Abstract
OBJECTIVE
The purpose of our study was to quantitatively evaluate calcified atherosclerotic burden in the cervical carotid arteries using MDCT to determine the relationship of scores with luminal stenosis and symptomatology.
MATERIALS AND METHODS
Calcium plaque volume was measured in 106 cervical carotid arteries (53 patients) using MDCT angiography. The study group included 32 asymptomatic patients (mean age, 70.2 ± 8.7 [SD] years; 15 women, 17 men) and 21 patients with ischemic neurologic symptoms (69.6 ± 12.9 years; eight women, 13 men). By vessel, there were 43 high-grade stenotic (≥ 60% by North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria), 15 moderate-grade stenotic (30–59%), and 44 mild-grade stenotic or normal (0–29%) vessels, with four excluded for prior carotid endarterectomy. Volume scores were calculated by summing the area of calcium in the common and extracranial internal carotid arteries on axial slices and multiplying by the slice increment.
RESULTS
Controlling for cardiovascular risk factors and luminal stenosis, we found that scores were significantly related to the occurrence of symptoms (p = 0.003). Even with patient age as a covariant, patients with high-grade stenosis had significantly higher scores than those without high-grade disease (p = 0.004). Moreover, quantitative burden was associated with luminal stenosis on adjusted multivariate analysis (p = 0.034). The specificity and positive predictive value for high-grade luminal narrowing were notably lower on individual vessel analysis than on total score analysis, likely secondary to variability in vascular remodeling.
CONCLUSION
Calcium scores in the cervical carotid arteries may represent an independent marker for luminal stenosis and ischemic symptoms. A prospective longitudinal study examining calcium levels and morbidity may be warranted to examine whether burden has a role in risk stratification.
Keywords: CT arteriography, calcium, carotid, stroke
Vascular calcium deposits have recently become a major research and public interest secondary to increasing evidence of the relationship of calcium scores with atherosclerotic burden and clinical outcome. The prevailing example involves coronary calcium scores measured on CT, which have been associated with luminal stenosis [1], overall coronary atherosclerotic load [2], and increased morbidity and mortality [3, 4]. Furthermore, abdominal aortic calcium deposits on lateral lumbar radiographs carry an increased risk of coronary heart disease and cardiovascular disease and mortality [5], and aortic arch calcifications on chest radiographs have been related to coronary heart disease and ischemic stroke [6]. Given the systemic nature of atherosclerosis, cervical carotid artery calcium burden may have similar importance in terms of luminal stenosis and ischemic symptoms.
However, limited studies [7] have been performed to examine the importance of carotid artery calcium scores. This may partly be because of the predominant use of sonography and MR angiography in imaging studies involving the neck vasculature, but these techniques are significantly limited in the evaluation of calcium. MDCT angiography is a robust technique for assessing calcification and can potentially depict and allow quantification of load in the carotid arteries in a manner similar to coronary artery scoring, while being highly accurate for measuring luminal stenosis [8–13].
The purpose of our study was to quantitatively evaluate calcified atherosclerotic burden in the common and extracranial internal carotid arteries using MDCT to determine the relationship of scores with luminal stenosis and ischemic symptoms.
Materials and Methods
Patient Population
After the protocol was approved by the local institutional review board, we retrospectively identified all patients 45 years and older who underwent MDCT angiography of the carotid arteries between September 2001 and February 2004. Medical notes, laboratory data, images and imaging reports, and discharge summaries were reviewed for relevant clinical history in 94 consecutive patients. Exclusion criteria, which led to the elimination of 41 patients, were patients with concomitant conflicting causes of neurologic symptoms, such as cardiac thrombus, intracranial masses, or intracranial small vessel disease (as evidenced by lacunar infarction, defined as a lesion < 1.5 cm in diameter in the subcortical or brainstem area in the territory of a small penetrating artery on CT or MRI of the brain); bilateral events; posterior circulation symptoms; and stenosis from nonatherosclerotic causes, such as fibromuscular dysplasia or radiation. Of the 53 patients examined, there were 16 asymptomatic patients with high-grade stenosis (≥ 60%), two asymptomatic patients with moderate-grade stenosis (30–59%), 14 asymptomatic patients with mild-grade stenosis or no significant stenosis (0–29%), 19 symptomatic patients with high-grade stenosis, and two symptomatic patients with moderate-grade stenosis.
All 21 patients with symptomatic disease had retinal or hemispheric neurologic events: 12 with transient ischemic attacks (TIA) and nine with stroke, which was relevant to the carotid disease within 2 weeks of undergoing CT angiographic imaging. TIA and stroke were defined according to previously published criteria [14]. Patients classified as asymptomatic had no history of symptoms neither remote nor at the time of examination. Information for the asymptomatic group (mean age ± SD, 70.2 ± 8.7 years) and symptomatic group (69.6 ± 12.9 years)—including the presence of coronary artery disease (CAD: angina, myocardial infarction, or coronary revascularization), diabetes mellitus, hypertension, remote or present history of smoking, elevated cholesterol, aspirin use, and statin use—is summarized in Table 1. Referral for MDCT angiography was for confirmation of stenosis after sonography as an alternative to digital subtraction angiography in 33 patients and nondiagnostic sonography or MRI in eight patients and after trauma in 12 patients. None of the patients referred for MDCT for trauma had neurologic ischemic events (confirmed with CT or MRI and clinically); they were predominantly evaluated for possible involvement of vessels after osseous or soft-tissue injury to the neck.
TABLE 1.
Clinical Characteristics of Study Population
| Characteristic | No. (%) of Patients | |
|---|---|---|
| Asymptomatic (n = 32) | Symptomatic (n = 21) | |
| Female | 15 (47) | 8 (38) |
| High-grade stenosis | 16 (50) | 19 (90) |
| Hypertension | 21 (66) | 15 (71) |
| Diabetes | 9 (28) | 5 (24) |
| Smoking | 11 (34) | 13 (62) |
| Hypercholesterolemia | 19 (59) | 13 (62) |
| Coronary artery disease | 15 (47) | 10 (48) |
| Aspirin use | 19 (59) | 14 (67) |
| Statin use | 13 (41) | 12 (57) |
The degree of luminal stenosis was measured by an experienced neuroradiologist on the basis of axial slices, multiplanar reconstructions, maximum intensity projections, and 3D volume-rendering reconstructions for optimal assessment [15] using North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria [16]. Significant carotid artery disease was defined as equal to or greater than 60% stenosis given the controversy regarding therapy for asymptomatic patients with stenosis above this value. Among the 106 cervical carotid arteries of 53 patients, there were 43 high-grade stenotic, 15 moderate-grade stenotic, and 44 mild-grade or normal vessels. Four vessels were excluded secondary to prior carotid endarterectomy.
Scan Parameters
MDCT was performed on either an 8-MDCT scanner (LightSpeed Ultra, GE Healthcare) or 16-MDCT scanner (LightSpeed 16, GE Healthcare) in all patients. After a delay determined by an automated bolus-timing program for the injection of 120 mL of nonionic contrast medium at a rate of 4 mL/sec, helical acquisition was performed from the aortic arch to the supraventricular white matter. Scan parameters were identical for both scanners and were as follows: 1.25-mm slice thickness, increment of 0.625 or 1.25 mm, table speed of 6.25 mm per rotation, and 0.8 sec gantry rotation period. The imaging data were transferred to a computer workstation (Leonardo workstation, Siemens Medical Solutions) for postprocessing.
Carotid Calcium Score Determination
To determine the presence and quantity of carotid calcium, an observer blinded to the clinical history evaluated the axial images of the MDCT data sets. All images of the common and extracranial internal carotid artery were visually examined for the presence of calcium. Calcium was defined as structures with a density greater than 130 H within the vessel wall that were hyperdense to the contrast-enhanced lumen and surrounding parenchyma (Fig. 1), similar to methods reliably used in the coronary circulation [17]. Wide window settings were used for most analyses, but several cases required individual changes to the window level for optimal visualization (range of window width settings, 600–1,000 H; range of window level settings, 150–400 H). Calcium scores were assigned for all the vessels from the origin of the common carotid artery to the internal carotid artery at the base of the skull. The intracranial internal carotid artery (e.g., cavernous), external carotid artery, and aortic arch calcifications were not included.
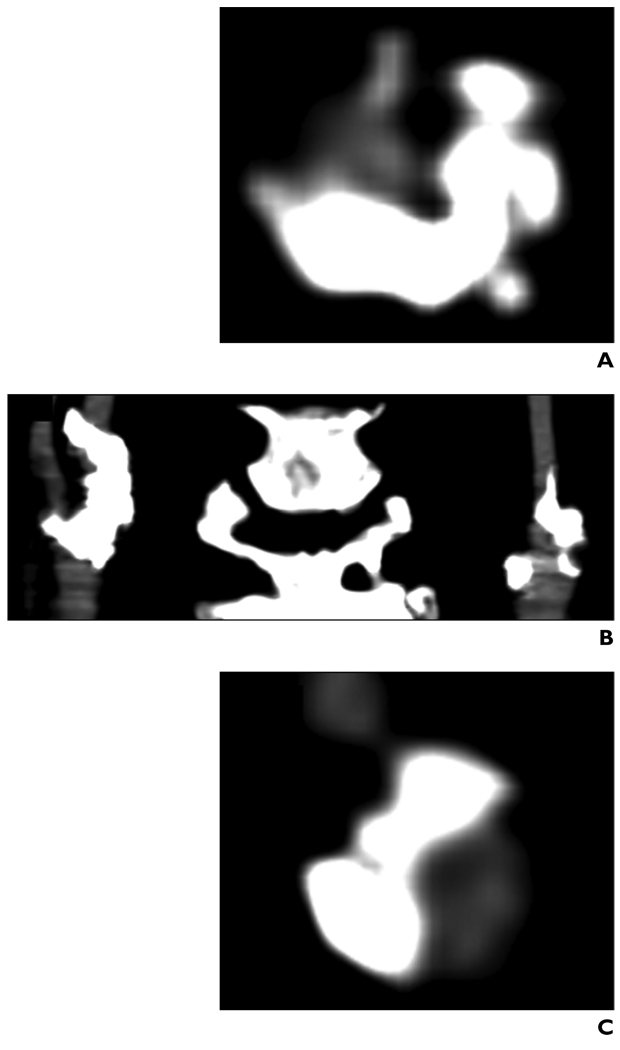
Fig. 1.
Contrast-enhanced MDCT images of heavily calcified cervical carotid arteries in 67-year-old woman.
A, Axial view shows right internal carotid artery just superior to bifurcation.
B, Coronal reconstruction.
C, Axial view shows left internal carotid artery just superior to bifurcation.
Calcium on axial slices was manually traced for area measurements. The sum of the areas of calcium for the common and relevant internal carotid arteries was tallied and multiplied by the slice increment to determine volume in cubic millimeters. The total score for each patient was the sum of the bilateral cervical carotid arteries. Vessel scores were defined as burden in the unilateral cervical carotid artery (common plus extracranial internal carotid arteries). Reproducibility was assessed by an independent blinded observer who measured total scores for 30 random patients.
Statistical Analysis
Continuous data are described as the mean value ± SD. For comparing variances, we performed the square root transformation on the total scores and vessel scores to normalize the positive skew. Luminal stenosis was classified into one of two groups for total scores (1, high-grade; 2, moderate-grade, mild-grade, or normal) and into one of three categories for vessel scores (1, high-grade; 2, moderate-grade; 3, mild-grade or normal). Patient age was classified into one of three groups (1, < 65 years; 2, 65–74 years; or 3, ≥ 75 years). Two-way analysis of variance was performed for comparing subgroups of total scores and of vessel scores. For vessel analysis, each artery was treated as an independent experimental unit.
The associative value of cardiovascular risk factors, numeric age, and raw total carotid scores for both high-grade stenosis and symptomatology was examined using unadjusted simple and multiple logistic regression. Pearson’s correlation coefficient was calculated to evaluate the relationship between left and right carotid artery scores and age and total scores. Interobserver agreement was evaluated with Bland-Altman analysis [18]. Statistical significance was assumed at a p value of less than 0.05. All analyses were performed using SigmaStat 2.03 software (Access Softek).
Results
Total Patient Scores
Of the 49 patients (four excluded from totals secondary to unilateral carotid endarterectomy), 43 (87.8%) had calcium present. Two patients with high-grade stenosis and four without significant stenosis had scores of 0. There was a modest but significant correlation between age and scores (r = 0.56, p < 0.001). With age as a cofactor, patients with high-grade stenosis had significantly higher scores than those with less than 60% luminal narrowing (p = 0.004). Symptomatic patients had significantly higher scores than asymptomatic patients (p < 0.001) without stenosis taken into account. No interaction with patient age was present.
Table 2 shows the relationship of carotid calcium scores to high-grade luminal stenosis and symptoms with patient age and traditional cardiovascular risk factors taken into account. The multifactorial column indicates the odds ratio by patient characteristic for the two outcomes, stenosis in the top half and symptoms in the bottom half, after adjustment for the odds ratio conferred by each of the other variables—that is, the odds ratio independent of the other independent variables. The multifactorial odds ratio greater than 1.0, without the inclusion of 1.0 on the 95% confidence intervals (CIs), for raw calcium scores indicates their positive association with luminal stenosis and symptoms independent of patient age, blood pressure, diabetes status, and so on. There was a significant association between raw scores and luminal stenosis on multifactorial (p = 0.034) and also on unifactorial (p = 0.009) analyses. In addition, similar examination for scores relative to symptoms, with luminal stenosis also taken into consideration, found a significant association on unifactorial (p = 0.004) and multifactorial (p = 0.003) analyses. The only other factor significantly positive for symptoms on both analyses was high-grade luminal stenosis (unifactorial, p = 0.004; multifactorial, p = 0.0.047). This relationship has been firmly established by trials such as NASCET [16].
TABLE 2.
Multifactorial Association Between Cardiovascular Risk Factors and Calcium Scores for High-Grade Luminal Stenosis and Symptoms
| Characteristic | Multifactorial | |
|---|---|---|
| Odds Ratio | 95% CI | |
| High-grade stenosis | ||
| Calcium scores (raw numeric) | 1.005a | 1.001–1.010 |
| Age (numeric) | 1.03 | 0.93–1.15 |
| High cholesterol (yes/no) | 0.18 | 0.03–1.24 |
| Hypertension (yes/no) | 0.41 | 0.06–2.95 |
| Diabetes (yes/no) | 4.67 | 0.63–34.8 |
| Smoking (yes/no) | 0.18 | 0.03–1.24 |
| Symptoms | ||
| Calcium scores (raw numeric) | 1.008a | 1.003–1.013 |
| Age (numeric) | 0.84a | 0.74–0.96 |
| High-grade stenosis (yes/no) | 9.29a | 1.03–84.0 |
| High cholesterol (yes/no) | 4.02 | 0.40–40.8 |
| Hypertension (yes/no) | 0.43 | 0.04–4.57 |
| Diabetes (yes/no) | 2.28 | 0.36–14.3 |
| Smoking (yes/no) | 0.94 | 0.16–5.56 |
Note—The multivariable regression model simultaneously included all variables listed. CI = confidence interval.
p < 0.05.
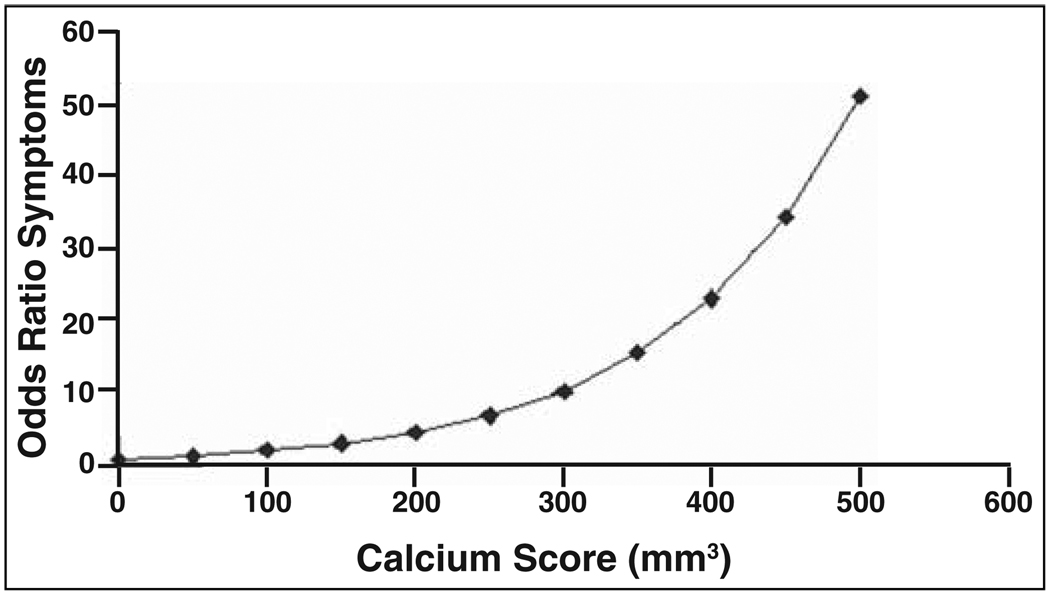
Figure 2, based on data from adjusted analysis, shows the drastic increase in the odds ratio for the occurrence of TIA or stroke as calcium scores increase. Calcium burden had a strong independent relationship with symptoms, especially at higher levels. For example, a patient with a calcium score of 300 mm3 was approximately 10 times more likely to be symptomatic, similar to the odds for symptoms conferred by high-grade luminal stenosis.
Fig. 2.
Scatterplot with data points connected by line shows odds ratio for symptoms plotted against calcium scores based on exponential of B coefficient from adjusted multiple logistic regression (0.0079) multiplied by raw calcium score in cubic millimeters. There was considerable increase in odds for occurrence of symptoms with increasing calcium scores.
Among the 30 scores measured by both observers (observer 1, 308.6 ± 297.5; observer 2, 315.4 ± 307.2), the coefficient of repeatability of 37.34 and limits of agreement (−44.37, 30.31) were acceptably small indicating good interobserver agreement (raw score intraclass correlation coefficient, 0.98; 95% CI, 0.97–0.99).
Individual Vessel Scores
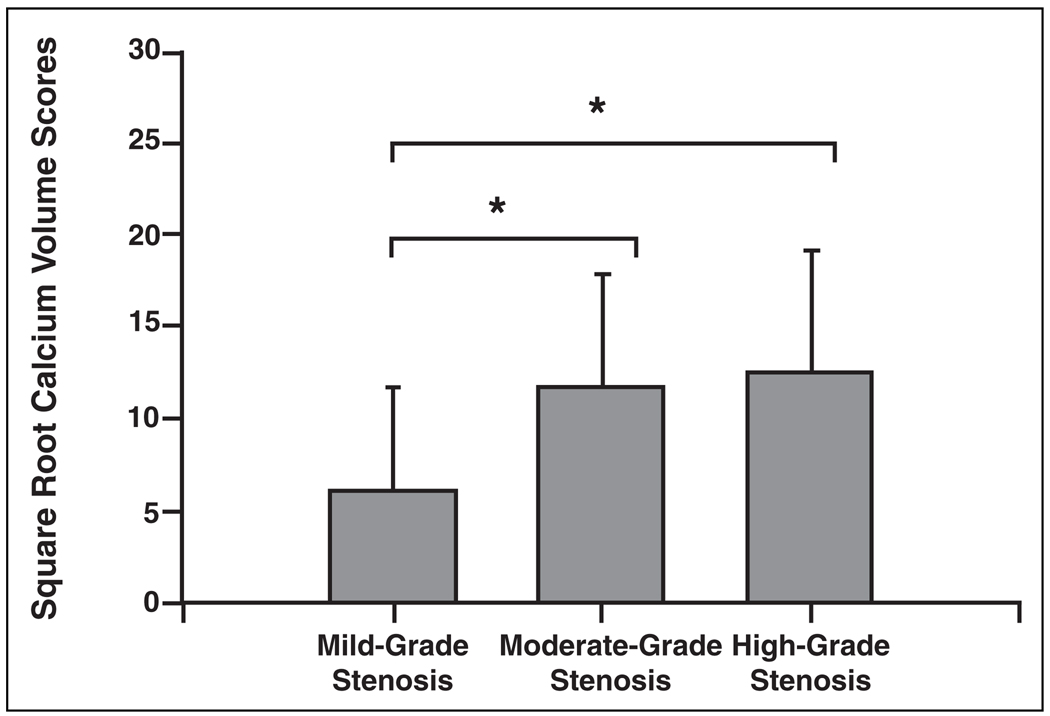
After transformation, the mean score for all vessels was 9.7 ± 6.8. Vessel scores grouped by degree of stenosis are summarized in Figure 3. Comparing the three categories showed a significant difference among the groups (p < 0.001), with pairwise comparison indicating high-grade (p < 0.001) and moderate-grade (p = 0.002) stenotic vessels have significantly higher scores than mild-grade stenotic or normal vessels without interaction with patient age. No significant difference was found between high-grade and moderate-grade stenotic vessels (p = 0.82).
Fig. 3.
Bar graph with SD error bars shows mean vessel scores by degree of stenosis: mild-grade stenosis or normal (0–29%), n = 44; moderate-grade stenosis (30–59%), n = 15; and high-grade stenosis (60%), n = 43. Vessels with high- and moderate-grade stenosis had significantly higher scores than those with mild-grade stenosis or normal vessels (p < 0.005). There was no significant difference between scores of high- and moderate-grade stenotic vessels (p = 0.82). Asterisks indicate p < 0.05.
There was a strong and significant relationship between a patient’s right and left carotid artery scores (r = 0.79, p < 0.001). Moreover, an individual raw vessel score of 125 mm3 (half of the cutoff score used for a high specificity of 94.4% (17/18) and positive predictive value (PPV) of 95.0% (19/20) with total scores for high-grade stenosis) had a sensitivity of 55.8% (24/43), specificity of 78.0% (46/59), PPV of 64.9% (24/37), and negative predictive value (NPV) of 70.8% (46/65) for significant luminal narrowing. The PPV and specificity are notably lower than the values for similar comparison with total scores.
Discussion
The significance of carotid calcium scores has been incompletely studied, especially their relationship to symptoms. Culebras et al. [7] measured calcium deposits in the cervical carotid arteries of 40 symptomatic patients using conventional CT and found a correlation between patient age and calcium scores. They also concluded that because there was no significant difference in scores between the symptomatic and asymptomatic sides, calcium has no appreciable association with symptomatology. This study was limited by the semiquantitative measurement methods; use of conventional CT with thick sections; and, most notably, by the lack of inclusion of asymptomatic patients. We found that overall cervical calcium load may have value regarding the occurrence of symptoms. To our knowledge, this study is the first to show carotid calcium scores as a potential risk marker for TIA and stroke. In addition, our results show a relationship between calcium burden and stenosis.
The value of calcium scores as a marker for symptoms is controversial. Our cross-sectional study found that carotid artery calcium scores have a significant and independent relationship with symptoms, even with stenosis and other cardiovascular risk factors taken into account. Although differing in hemodynamics and caliber from the carotid arteries, the coronary arteries have been examined on follow-up of CT scoring, and researchers of several studies with a large number of patients found that calcium burden may provide incremental prognostic information over patient age and other risk factors [3, 4]. Furthermore, using electron beam CT, researchers found a higher temporal progression of calcium volume to be associated with increased risk of myocardial infarction [19], and lipid-lowering therapy has been shown to slow the progression of coronary calcium [20, 21]. CT may be a useful tool to monitor the effectiveness of drug therapy and for risk stratification. Given our promising initial results and the emerging evidence in other vascular beds, larger prospective series may be warranted to examine the relative risk conferred by elevated carotid calcium burden.
Although calcium scores may represent a marker for symptoms, the mechanism responsible for this association has yet to be established. The relationship may be connected to atherosclerotic burden and activity and is unlikely due to calcification conferring instability of particular plaques causing stenosis. Calcified “culprit” plaques are likely more biomechanically stable and less prone to disruption [22, 23]. Retrospective in vitro and in vivo studies of human carotid artery obstructive plaques have found that calcified plaques are less often associated with ischemic symptoms [24, 25]. Further studies are necessary to elucidate the complex association between calcium burden and symptoms.
Arterial intimal calcifications are almost invariably an indicator of atherosclerotic disease [1], and a linear relationship exists between calcium area and total plaque area [26, 27]. With increasing burden, compensatory remodeling with arterial enlargement can occur, which prevents luminal stenosis [28]. However, if this mechanism is overwhelmed, atherosclerotic disease can progressively become obstructive and lead to ischemic symptoms through hypoperfusion, thrombosis, or emboli. Our study found that even with patient age as a cofactor, total calcium scores are significantly higher in patients with high-grade stenosis than in those without significant disease. In addition, scores have a significant associative value for luminal stenosis on adjusted analysis. These findings suggest that burden is a marker of luminal narrowing. However, as evidenced by the symmetric nature of scores and decreased predictive values with individual vessel analysis, calcified atherosclerotic load is not necessarily specific for the anatomic location of stenosis. Moreover, given the similar scores in high-grade and moderate-grade stenotic vessels and high scores in several patients without stenosis, the relationship between scores and luminal narrowing is also not completely linear. Similar to the coronary circulation, calcium burden in the carotid arteries likely reflects overall atherosclerotic burden and secondarily luminal stenosis, depending on individual variability in vascular remodeling.
Clinically, given the relative cost, lack of radiation, and high diagnostic accuracy of B-mode sonography, carotid calcium measurement by MDCT is not recommended as a routine method for the evaluation of carotid artery stenosis. However, in patients who incidentally have a large burden of cervical carotid artery calcium on unenhanced neck CT performed for other reasons such as cervical spine evaluation or airway assessment, the use of contrast material or sonography may be justified to examine for the presence of carotid stenosis.
If confirmed longitudinally, there are significant potential clinical benefits for calcium scores as a risk factor for ischemic neurologic symptoms.The ideal management for asymptomatic carotid artery stenosis of greater than 60% is still under debate. Thus, our finding that the odds for the occurrence of symptoms increases with increasing calcium burden, even after multivariate adjustment for factors such as stenosis, suggests that scores are an independent marker for ischemic events and could be used to stratify patients on the basis of risk and allow the most appropriate treatment—less aggressive medical treatment versus more invasive therapy such as stent placement or endarterectomy— to be selected.
MDCT angiography may have an expanding role in the diagnostic algorithm for carotid artery disease: it could help not only to confirm results after sonography, as is often requested by clinicians, but also to quantify calcium levels to further classify asymptomatic patients as being at low or high risk for developing symptoms. This capability would confer a distinct advantage for MDCT angiography over MRI, which is less robust in the evaluation of calcium. Moreover, the effects of drug therapy, specifically statins, could possibly be monitored by serially evaluating carotid calcium levels instead of or in addition to coronary calcium scores. Coronary artery calcium scores are hampered by poor interexamination reproducibility secondary to coronary arterial motion artifacts and cardiac position changes [29]. Carotid burden is less prone to motion artifacts and consequently may be more reproducible from examination to examination.
The study has several limitations including examining only patients with symptoms likely due to complications from extracranial carotid artery atherosclerotic disease and not assessing the odds for neurologic symptoms conferred by calcifications due to other potential causes of ischemia. However, carotid artery disease accounts for approximately one half of ischemic strokes [30]. In addition, the exclusion of patients with symptoms from other causes allowed a more direct examination of the relationship between vascular and cerebral territory and thus presumably scores with symptoms. Several of the CIs for the risk factors were relatively wide, likely because of the limited number of patients in our study. Consequently, larger studies would help to validate our results.
The use of MDCT angiography as the gold standard for luminal stenosis in our study could also have led to potential error. CT has been found in multiple trials to be accurate in the diagnosis of carotid artery stenosis but can lead to miscategorization of the degree of stenosis, especially if not performed and evaluated properly or if the plaques causing stenosis are heavily calcified [8–13]. However, our groups for classification of the percentage luminal stenosis for total vessel and individual vessel scores were relatively broad, making error in categorization less likely. We also used MDCT, rather than single-detector CT for which most data are currently published, which likely resulted in improved accuracy from better resolution.
Another potential limitation is the possibility of contrast material obscuring small amounts of calcium, but Achenbach et al. [17] found that MDCT angiography had a sensitivity of 94% and specificity of 94% for calcified plaque despite the presence of contrast material. Moreover, Hong et al. [31] found a very high correlation between coronary calcium scores based on 1.25-mm-section-width CT angiography and traditional 3-mm-section-width unenhanced CT. Finally, we did not examine the contribution of intracranial carotid artery calcifications as seen on routine head CT in addition to or in comparison with extracranial carotid calcium burden for the development of symptoms; future studies looking at this relationship would be of interest.
In conclusion, cervical calcium burden can be reliably quantified by MDCT angiography and likely represents a marker for luminal stenosis. More important, calcium scores are a potential independent risk marker for TIA and stroke that could be used to stratify patients on the basis of risk and to monitor the effects of therapy.
References
- 1.O’Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association Expert Consensus document on electron-beam computed tomography for the diagnosis and prognosis of coronary artery disease. Circulation. 2000;102:126–140. doi: 10.1161/01.cir.102.1.126. [DOI] [PubMed] [Google Scholar]
- 2.Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 3.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology. 2003;228:826–833. doi: 10.1148/radiol.2283021006. [DOI] [PubMed] [Google Scholar]
- 4.Kondos GT, Hoff JA, Sevrukov A, et al. Electron-beam tomography coronary artery calcium and cardiac events: a 37-month follow-up of 5635 initially asymptomatic low- to intermediate-risk adults. Circulation. 2003;107:2571–2576. doi: 10.1161/01.CIR.0000068341.61180.55. [DOI] [PubMed] [Google Scholar]
- 5.Wilson PW, Kauppila LI, O’Donnell CJ, et al. Abdominal aortic calcific deposits are an important predictor of vascular morbidity and mortality. Circulation. 2001;103:1529–1534. doi: 10.1161/01.cir.103.11.1529. [DOI] [PubMed] [Google Scholar]
- 6.Iribarren C, Sidney S, Sternfeld B, Browner WS. Calcification of the aortic arch: risk factors and association with coronary heart disease, stroke, and peripheral vascular disease. JAMA. 2000;283:2810–2815. doi: 10.1001/jama.283.21.2810. [DOI] [PubMed] [Google Scholar]
- 7.Culebras A, Otero C, Toledo JR, Rubin BS. Computed tomographic study of cervical carotid calcification. Stroke. 1989;20:1472–1476. doi: 10.1161/01.str.20.11.1472. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz RB, Jones KM, Chernoff DM, et al. Common carotid artery bifurcation: evaluation with spiral CT—work in progress. Radiology. 1992;185:513–519. doi: 10.1148/radiology.185.2.1410365. [DOI] [PubMed] [Google Scholar]
- 9.Marks MP, Napel S, Jordan JE, Enzmann DR. Diagnosis of carotid artery disease: preliminary experience with maximum-intensity-projection spiral CT angiography. AJR. 1993;160:1267–1271. doi: 10.2214/ajr.160.6.8498231. [DOI] [PubMed] [Google Scholar]
- 10.Cumming MJ, Morrow IM. Carotid artery stenosis: a prospective comparison of CT angiography and conventional angiography. AJR. 1994;163:517–523. doi: 10.2214/ajr.163.3.8079836. [DOI] [PubMed] [Google Scholar]
- 11.Cinat M, Lane CT, Pham H, Lee A, Wilson SE, Gordon I. Helical CT angiography in the preoperative evaluation of carotid artery stenosis. J Vasc Surg. 1998;28:290–300. doi: 10.1016/s0741-5214(98)70165-x. [DOI] [PubMed] [Google Scholar]
- 12.Marcus CD, Ladam-Marcus VJ, Bigot JL, Clement C, Baehrel B, Menanteau BP. Carotid arterial stenosis: evaluation at CT angiography with the volume-rendering technique. Radiology. 1999;211:775–780. doi: 10.1148/radiology.211.3.r99jn42775. [DOI] [PubMed] [Google Scholar]
- 13.Randoux B, Marro B, Koskas F, et al. Carotid artery stenosis: prospective comparison of CT, three-dimensional gadolinium-enhanced MR, and conventional angiography. Radiology. 2001;220:179–185. doi: 10.1148/radiology.220.1.r01jl35179. [DOI] [PubMed] [Google Scholar]
- 14.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack: proposal for a new definition. N Engl J Med. 2002;347:1713–1716. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 15.Dix JE, Evans AJ, Kallmes DF, Sobel AH, Phillips CD. Accuracy and precision of CT angiography in a model of carotid artery bifurcation stenosis. Am J Neuroradiol. 1997;18:409–415. [PMC free article] [PubMed] [Google Scholar]
- 16.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [No authors listed]. [DOI] [PubMed] [Google Scholar]
- 17.Achenbach S, Moselewski F, Ropers D, et al. Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation. 2004;109:14–17. doi: 10.1161/01.CIR.0000111517.69230.0F. [DOI] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 19.Raggi P, Callister TQ, Shaw LJ. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler Thromb Vasc Biol. 2004;24:1272–1277. doi: 10.1161/01.ATV.0000127024.40516.ef. [DOI] [PubMed] [Google Scholar]
- 20.Callister TQ, Raggi P, Cooil B, Lippolis NJ, Russo DJ. Effect of HMG-CoA reductase inhibitors on coronary artery disease as assessed by electron-beam computed tomography. N Engl J Med. 1998;339:1972–1978. doi: 10.1056/NEJM199812313392703. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach S, Ropers D, Pohle K, et al. Influence of lipid-lowering therapy on the progression of coronary artery calcification: a prospective evaluation. Circulation. 2002;106:1077–1082. doi: 10.1161/01.cir.0000027567.49283.ff. [DOI] [PubMed] [Google Scholar]
- 22.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications: a statement for health professionals from the American Heart Association Writing Group. Circulation. 1996;94:1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 23.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 24.Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 25.Nandalur KR, Baskurt E, Hagspiel KD, Phillips CD, Kramer CM. Calcified carotid atherosclerotic plaque is associated less with ischemic symptoms than is noncalcified plaque on MDCT. AJR. 2005;184:295–298. doi: 10.2214/ajr.184.1.01840295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 27.Rumberger JA, Schwartz RS, Simons DB, Sheedy PF, 3rd, Edwards WD, Fitzpatrick LA. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73:1169–1173. doi: 10.1016/0002-9149(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 28.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 29.Lu B, Zhuang N, Mao SS, et al. EKG-triggered CT data acquisition to reduce variability in coronary arterial calcium score. Radiology. 2002;224:838–844. doi: 10.1148/radiol.2242011332. [DOI] [PubMed] [Google Scholar]
- 30.Gillard JH. Imaging of carotid artery disease: from luminology to function? Neuroradiology. 2003;45:671–680. doi: 10.1007/s00234-003-1054-5. [DOI] [PubMed] [Google Scholar]
- 31.Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF. Coronary artery calcium: absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology. 2002;223:474–480. doi: 10.1148/radiol.2232010919. [DOI] [PubMed] [Google Scholar]