Abstract
BACKGROUND AND PURPOSE: Atherosclerotic arterial remodeling has been described in the coronary circulation but has not been studied extensively for carotid atherosclerosis. The purpose of our study was to examine the association between carotid artery remodeling and clinical presentation in patients with significant stenosis by using multidetector row CT (MDCT).
MATERIALS AND METHODS: One hundred eight patients with ≥50% stenosis (North American Symptomatic Carotid Endarterectomy Trial criteria) by MDCT angiography between January 2004 and June 2006 were identified. The study group included 37 symptomatic (65.9 ± 13.0 years; 12 women; stenosis, 81.5 ± 12.2%; 17 with stroke; 15 with transient ischemic attack; 5 with amaurosis fugax) and 71 asymptomatic patients (70.5 ± 10.5 years; 28 women; stenosis, 78.8 ± 11.1%). Remodeling ratio (RR) was calculated by dividing the outer vessel circumference at the site of greatest stenosis by a normal reference-segment vessel circumference. Maximum vessel thickness (MxVT) and eccentricity index (EI) of the plaque, defined as maximal thickness/minimal thickness at the site of greatest luminal narrowing, were also determined. Data were analyzed by using an independent t test.
RESULTS: The RR was significantly higher in symptomatic patients (1.64 ± 0.44) than in asymptomatic patients (1.41 ± 0.5) (P=.02). There was no significant difference in MxVT in symptomatic (5.9 ± 2.1 mm) and asymptomatic patients (5.6 ± 2.4 mm) (P=.45) and no significant difference in EI (symptomatic, 4.7 ± 2.7; asymptomatic, 4.3 ± 2.2; P=.38).
CONCLUSION: In this series of subjects with significant internal carotid artery stenosis, expansive carotid remodeling was significantly greater in patients with cerebral ischemic symptoms than in asymptomatic patients. The extent of expansive remodeling may indicate underlying atherosclerotic plaque vulnerability. MDCT has a role in the evaluation of carotid artery disease beyond examining luminal stenosis.
Atherosclerotic disease of the extracranial internal carotid arteries is a common treatable cause of symptomatic cerebral vascular disease. Currently, the decision to intervene, by using surgical or endovascular techniques, is based primarily on the percent luminal narrowing of the vessel. The benefit of intervention in both symptomatic and asymptomatic patients with a high degree of carotid luminal narrowing has been previously established by large multicenter trials.1,2 At present, plaque characteristics other than the degree of luminal narrowing are not routinely considered. However, the internal composition of atherosclerotic plaque has been shown to correlate with increased risk of thromboembolic disease in multiple vascular territories.3–5 Several imaging techniques have demonstrated the ability to image the internal constituents of carotid plaque, including sonography, MR imaging, and multidetector row CT (MDCT).6–8 A method to distinguish noninvasively stable carotid atherosclerotic plaque from potentially unstable forms might improve the ability to select patients who are likely to benefit most from intervention. Plaque characterization by imaging also could potentially provide more accurate risk stratification for future cerebral ischemic events and monitor the effects of medical treatment on patients who are not operative candidates.
Glagov et al9 initially described expansive remodeling of the vessel in response to atherosclerotic plaque formation, which is one mechanism by which luminal encroachment or stenosis is delayed during the progression of atherosclerosis. However, recent data in the coronary circulation using intravascular sonography suggest that this compensatory expansive remodeling may also contribute to underlying plaque vulnerability, making it more prone to rupture.10 To our knowledge, an association between the extent of expansive carotid artery remodeling and cerebral ischemic events has not been previously assessed by any noninvasive technique. The purpose of our study was to examine the association between carotid artery remodeling and clinical presentation in patients with significant stenosis by using MDCT angiography.
Materials and Methods
The study received prior institutional review board approval. Informed patient consent was not required.
Patient Population
The study group was selected following a search of the patient database of the University of Virginia Health System from January 2004 to June 2006 for patients who had undergone dedicated MDCT angiography of the neck and demonstrated significant stenosis, defined as ≥50% secondary to atherosclerotic disease. During the study period, 1109 CT studies were performed; 214 patients (19%) in these studies had significant internal carotid stenosis. Twenty-two patients were excluded from the study due to previous surgical endarterectomy with subsequent restenosis. One patient was excluded on the basis of previous placement of a carotid stent. Ten patients with tandem lesions were excluded. Seventy-three patients were excluded on the basis of the presence of another potential cause for neurologic symptoms (possible cardiac source of emboli or lacunar infarctions resulting from small vessel disease). The study group was composed of 108 patients including 37 symptomatic (17 with stroke, 15 with transient ischemic attack [TIA], and 5 with amaurosis fugax) and 71 asymptomatic patients referred for MDCT angiography. The reason for referral for MDCT was the presence of neurologic symptoms (n=37), prior sonography demonstrating stenosis (n=66), or patients undergoing coronary bypass being evaluated for comorbid disease (n=5). Only patient age (P=.05) and presence of hypertension (P=.002) were significantly different between the symptomatic and asymptomatic groups. The Table summarizes the study group demographics.
Clinical characteristics of the study population
| No. of asymptomatic patients (%) | No. of symptomatic patients (%) | P value | |
|---|---|---|---|
| Age (years) | 70.5 ± 10.5 | 65.9 ± 13.0 | .05* |
| Female | 28 (39) | 12 (32) | ns |
| % stenosis | 78.8 ± 11.1 | 81.5 ± 12.2 | ns |
| Hypertension | 44 (62) | 34 (92) | .002* |
| Diabetes | 16 (23) | 13 (35) | ns |
| Smoking | 36 (50) | 25 (68) | ns |
| Dyslipidemia | 40 (56) | 25 (68) | ns |
| Coronary artery disease | 34 (48) | 11 (30) | ns |
Note:—ns indicates no significance.
P < .05 is statistically significant.
Definition of Symptomatic and Asymptomatic Patients
The study patients were divided into 1) symptomatic with a documented neurologic event (stroke, TIA, or amaurosis fugax) within 7 days of undergoing the study and in a vascular distribution concordant with the affected carotid artery, and 2) asymptomatic patients. Relevant clinical information was obtained from the electronic medical record at our institution for all patients, including the presence or absence of coronary artery disease, diabetes mellitus, hypertension, hyperlipidemia, and history of smoking. TIA and stroke were defined on the basis of previously published criteria.11,12 Amaurosis fugax was defined as acute onset of transient complete or partial monocular loss of vision.
Imaging Parameters
MDCT angiography was performed on all study patients by using a 16–detector row CT scanner (LightSpeed 16; GE Healthcare, Milwaukee, Wis). Images were obtained from the aortic arch to the level of the inferior orbits by using a helical acquisition with 6.25 mm/rotation and 1.25 × 1.25 mm collimation (120 kVp, 350 mA). A total of 100- to 125-mL iohexol (Omnipaque 300; GE Healthcare) was injected at a rate of 4.0–4.5 mL/s following a time delay determined by an automated bolus-timing program. The data were transferred to a computer workstation (PACS, Version 5.2.1, Kodak, Tel Aviv, Israel) for image postprocessing.
Image Interpretation
A single experienced neuroradiologist, blinded to the clinical information, performed all measurements of luminal stenosis. The degree of luminal stenosis was measured on the basis of the North American Symptomatic Carotid Endarterectomy Trial Collaborators criteria1 by using a combination of axial images and multiplanar reconstruction techniques.
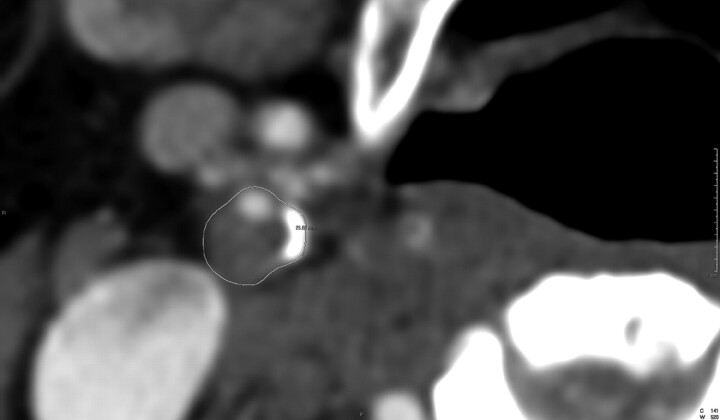
Plaque remodeling ratio (RR) was determined by 1) measurement of the outside vessel circumference of the extracranial internal carotid artery at the point of maximal luminal stenosis, and 2) was divided by the outside vessel circumference at a region unaffected by atherosclerotic disease (Figs 1 and 2). The rationale for this method is based on earlier work by Nakamura et al,13 who evaluated plaque remodeling in the coronary circulation. All circumference measurements were performed by a single blinded investigator. The reproducibility was assessed by measurements performed by a second blinded investigator in 50 consecutive patients.
Fig 1.
A line has been drawn around the vessel to measure the circumference in this plaque with marked expansion of the carotid artery.
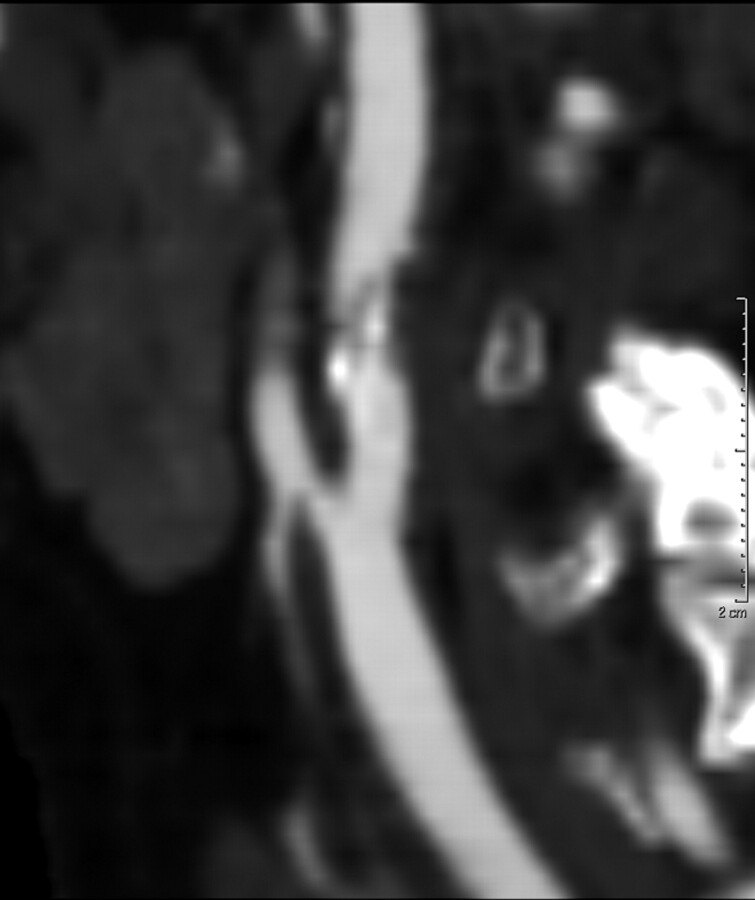
Fig 2.
Sagittal reconstruction demonstrates plaque with minimal expansion of the vessel.
The maximal vessel thickness (MxVT) in millimeters was determined by measuring from the luminal-intimal boundary to the outer vessel wall at the site of greatest wall thickness anywhere throughout the length of the plaque. Finally, the eccentricity index (EI) was determined by dividing the MxVT by the minimal vessel thickness at the site of greatest luminal stenosis.
Statistics
Continuous data are described as the mean value ± SD. For comparing variances of numeric values, we performed the independent t test. Categoric data were evaluated by using the chi-square or the Fisher exact test. Interobserver agreement was evaluated with a Bland-Altman analysis.14 MedCalc software (Version 8.2, Mariakerke, Belgium) was used for statistical analyses. A P value of less than .05 was regarded as statistically significance.
Results
Plaque Remodeling Measures
The RR was significantly higher in symptomatic patients (1.64 ± 0.44) than in asymptomatic patients (1.41 ± 0.50) (P=.02). There was no significant difference in MxVT in symptomatic (5.9 ± 2.1 mm) and asymptomatic patients (5.6 ± 2.4 mm) (P=.45) and no significant difference in EI (symptomatic, 4.7 ± 2.7; asymptomatic, 4.3 ± 2.2; P=.38).
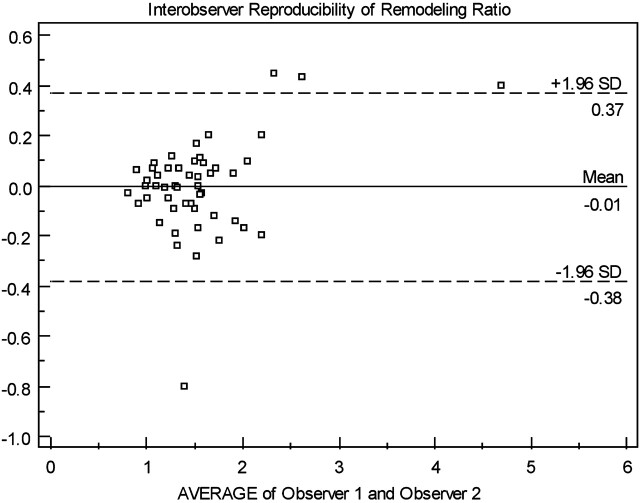
Interobserver Agreement
Interobserver agreement of RR was assessed in 50 consecutive patients by using Bland-Altman plots, which demonstrated a moderate-good interobserver agreement (Fig 3).
Fig 3.
Interobserver variability in RR.
Discussion
Our results demonstrate a relationship between the extent of expansive carotid artery plaque remodeling and the presence of symptoms. In our study, patients with carotid artery plaque causing significant stenosis (≥50%) who presented with symptomatic cerebral vascular disease (stroke, TIA, or amaurosis fugax) were more likely to have plaque with a greater degree of expansion of the vessel than asymptomatic patients. These results indicate the potential contribution of the RR, as determined by MDCT, to the prediction of a patient's risk for a future cerebral ischemic event. Although a larger prospective trial would be necessary to evaluate its overall impact, assessment of the RR could potentially alter selection criteria for carotid intervention. Aside from the clinical implications on the selection for intervention, measurement of the RR could contribute to more accurate risk stratification of patients for future cerebral ischemic events. Plaque with a higher RR may be an indication for more aggressive medical preventative treatment.
Our findings agree with those of Schoenhagen et al,10 who demonstrated a relationship between expansive remodeling and symptoms in the coronary circulation. Although there are inherent differences in the carotid and coronary vasculature, thromboembolic phenomena are believed to be a shared common pathway to symptomatic disease in both vascular distributions.15,16 As has been suggested in the coronary circulation, expansive vascular remodeling may be an indicator of potential plaque vulnerability to rupture. Plaques with expansive remodeling have been shown to have a significantly larger lipid core and a higher macrophage count than negatively or less positively remodeled plaques. Both of these features have been found to be associated with instability.17 The increased vulnerability of plaque with greater expansive remodeling is likely due to a combination of functional and morphologic mechanisms. In a histologic specimen study, Pasterkamp et al18 examined the relationship between inflammatory markers such as CD68 and CD45RO, which have been linked to plaque vulnerability and plaque area. They found that these immunohistologic markers were significantly greater in plaques with larger areas.
MDCT angiography is noninvasive and can be performed rapidly, making it a good candidate for routine imaging evaluation of carotid disease. The technique has previously been established as accurate for the assessment of percent luminal narrowing in carotid artery disease.19 It has also recently been validated for its ability to differentiate internal plaque components compared with histology.20 Although already supplanting invasive angiography in many areas within the United States in the evaluation of stenosis, MDCT is also capable of delineating plaques on the basis of attenuation values and measuring calcium scores within the carotid arteries, which are potential markers of ischemic symptoms.21,22 An additional new role may be the assessment of carotid wall expansion secondary to atherosclerosis with the RR. Together, these atherosclerotic plaque characteristics may lead to better risk stratification of patients with asymptomatic carotid stenosis, in whom management remains controversial. With a model taking into account more risk factors, patients could be better distinguished as to the need for carotid endarterectomy/endovascular intervention versus medical therapy.
There are several potential limitations of our study. The retrospective design of the study introduces the possibility that the parameter being measured (remodeling) could be the result of and not the cause for the outcome (cerebral ischemic event). Conceivably, a plaque that demonstrated a high RR in a symptomatic patient could have represented an acute hemorrhage into an unstable plaque. Although this plaque may have been the source of the symptoms, the imaging may only be a representation of the previously ruptured plaque. This would lessen the potential predictive value of measuring RR. The presence of increased hemorrhage within plaque in symptomatic patients has been previously described using MR imaging.23,24
Next, though MDCT angiography has been established as accurate for the measurement of the vascular lumen,19 its capabilities in evaluating the vessel wall have yet to be fully explored. In our experience, the outer vascular diameter could be reliably visualized, as demonstrated by the moderate-good interobserver agreement noted in the measurement of RR, but studies correlating histologic vessel wall measurements with MDCT values are still lacking. Second, unlike in the coronary vasculature, the internal carotid artery has a segment that is notably normally expanded, the carotid bulb. In theory, any significant variability conferred by the naturally larger caliber of the carotid bulb relative to the distal vessel should be accounted for by the equation and equalized between the 2 groups (symptomatic and asymptomatic). This should hold true even if the inherent ratio of the circumference of the bulb to the circumference of the distal vessel in healthy patients varies among individuals, because this natural variance would be similar in the 2 groups. Additionally, any variance in location of plaque within the vessel would likely again be accounted for, given that plaque in all patients almost exclusively occurs near the bulb region. Therefore, we believe this assumption in the method used for calculation of RR does not discount the presence of a significantly higher ratio in the symptomatic group, though it is difficult to fully assess the effect of plaque location within the vessel on our results.
Conclusion
In the setting of significant (≥50%) carotid stenosis in our series of patients, the degree of expansive vascular remodeling as measured by the RR was significantly greater in patients with cerebral ischemic symptoms than in asymptomatic patients. Atherosclerotic plaque which causes a greater degree of expansive remodeling may indicate underlying plaque vulnerability. Our results suggest a potential additional benefit of using MDCT angiography in the evaluation of carotid atherosclerotic disease beyond simply the measurement of luminal stenosis.
References
- 1.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1991;325:445–53 [DOI] [PubMed] [Google Scholar]
- 2.Endarterectomy for asymptomatic carotid artery stenosis: Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–28 [PubMed] [Google Scholar]
- 3.Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation 1995;92:657–71 [DOI] [PubMed] [Google Scholar]
- 4.Fuster V. Elucidation of the role of plaque instability and rupture in acute coronary events. Am J Cardiol 1995;76:24C–33C [DOI] [PubMed] [Google Scholar]
- 5.Nandalur KR, Hardie AD, Raghavan P, et al. Composition of the stable carotid plaque: insights from a multidetector computed tomography study of plaque volume. Stroke 2007;38:935–40. Epub 2007 Feb 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappendijk VC, Cleutjens KBJM, Kessels AGH, et al. Assessment of human atherosclerotic carotid plaque components with multisequence MR imaging: initial experience. Radiology 2005;234:487–92 [DOI] [PubMed] [Google Scholar]
- 7.Polak JF, Shemanski L, O'Leary DH, et al. Hypoechoic plaque at US of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older–Cardiovascular Health Study. Radiology 1998;208:649–54. Erratum in: Radiology 1998;209:288–89 [DOI] [PubMed] [Google Scholar]
- 8.Leeson MD, Cacayorin ED, Iliya AR, et al. Atheromatous extracranial carotid arteries: CT evaluation correlated with arteriography and pathologic examination. Radiology 1985;156:397–402 [DOI] [PubMed] [Google Scholar]
- 9.Glagov S, Weisenberg E, Zarins CK, et al. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 1987;316:1371–75 [DOI] [PubMed] [Google Scholar]
- 10.Schoenhagen P, Ziada KM, Kapadia SR, et al. Extent and direction of arterial remodeling in stable versus unstable coronary syndromes: an intravascular ultrasound study. Circulation 2000;101:598–603 [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack: proposal for a new definition. N Engl J Med 2002;347:1713–16 [DOI] [PubMed] [Google Scholar]
- 12.Inzitari D, Eliasziw M, Gates P, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid-artery stenosis: North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 2000;342:1693–700 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura M, Nishikawa H, Mukai S, et al. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol 2001;37:63–69 [DOI] [PubMed] [Google Scholar]
- 14.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–10 [PubMed] [Google Scholar]
- 15.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies—Part I. Circulation 2003;108:1664–72 [DOI] [PubMed] [Google Scholar]
- 16.Spagnoli LG, Mauriello A, Sangiorgi G, et al. Extracranial thrombotically active carotid plaque as a risk factor for ischemic stroke. JAMA 2004;292:1845–52 [DOI] [PubMed] [Google Scholar]
- 17.Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation 2002;105:939–43 [DOI] [PubMed] [Google Scholar]
- 18.Pasterkamp G, Schoneveld AH, van der Wal AC, et al. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability:the remodeling paradox. J Am Coll Cardiol 1998;32:655–62 [DOI] [PubMed] [Google Scholar]
- 19.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology 2001;220:179–85 [DOI] [PubMed] [Google Scholar]
- 20.de Weert TT, Ouhlous M, Meijering E, et al. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol 2006;26:2366–72 [DOI] [PubMed] [Google Scholar]
- 21.Nandalur KR, Baskurt E, Hagspiel KD, et al. Carotid artery calcification on CT may independently predict stroke risk. AJR Am J Roentgenol 2006;186:547–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nandalur KR, Baskurt E, Hagspiel KD, et al. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR Am J Roentgenol 2005;184:295–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology 2006;240:464–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque. Stroke 2004;35:1079. [DOI] [PubMed] [Google Scholar]