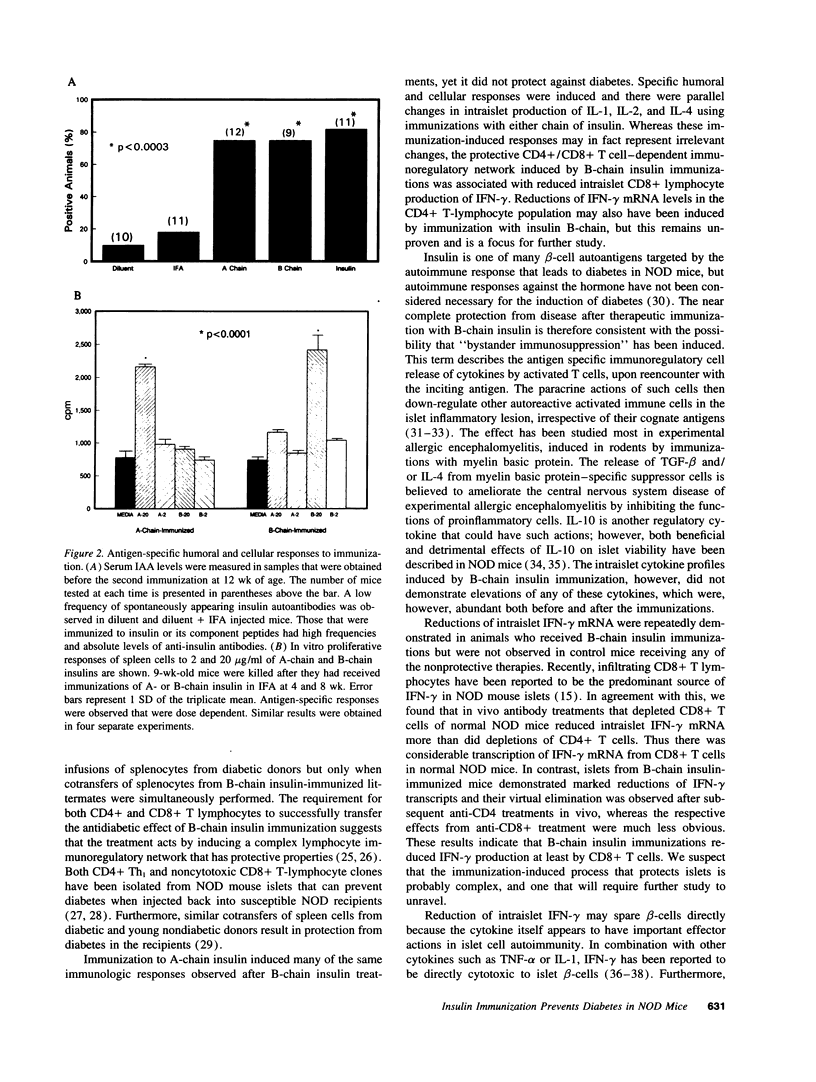
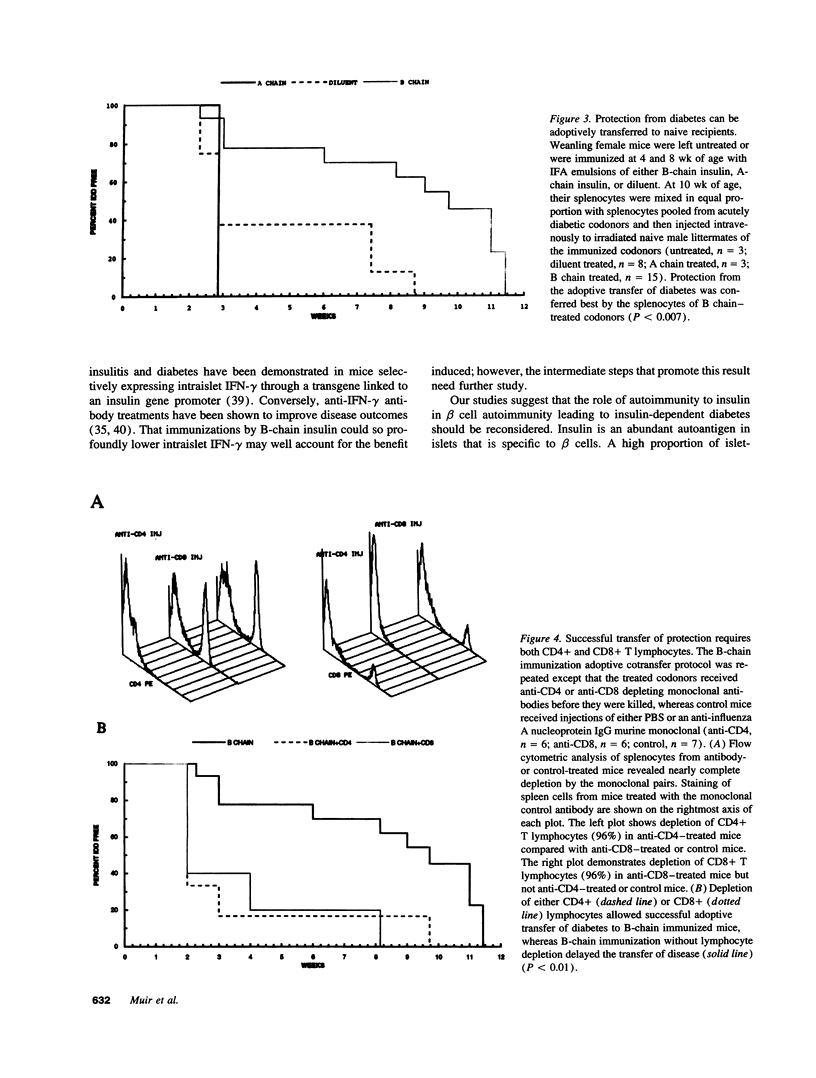
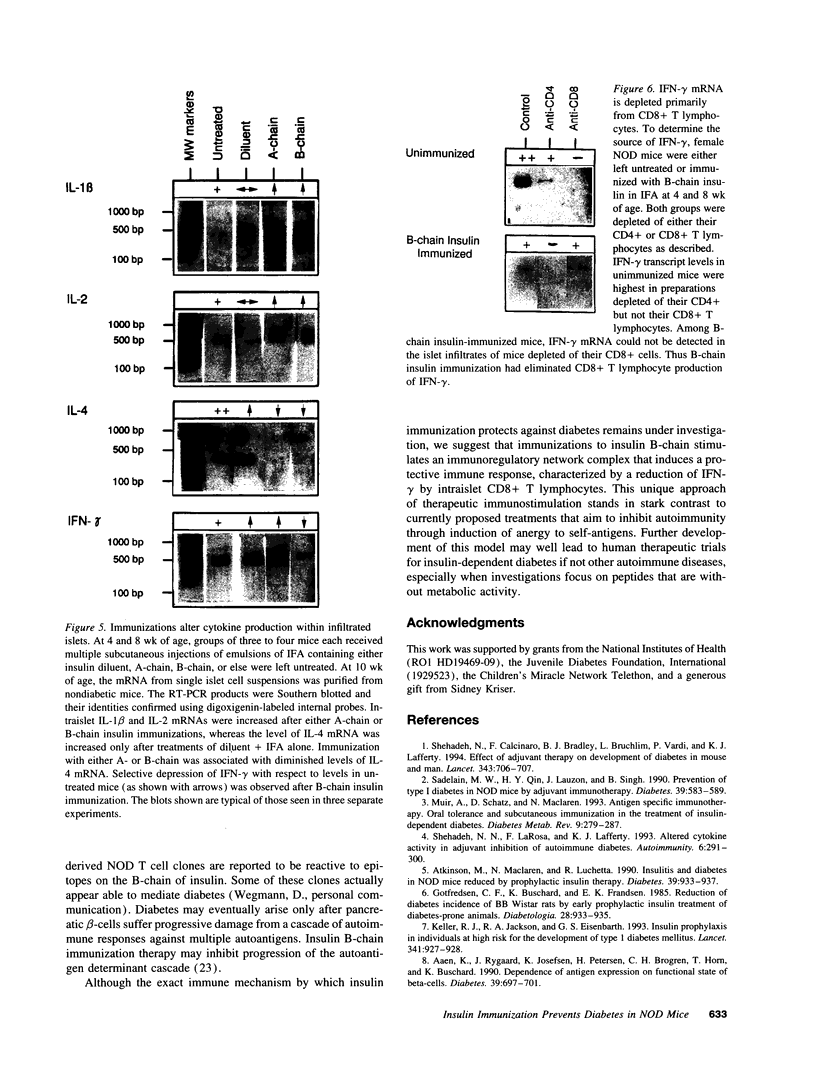
We reported previously that daily injections of isophane insulin prevented both hyperglycemia and insulitis in nonobese diabetic (NOD) mice (Atkinson, M., N. Maclaren; and R. Luchetta. 1990. Diabetes. 39:933-937). The possible mechanisms responsible include reduced immunogenicity of pancreatic beta-cells from "beta-cell rest" and induced active immunoregulation to insulin (Aaen, IK., J. Rygaard, K. Josefsen, H. Petersen, C. H. Brogren, T. Horn, and K. Buschard. 1990. Diabetes. 39:697-701). We report here that intermittent immunizations with insulin or its metabolically inactive B-chain in incomplete Freund's adjuvant also prevent diabetes in NOD mice, whereas immunizations with A-chain insulin or with BSA do not. Adoptive transfer of splenocytes from B-chain insulin-immunized mice prevented diabetes in recipients co-infused with diabetogenic spleen cells, an effect that was abolished by prior in vivo elimination of either CD4+ or CD8+ cells. Insulin immunization did not reduce the extent of intraislet inflammation (insulitis); however, it did abolish expression of IFN-gamma mRNA within the insulitis lesions. Immunizations with insulin thus induce an active suppressive response to determinants on the B-chain that converts the insulitis lesion from one that is destructive to one that is protective.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaen K., Rygaard J., Josefsen K., Petersen H., Brogren C. H., Horn T., Buschard K. Dependence of antigen expression on functional state of beta-cells. Diabetes. 1990 Jun;39(6):697–701. doi: 10.2337/diab.39.6.697. [DOI] [PubMed] [Google Scholar]
- Adorini L., Barnaba V., Bona C., Celada F., Lanzavecchia A., Sercarz E., Suciu-Foca N., Wekerle H. New perspectives on immunointervention in autoimmune diseases. Immunol Today. 1990 Nov;11(11):383–386. doi: 10.1016/0167-5699(90)90148-3. [DOI] [PubMed] [Google Scholar]
- Anderson J. T., Cornelius J. G., Jarpe A. J., Winter W. E., Peck A. B. Insulin-dependent diabetes in the NOD mouse model. II. Beta cell destruction in autoimmune diabetes is a TH2 and not a TH1 mediated event. Autoimmunity. 1993;15(2):113–122. doi: 10.3109/08916939309043886. [DOI] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K. Islet cell autoantigens in insulin-dependent diabetes. J Clin Invest. 1993 Oct;92(4):1608–1616. doi: 10.1172/JCI116745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson M. A., Maclaren N. K., Luchetta R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes. 1990 Aug;39(8):933–937. doi: 10.2337/diab.39.8.933. [DOI] [PubMed] [Google Scholar]
- Björk E., Kämpe O., Grawé J., Hallberg A., Norheim I., Karlsson F. A. Modulation of beta-cell activity and its influence on islet cell antibody (ICA) and islet cell surface antibody (ICSA) reactivity. Autoimmunity. 1993;16(3):181–188. doi: 10.3109/08916939308993326. [DOI] [PubMed] [Google Scholar]
- Boitard C., Yasunami R., Dardenne M., Bach J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J Exp Med. 1989 May 1;169(5):1669–1680. doi: 10.1084/jem.169.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. L., Iscaro A., Harrison L. C. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988 Oct 1;141(7):2325–2329. [PubMed] [Google Scholar]
- Campbell I. L., Kay T. W., Oxbrow L., Harrison L. C. Essential role for interferon-gamma and interleukin-6 in autoimmune insulin-dependent diabetes in NOD/Wehi mice. J Clin Invest. 1991 Feb;87(2):739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Elias D., Cohen I. R. Peptide therapy for diabetes in NOD mice. Lancet. 1994 Mar 19;343(8899):704–706. doi: 10.1016/s0140-6736(94)91582-2. [DOI] [PubMed] [Google Scholar]
- Foulis A. K., McGill M., Farquharson M. A. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man--macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol. 1991 Oct;165(2):97–103. doi: 10.1002/path.1711650203. [DOI] [PubMed] [Google Scholar]
- Gotfredsen C. F., Buschard K., Frandsen E. K. Reduction of diabetes incidence of BB Wistar rats by early prophylactic insulin treatment of diabetes-prone animals. Diabetologia. 1985 Dec;28(12):933–935. doi: 10.1007/BF00703140. [DOI] [PubMed] [Google Scholar]
- Karpus W. J., Swanborg R. H. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991 Feb 15;146(4):1163–1168. [PubMed] [Google Scholar]
- Kawamura T., Nagata M., Utsugi T., Yoon J. W. Prevention of autoimmune type I diabetes by CD4+ suppressor T cells in superantigen-treated non-obese diabetic mice. J Immunol. 1993 Oct 15;151(8):4362–4370. [PubMed] [Google Scholar]
- Keller R. J., Eisenbarth G. S., Jackson R. A. Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet. 1993 Apr 10;341(8850):927–928. doi: 10.1016/0140-6736(93)91215-8. [DOI] [PubMed] [Google Scholar]
- Khoury S. J., Hancock W. W., Weiner H. L. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992 Nov 1;176(5):1355–1364. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Y. M., Giraldo A. A., Waldmann H., Cobbold S. P., Fuller B. E. Resistance to experimental autoimmune thyroiditis: L3T4+ cells as mediators of both thyroglobulin-activated and TSH-induced suppression. Clin Immunol Immunopathol. 1989 Apr;51(1):38–54. doi: 10.1016/0090-1229(89)90204-3. [DOI] [PubMed] [Google Scholar]
- Lider O., Santos L. M., Lee C. S., Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein. II. Suppression of disease and in vitro immune responses is mediated by antigen-specific CD8+ T lymphocytes. J Immunol. 1989 Feb 1;142(3):748–752. [PubMed] [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966 Mar;50(3):163–170. [PubMed] [Google Scholar]
- Miller A., Lider O., Roberts A. B., Sporn M. B., Weiner H. L. Suppressor T cells generated by oral tolerization to myelin basic protein suppress both in vitro and in vivo immune responses by the release of transforming growth factor beta after antigen-specific triggering. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):421–425. doi: 10.1073/pnas.89.1.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A., Schatz D., Maclaren N. Antigen-specific immunotherapy: oral tolerance and subcutaneous immunization in the treatment of insulin-dependent diabetes. Diabetes Metab Rev. 1993 Dec;9(4):279–287. doi: 10.1002/dmr.5610090408. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Asplin C. M., Clemons P., Lyen K., Tatpati O., Raghu P. K., Paquette T. L. Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science. 1983 Dec 23;222(4630):1337–1339. doi: 10.1126/science.6362005. [DOI] [PubMed] [Google Scholar]
- Pankewycz O., Strom T. B., Rubin-Kelley V. E. Islet-infiltrating T cell clones from non-obese diabetic mice that promote or prevent accelerated onset diabetes. Eur J Immunol. 1991 Apr;21(4):873–879. doi: 10.1002/eji.1830210403. [DOI] [PubMed] [Google Scholar]
- Peck A. B., Bach F. H. A miniaturized mouse mixed leukocyte culture in serum-free and mouse serum supplemented media. J Immunol Methods. 1973 Oct;3(2):147–163. doi: 10.1016/0022-1759(73)90030-6. [DOI] [PubMed] [Google Scholar]
- Pennline K. J., Roque-Gaffney E., Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994 May;71(2):169–175. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- Qin S., Cobbold S., Tighe H., Benjamin R., Waldmann H. CD4 monoclonal antibody pairs for immunosuppression and tolerance induction. Eur J Immunol. 1987 Aug;17(8):1159–1165. doi: 10.1002/eji.1830170813. [DOI] [PubMed] [Google Scholar]
- Rabinovitch A., Sumoski W., Rajotte R. V., Warnock G. L. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990 Jul;71(1):152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- Reich E. P., Scaringe D., Yagi J., Sherwin R. S., Janeway C. A., Jr Prevention of diabetes in NOD mice by injection of autoreactive T-lymphocytes. Diabetes. 1989 Dec;38(12):1647–1651. doi: 10.2337/diab.38.12.1647. [DOI] [PubMed] [Google Scholar]
- Sadelain M. W., Qin H. Y., Lauzon J., Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990 May;39(5):583–589. doi: 10.2337/diab.39.5.583. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Shizuru J., Liggitt D., Martin L., McIntyre B., Gregory A., Parslow T., Stewart T. Loss of pancreatic islet tolerance induced by beta-cell expression of interferon-gamma. Nature. 1990 Aug 30;346(6287):844–847. doi: 10.1038/346844a0. [DOI] [PubMed] [Google Scholar]
- Serreze D. V., Hamaguchi K., Leiter E. H. Immunostimulation circumvents diabetes in NOD/Lt mice. J Autoimmun. 1989 Dec;2(6):759–776. doi: 10.1016/0896-8411(89)90003-6. [DOI] [PubMed] [Google Scholar]
- Shehadeh N. N., LaRosa F., Lafferty K. J. Altered cytokine activity in adjuvant inhibition of autoimmune diabetes. J Autoimmun. 1993 Jun;6(3):291–300. doi: 10.1006/jaut.1993.1025. [DOI] [PubMed] [Google Scholar]
- Shehadeh N., Calcinaro F., Bradley B. J., Bruchim I., Bruchlim I., Vardi P., Lafferty K. J. Effect of adjuvant therapy on development of diabetes in mouse and man. Lancet. 1994 Mar 19;343(8899):706–707. doi: 10.1016/s0140-6736(94)91583-0. [DOI] [PubMed] [Google Scholar]
- Steinman L. Development of antigen-specific therapies for autoimmune disease. Mol Biol Med. 1990 Aug;7(4):333–339. [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Wogensen L., Lee M. S., Sarvetnick N. Production of interleukin 10 by islet cells accelerates immune-mediated destruction of beta cells in nonobese diabetic mice. J Exp Med. 1994 Apr 1;179(4):1379–1384. doi: 10.1084/jem.179.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]