Abstract
Background
Hydrogen sulfide (H2S) is an endogenous signaling molecule with potent cytoprotective effects. The present study evaluated the therapeutic potential of H2S in murine models of heart failure.
Methods and Results
Heart failure was induced by subjecting mice either to permanent ligation of the left coronary artery for 4 weeks or to 60 minutes of left coronary artery occlusion followed by reperfusion for 4 weeks. Transgenic mice with cardiac-restricted overexpression of the H2S-generating enzyme cystathione γ-lyase (αMHC-CGL-Tg+) displayed a clear protection against left ventricular structural and functional impairment as assessed by echocardiography in response to ischemia-induced heart failure, as well as improved survival in response to permanent myocardial ischemia. Exogenous H2S therapy (Na2S; 100 μg/kg) administered at the time of reperfusion (intracardiac) and then daily (intravenous) for the first 7 days after myocardial ischemia also protected against the structural and functional deterioration of the left ventricle by attenuating oxidative stress and mitochondrial dysfunction. Additional experiments aimed at elucidating some of the protective mechanisms of H2S therapy found that 7 days of H2S therapy increased the phosphorylation of Akt and increased the nuclear localization of 2 transcription factors, nuclear respiratory factor 1 and nuclear factor-E2-related factor (Nrf2), that are involved in increasing the levels of endogenous antioxidants, attenuating apoptosis, and increasing mitochondrial biogenesis.
Conclusions
The results of the present study suggest that either the administration of exogenous H2S or the modulation of endogenous H2S production may be of therapeutic benefit in the treatment of ischemia-induced heart failure.
Keywords: cystathionine γ-Lyase, heart failure, hydrogen sulfide, ischemia, myocardial infarction
Heart failure continues to be a major health problem in the United States, especially in the elderly population.1,2 Unfortunately, current treatments for heart failure are insufficient, and the availability of hearts for transplantation is severely inadequate.3 Therefore, adjunct pharmacotherapies designed to coincide with the standard means of care are needed to decrease the extent of injury leading to the development of heart failure. Small gaseous signaling molecules are labile biological mediators that are able to freely diffuse through cell membranes to invoke cellular signaling, thus alleviating the need for membrane receptors and second messengers. Hydrogen sulfide (H2S), a recently classified small molecule effector,4 is produced in the body by the enzymes cystathionine γ-lyase (CGL; cytstathione, CTH), cystathionine β-synthase, and 3-mercaptopyruvate sulfurtransferase. H2S has been reported to provide cardioprotection in various models of cardiac injury through its ability to preserve mitochondrial function and to reduce cardiomyocyte apoptosis.5,6 Although the cytoprotective effects of H2S have been demonstrated in models of acute cardiac injury, the effects of H2S therapy on cardiac function in the setting of chronic heart failure are currently unknown. Therefore, the purpose of the present study was to investigate the potential cardioprotective effects of endogenous and exogenous H2S on survival and cardiac function in 2 murine models of ischemia-induced heart failure.
Methods
Animals
Male C57BL6/J mice, 8 to 10 weeks of age, were used (Jackson Laboratories, Bar Harbor, Me). The generation of cardiac-specific transgenic mice overexpressing CGL (αMHC-CGL-Tg+, FVB background) has been described previously.6 αMHC-CGL-Tg+ and nontransgenic littermates were bred and used at 8 to 10 weeks of age. All experimental mouse procedures were approved by the Institute for Animal Care and Use Committee at Emory University and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86–23, revised 1996), and to federal and state regulations.
Materials
Sodium sulfide (Na2S) was produced by Ikaria Holdings, Inc (Seattle, Wash) by using H2S gas (Matheson, Newark, Calif) as a starting material as previously described.7 Na2S (100 μg/kg) was administered with a 32-gauge needle in a final volume of 50 μL as an intracardiac injection once at the time of reperfusion (Na2S) or once at the time of reperfusion followed by daily tail vein (intravenous) injections for the first 7 days of reperfusion (Na2S 7 days). This dose of Na2S was selected on the basis of our previous experience investigating Na2S in murine models of cardiac ischemia/reperfusion injury.7 Saline was administered in the same manner for the respective vehicle groups.
Heart Failure Protocols
Heart failure was induced either by permanent ligation of the left coronary artery (LCA) or by subjecting mice to 60 minutes of LCA occlusion followed by reperfusion for up to 4 weeks as described previously.8 All mice were randomly allocated to treatment groups. Myocardial infarct size assessment, echocardiographic assessment of left ventricular (LV) structure and function, and histological analysis of infarct scar were all performed as previously described.7,9
Lipid Hydroperoxide Assay
Quantification of lipid peroxidation was performed to assess the extent of cardiac oxidative stress as described previously.7
Quantitative Real-Time Polymerase Chain Reaction for Mitochondrial DNA
Mitochondrial DNA content was quantified by real-time reverse-transcription polymerase chain reaction with cardiac DNA as described previously.10
Cardiac Mitochondria Isolation, Mitochondrial Respiratory Rate, and ATP Synthesis
Cardiac mitochondria were isolated from the following groups of mice: sham-operated, vehicle-treated, and Na2S-treated mice. Oxygen consumption and ATP synthesis rates were determined as previously described.8
Western Blot Analysis
Western blot analysis was performed as described previously.7
Statistical Analysis
All data in this study are expressed as mean ±SEM. Means were compared by use of Prism 4 (GraphPad Software Inc) with a Student unpaired 2-tailed t test (Western blot analysis), 1-way ANOVA (ratio of heart to body weight, lipid hydroperoxidation [LPO] data, mitochondrial DNA, and mitochondrial respiration data), or 2-way ANOVA (echocardiography data) when appropriate. For the ANOVA, if a significant result was found, the Tukey (1-way ANOVA) or Bonferroni (2-way ANOVA) test was used as the posthoc analysis. Survival curves were compared by use of a log-rank (Mantel-Cox) test. For all data, a value of P<0.05 was considered significant.
Results
Endogenous Overexpression of the H2S-Generating Enzyme CGL Improves Survival After Permanent LCA Occlusion
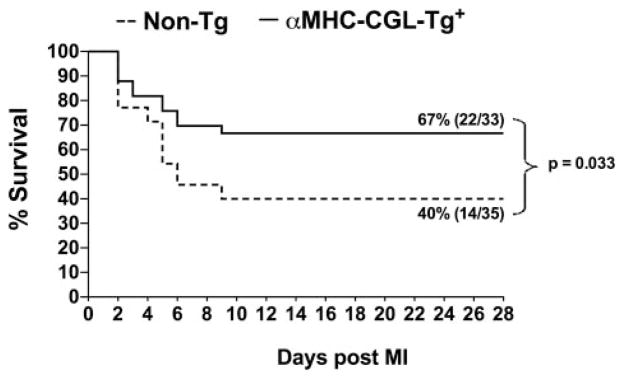
The effects of H2S on heart failure were first evaluated in mice that overexpress the H2S-generating enzyme CGL (αMHC-CGL-Tg+). These cardiac-specific transgenic mice have an ≈15-fold overexpression of CGL in their hearts, which results in a 2-fold increase in cardiac H2S production.6 For these experiments, αMHC-CGL-Tg+ and nontransgenic mice were subjected to permanent occlusion of the LCA. At 4 weeks after myocardial ischemia, both groups of mice exhibited significant mortality (Figure 1). The αMHC-CGL-Tg+ mice exhibited an overall survival rate of 67% (22 of 33), whereas the nontransgenic mice exhibited an overall survival rate of 40% (14 of 35). Therefore, cardiac-specific overexpression of CGL resulted in a 68% improvement in survival after myocardial ischemia (P=0.033 between groups).
Figure 1.
Overexpression of CGL improved survival after permanent occlusion of the LCA. Survival curve for αMHC-CGL-Tg+ and nontransgenic (Non-Tg) mice during the 4-week period after permanent occlusion of the LCA. The αMHC-CGL-Tg+ mice exhibited an overall survival rate of 67% (22 of 33) during the 4-week follow-up; the nontransgenic mice exhibited an overall survival rate of 40% (14 of 35). Comparisons between survival curves were made with a log-rank (Mantel-Cox) test.
Endogenous Overexpression of CGL Reduces LV Dilatation and Cardiac Hypertrophy but Does Not Improve Function After Permanent LCA Occlusion
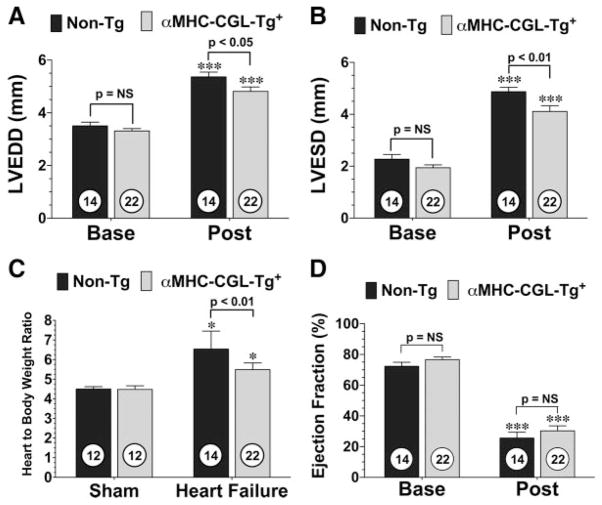
At the end of the 4-week follow-up period, the surviving mice were subjected to 2-dimensional, high-resolution echocardiography to determine the degree of LV dilatation and LV dysfunction. Analysis revealed that the LV end-diastolic (LVEDD) and end-systolic (LVESD) diameter of both the αMHC-CGL-Tg+ and nontransgenic mice were significantly higher than their respective baseline readings (P<0.001), suggesting that LV dilation had occurred (Figure 2A and 2B). However, the hearts of αMHC-CGL-Tg+ mice had significantly smaller increases in both LVEDD (P<0.05 versus nontransgenic) and LVESD (P<0.01 versus nontransgenic). Cardiac hypertrophy was also analyzed by determining the ratios of heart to body weight (Figure 2C). Both αMHC-CGL-Tg+ and nontransgenic mice displayed cardiac hypertrophy 4 weeks after myocardial ischemia compared with sham-operated animals (P<0.05), but αMHC-CGL-Tg+ mice displayed significantly less hypertrophy (P<0.01 versus nontransgenic). Despite these significant reductions in LV dilatation and cardiac hypertrophy, no improvement in LV ejection fraction was evident in the αMHC-CGL-Tg+ mice compared with the nontransgenic mice (Figure 2D). Additionally, the heart rate of the 2 groups of mice was evaluated at baseline and 4 weeks after myocardial ischemia (Figure IA in the online-only Data Supplement). No differences at baseline were observed, and both groups of mice exhibited an elevated heart rate 4 weeks after myocardial ischemia.
Figure 2.
Overexpression of CGL reduces LV dilatation and cardiac hypertrophy but does not improve LV function after permanent occlusion of LCA. LVEDD (A), LVESD (B), ratio of heart to body weight (C), and LV ejection fraction (D) for αMHC-CGL-Tg+ and nontransgenic (Non-Tg) mice 4 weeks after permanent LCA occlusion (Post). LVEDD, LVESD, and LV ejection fraction were calculated with 2-dimensional B-mode echocardiography images at baseline (Base) and after myocardial ischemia in all groups. Ratios of heart to body weight were used as a measure of cardiac hypertrophy. Values are mean±SEM. Numbers inside bars indicate the number of animals investigated in each group. Means for the echocardiography data were compared by use of a 2-way ANOVA with a Bonferroni test as the posthoc analysis. Means for the ratio of heart to body weight were compared through the use of a 1-way ANOVA with a Tukey test as the posthoc analysis. ***P<0.001 vs baseline; *P<0.05 vs sham.
We also measured the infarct area relative to the entire LV at 4 weeks after infarction (Figure IIA in the online-only Data Supplement). Analysis revealed that the nontransgenic mice displayed a 22±3% infarct area/LV and the αMHC-CGL-Tg+ mice displayed a 25±2% infarct area/LV. These findings suggest that overexpression of CGL improves survival after permanent LCA occlusion and that this survival benefit is independent of any effect on infarct size.
Endogenous Overexpression of CGL Improves LV Structure and Function After Ischemia-Induced Heart Failure
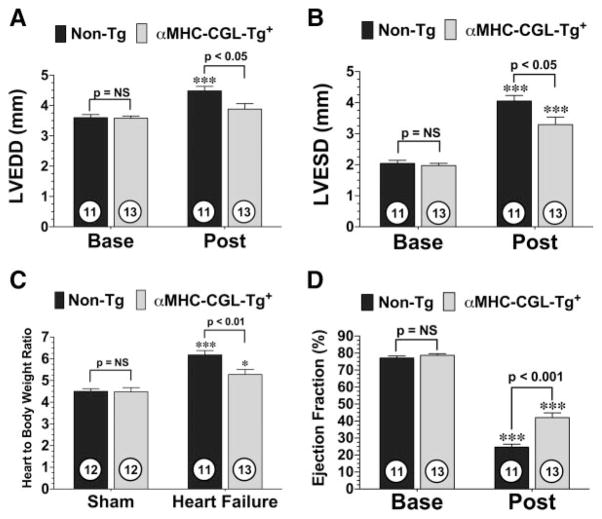
To study the effects of H2S in a more clinically relevant model of heart failure that mimics the effects of coronary revascularization therapy, αMHC-CGL-Tg+ and nontransgenic mice were subjected to 60 minutes of LCA occlusion followed by 4 weeks of reperfusion. Myocardial infarction was evaluated in 2 different groups of mice at 24 hours and 4 weeks of reperfusion with the Evans blue/triphenyltetrazolium chloride method and histologically, respectively. Following 24 hours of reperfusion, the area at risk per LV was similar (P=NS) in both groups, and the αMHC-CGL-Tg+ mice (n=7) displayed a 19% reduction (52±2% versus 42±4%; P<0.05) in infarct area relative to the area at risk and a 30% reduction (33±3% versus 23±2%; P<0.05) in infarct area/LV compared with the nontransgenic mice (n=10). Following 4 weeks of reperfusion, analysis revealed that the nontransgenic mice displayed an 11.8±1.2% infarct area/LV and the αMHC-CGL-Tg+ mice displayed a 7.3±1% infarct area/LV at 4 weeks of reperfusion, which corresponded to a 38% reduction in infarct area (P<0.01 versus nontransgenic; Figure IIB in the online-only Data Supplement). Following 4 weeks of reperfusion, LV dilatation, cardiac hypertrophy, and LV dysfunction were all prevalent in both groups of mice (Figure 3). However, αMHC-CGL-Tg+ mice displayed significantly smaller increases in LVEDD (P<0.05), LVESD (P<0.05), and ratio of heart to body weight (P<0.01) and displayed better LV ejection fraction (P<0.001) compared with nontransgenic mice. In addition, both groups of mice exhibited an elevated heart rate 4 weeks after myocardial I/R, but only the nontransgenic mice had a significant increase from baseline (P<0.05; Figure IB in the online-only Data Supplement). These findings suggest that increased production of H2S during the reperfusion phase has a positive impact on LV structure and function after ischemia-induced heart failure.
Figure 3.
Overexpression of CGL reduces LV dilatation, reduces cardiac hypertrophy, and improves LV function after myocardial ischemia and reperfusion. LVEDD (A), LVESD (B), ratio of heart to body weight (C), and LV ejection fraction (D) for αMHC-CGL-Tg+ and nontransgenic mice 4 weeks after 60 minutes of LCA occlusion and reperfusion (Post). Values are mean±SEM. Means for the echocardiography data were compared by use of a 2-way ANOVA with a Bonferroni test as the posthoc analysis. Means for the ratios of heart to body weight were compared by use of a 1-way ANOVA with a Tukey test as the posthoc analysis. ***P<0.001 vs baseline (Base) or sham; *P<0.05 vs sham.
Single Injection of Na2S Reduces Infarct Size but Does Not Improve LV Structure and Function
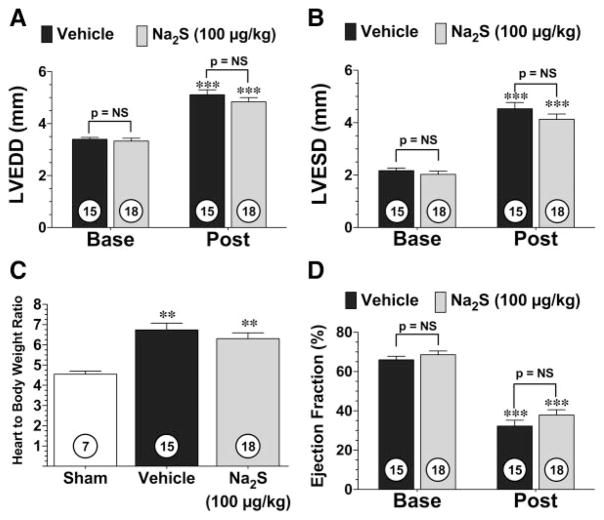
In an effort to translate these findings to a more clinically relevant model we next utilized pharmacologic administration of H2S (Figure 4). In these experiments, C57BL/6J mice were subjected to 60 minutes of LCA occlusion followed by 4 weeks of reperfusion. H2S (Na2S; 100 μg/kg) or vehicle (saline) was administered at the time of reperfusion (intracardiac). Again, myocardial infarction was evaluated in 2 different groups of mice at 24 hours and 4 weeks of reperfusion. Following 24 hours of reperfusion, Na2S (n=9) decreased infarct area/area at risk by 14% (69±2% versus 59±2%; P<0.05) and decreased infarct area/LV by 20% (41±2% versus 33±2%; P<0.05) compared with vehicle-treated mice (n=8). At 4 weeks of reperfusion, analysis revealed a similar 25% reduction in infarct area/LV (12±1% versus 9±1%; P<0.05) in the mice treated with Na2S compared with the vehicle-treated mice (Figure IIC in the online-only Data Supplement). However, following 4 weeks of reperfusion, the Na2S-treated mice did not show any improvements in LVEDD, LVESD, ratio of heart to body weight, LV ejection fraction, or heart rate compared with the vehicle-treated group (Figure 4 and Figure IC in the online-only Data Supplement). This finding suggests that a single administration of H2S at reperfusion is not sufficient to improve LV function at 4 weeks, even though a single administration of H2S reduces infarct size.
Figure 4.
Single administration of Na2S does not attenuate the development of ischemia-induced heart failure. LVEDD (A), LVESD (B), ratio of heart to body weight (C), and LV ejection fraction (D) for Na2S- and vehicle-treated mice 4 weeks after 60 minutes of LCA occlusion and reperfusion. (Post) Mice were treated with 100 μg/kg Na2S or vehicle at the time of reperfusion. Values are mean±SEM. Means for the echocardiography data were compared by use of a 2-way ANOVA with a Bonferroni test as the posthoc analysis. Means for the ratios of heart to body weight were compared by use of a 1-way ANOVA with a Tukey test as the posthoc analysis. ***P<0.001 vs baseline (Base); **P<0.01 vs sham.
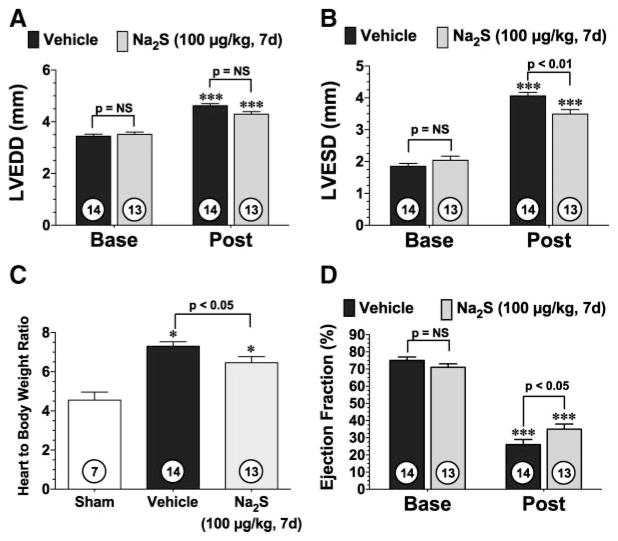
Daily Injections of Na2S During the First 7 Days of Reperfusion Improve LV Structure and Function
Subsequent experiments evaluated the effectiveness of daily administrations of H2S during the first 7 days of reperfusion (Figure 5). In these experiments, C57BL/6J mice were subjected to 60 minutes of LCA occlusion followed by 4 weeks of reperfusion. Analysis at 4 weeks of reperfusion revealed that treatment during the first 7 days of the reperfusion period led to a decrease in LV dilatation, a decrease in cardiac hypertrophy, and an improvement in cardiac function. No differences in heart rates were observed at baseline, and both groups of mice exhibited an elevated heart rate 4 weeks after myocardial ischemia (Figure ID in the online-only Data Supplement). To determine whether the 7-day treatment of Na2S had any additional effects on infarct size reduction, the area of infarction was evaluated at 4 weeks of reperfusion. Analysis revealed that the vehicle-treated mice displayed a 12±1% infarct area/LV and the Na2S-treated mice displayed a 9±1% infarct area/LV at 4 weeks of reperfusion, which corresponded to a 25% reduction in infarct area (P<0.01 versus vehicle; Figure IID in the online-only Data Supplement). These results suggest that treatment with exogenous H2S during the first 7 days of reperfusion is critical for sustained improvements in LV structure and function.
Figure 5.
Daily administrations of Na2S attenuate the development of ischemia-induced heart failure. LVEDD (A), LVESD (B), ratio of heart to body weight (C), and LV ejection fraction (D) for NA2S-and vehicle-treated mice 4 weeks after 60 minutes of LCA occlusion and reperfusion (Post). Mice were treated with 100 μg/kg Na2S at the time of reperfusion and then daily for the first 7 days of reperfusion. Values are mean±SEM. Means for the echocardiography data were compared by use of a 2-way ANOVA with a Bonferroni test as the posthoc analysis. Means for the ratios of heart to body weight were compared by use of a 1-way ANOVA with a Tukey test as the posthoc analysis. ***P<0.001 vs baseline (Base); *P<0.05 vs sham.
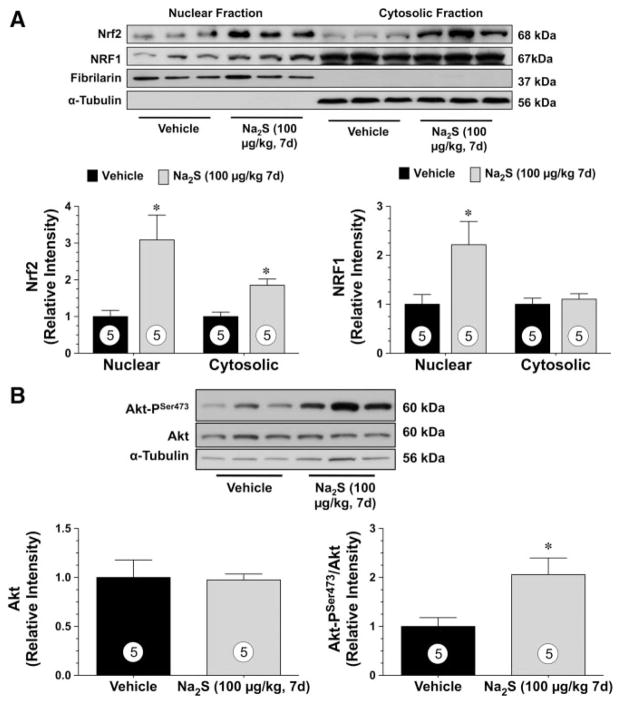
Daily Injections of Na2S Induce the Nuclear Localization of Nrf2 and NRF-1 and Increase the Phosphorylation of Akt
H2S has a diverse physiological profile, which may account for the cardioprotection observed in the current models of heart failure. Recently, nuclear factor-E2–related factor (Nrf2) has been identified as an important cellular target of H2S.7 Nrf2 is a key transcription factor that regulates antioxidant genes as an adaptive response to oxidative stress11–13 and regulates mitochondrial biogenesis through an upregulation of nuclear respiratory factor 1 (NRF-1).14 Therefore, experiments were conducted to evaluate Nrf2 signaling after H2S treatment. For these experiments, Na2S was administered to mice for 7 days (intravenous), at which time hearts were excised and processed for Western blot analysis (Figure 6). Because Nrf2 is a transcription factor, its protein expression was evaluated in both cytosolic and nuclear fractions. Analysis revealed that Nrf2 protein levels were increased (P<0.05) in both the cytosolic and nuclear fractions in the hearts treated with Na2S compared with the sham hearts (Figure 6A). Subsequently, the nuclear expression, but not the cytosolic expression, of NRF-1 was elevated in the hearts of mice treated with Na2S. Recently, NRF-1 transcriptional activity was reported to be regulated by Akt.15 Therefore, the ability of Na2S to increase the phosphorylation of Akt at serine residue 473 (Akt-PSer473) was evaluated (Figure 6B). A significant (P<0.05 versus sham) increase in the phosphorylation of Akt at serine residue 473 (AktSer473) was observed in the hearts of mice treated with Na2S for 7 days. No differences in total Akt levels were noted.
Figure 6.
Daily administrations of Na2S induce the nuclear localization of Nrf2 and NRF-1 and increase the phosphorylation of Akt. A, Representative immunoblots and densitometric analysis of cardiac Nrf2 and NRF-1 in the cytosolic and nuclear fractions after 1 week of Na2S treatment. B, Representative immunoblots and densitometric analysis of phosphorylated Akt-Ser473 and total Akt after 1 week of Na2S treatment. Values are mean±SEM. Means for all data were compared by use of an unpaired t test. *P<0.05 vs vehicle.
To determine whether Na2S could alter the expression levels of Nrf2, NRF-1 and Akt in nonvascular tissue, additional studies were performed using hepatic tissue taken from mice administered Na2S for 7 days. These studies revealed that Na2S therapy increased the nuclear expression of both Nrf2 and NRF-1 (P<0.05 versus vehicle) but did not increase the levels of Akt or alter its phosphorylation status (Figure III in the online-only Data Supplement), suggesting that the activation of Nrf2 and NRF-1 by Na2S was not restricted to the heart. Additionally, we have previously reported that hearts from αMHC-CGL-Tg+ mice have an increased nuclear expression of Nrf2.7 Further analysis in the present study revealed that hearts from αMHC-CGL-Tg+ mice had an increased nuclear expression of NRF-1 (P<0.01 versus nontransgenic) and an increased expression of Akt (P<0.05 versus nontransgenic) but no changes in Akt-PSer473 (Figure IV in the online-only Data Supplement). No changes in Nrf2, NRF-1, and Akt were observed in the livers of αMHC-CGL-Tg+ mice (Figure V in the online-only Data Supplement), which confirms our previous findings that the increased generation of H2S is confined to the heart.6
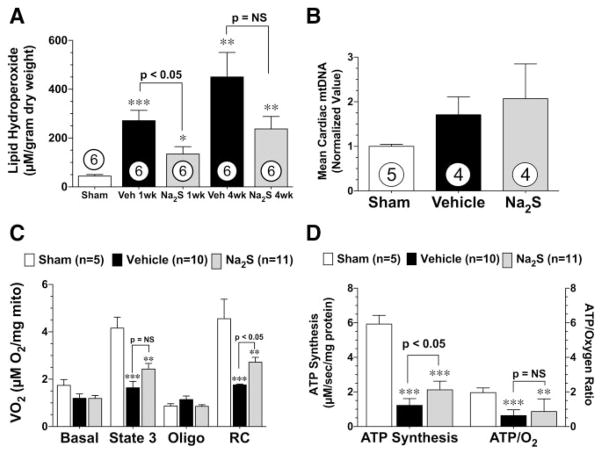
Daily Injections of Na2S Attenuate Oxidative Stress
Lipid hydroperoxidation (LPO) was used as a measure of cardiac oxidative stress during the development of heart failure. In these experiments (Figure 7A), 2 groups of C57BL/6J mice were subjected to 60 minutes of LCA occlusion followed by 1 and 4 weeks of reperfusion. At 1 week of reperfusion, both the vehicle-treated (P<0.001) and 7-day Na2S-treated (P<0.05) mice exhibited significantly higher levels of LPO compared with sham-operated controls. However, the Na2S-treated mice displayed significantly lower levels of LPO compared with the vehicle-treated mice (P<0.05). LPO levels remained elevated above sham levels in both groups of mice at 4 weeks of reperfusion (P<0.01), and although not statistically significant, there was a trend for lower LPO levels in the hearts of mice treated with Na2S compared with the vehicle-treated mice. These findings suggest that treatment with H2S during the first 7 days of reperfusion reduces oxidative stress associated with heart failure.
Figure 7.
Daily administrations of Na2S attenuate oxidative stress and mitochondrial dysfunction during the development of ischemia-induced heart failure. A, Lipid hydroperoxide levels (μmol/L) from sham controls and vehicle (Veh)- and Na2S-treated mice at 1 and 4 weeks of reperfusion after myocardial ischemia. B, Mean cardiac mitochondrial DNA levels (mtDNA) determined by real-time polymerase chain reaction analysis shown as arbitrary units normalized to the sham value (1.0), C, Mitochondrial oxygen consumption rates, oligomycin (oligo)-inhibited respiration, and respiratory control (RC) ratios of mitochondria isolated from sham-operated, vehicle-treated, and Na2S-treated mice at 4 weeks of reperfusion after myocardial ischemia. Mean±SEM values are shown for state 3 (ADP-stimulated) respiration in the presence of succinate and glycerol-3-phospate. D, ATP synthesis rates and the ratio of ATP synthesis to maximal oxygen consumption obtained from the same isolated mitochondria shown in C. Values are mean±SEM. Means for all data were compared by use of a 1-way ANOVA with a Tukey test as the posthoc analysis. *P<0.05, **P<0.01, and ***P<0.001 vs sham.
Daily Injections of Na2S Did Not Increase Mitochondrial Biogenesis but Did Improve Mitochondrial Respiration and ATP Synthesis
NRF-1 regulates the expression of several genes responsible for mitochondrial biogenesis.14,16 Because H2S increased the nuclear accumulation of NRF-1, the next series of experiments evaluated mitochondrial biogenesis. For these experiments, mice were subjected to 60 minutes of LCA occlusion followed by 4 weeks of reperfusion. Na2S or vehicle was administered at the time of reperfusion (intracardiac) and then daily for 7 days (intravenous). At 4 weeks of reperfusion, the hearts from all groups of mice were found to have similar ratios of cytochrome b DNA to β-actin DNA quantity, suggesting that H2S did not induce mitochondrial biogenesis (Figure 7B).
H2S can preserve mitochondrial function after acute myocardial ischemia/reperfusion injury.6 Therefore, we investigated the effects of H2S on mitochondrial function during the development of heart failure (Figure 7C and 7D). Mitochondria isolated from the hearts of vehicle-treated mice were found to have a 61% reduction (P<0.001 versus sham) in maximal ADP-stimulated (state 3) oxygen consumption, a slightly increased oligomycin-inhibited respiration, and reduced respiratory control ratio (P<0.001 versus sham), suggestive of uncoupling. ATP synthesis rates and the ATP/oxygen consumption ratios in the mitochondria from vehicle-treated mice were significantly (P<0.001) reduced compared with sham-operated mice (Figure 7D). Mitochondria isolated from the hearts of Na2S-treated mice were found to have slightly higher rates of ADP-stimulated oxygen consumption compared with vehicle-treated mice (P=0.07). Despite similar rates of oligomycin-inhibited respiration, the mitochondria isolated from Na2S-treated mice displayed higher respiratory control ratios (P<0.05), greater ATP synthesis rates (P<0.05), and slightly higher ATP/oxygen consumption ratios compared with vehicle-treated mice. These data indicate that the respiration of cardiac mitochondria during heart failure was inefficient, likely a result of uncoupled respiration, and that H2S treatment is able to limit this dysfunction.
Discussion
In recent years, the cardioprotective effects of H2S have been demonstrated in various models of myocardial injury.17 These studies have provided important mechanistic insights into its cardioprotective actions and important information on dosage, timing, and route of administration. For instance, a single administration of H2S before, during, or after myocardial ischemia decreases myocardial infarct size and attenuates LV dysfunction in both rodents and pigs.6,7,18 Although these studies provide strong evidence for the cardioprotective effects of short-term H2S therapy (ie, single treatment, short follow-up), these studies do not offer any insights into the long-term effects (ie, daily administration, long follow-up) of H2S therapy. Thus, the present study provides the first evidence that H2S therapy can provide long-term protection against myocardial injury. Importantly, the present study demonstrates that although a single administration of H2S at the time of reperfusion is beneficial in attenuating infarct size, this alone is not sufficient to cause a significant improvement in cardiac function. On the other hand, daily H2S therapy initiated at the time of reperfusion and continued for the first 7 days of reperfusion provided significant improvements in cardiac function and LV dimensions despite not providing any additional infarct-sparing benefits over a single treatment of H2S. This suggests that the first 7 days of reperfusion is a critical period for the development of heart failure in this murine model and that initiating therapeutic interventions during this time is paramount for improvements in outcome. This is further supported by the additional findings that genetic overexpression of a critical H2S-producing enzyme, CGL, results in increased endogenous H2S production6 and a profound protection against ischemia-induced heart failure and decreased mortality. Together, these results suggest that H2S treatment could potentially be initiated at the time of coronary artery reperfusion and then continued daily to achieve a long-term improvement in cardiac function and to decrease the morbidity and mortality resulting from heart failure.
H2S possesses a diverse physiological profile that contributes to its cardioprotective actions.19 Of the reported physiological effects of H2S, several could provide protection during the development of heart failure. First, it has become evident that H2S itself serves over the short term as a potent antioxidant20,21 and under more long-term conditions upregulates antioxidant defenses20,22 through the activation of the transcription factor Nrf2.7 Nrf2, a member of the NF-E2 family of nuclear basic leucine zipper transcription factors, regulates the gene expression of a number of enzymes that serve to detoxify pro-oxidative stressors.11 This regulation is mediated by Nrf2 binding to the antioxidant responsive element found in the promoter region of genes12 such as heme oxygenase-1, thioredoxin, thioredoxin reductase, glutathione reductase, glutathione peroxidase, glutathione S-transferase, and catalase.13,23,24 The reported antioxidant effects of H2S may be of critical importance in the setting of heart failure because oxidative stress plays a prominent role in the development of LV remodeling and dysfunction associated with heart failure,25 suggesting that increasing the activity of cellular antioxidant enzymes should protect the failing myocardium.26 The results of the present study support the previous finding that the cardioprotective effects of H2S are related to a reduction in oxidative stress because it was observed that H2S reduced LPO levels at both 1 and 4 weeks of reperfusion. The results of the present study also support a role for Nrf2 in mediating the antioxidant effects of H2S because H2S treatment was observed to induce the nuclear localization of Nrf2. Together, these findings indicate that H2S therapy creates an environment in the heart that is resistant to the oxidative stress associated with the development of heart failure.
Another physiological characteristic of H2S that could provide protection in the failing heart relates to the evidence that H2S can alter the metabolic state of organisms by modulating mitochondrial function.27 In heart failure, there is a decrease in the activity of the complexes of the respiratory chain and Krebs cycle enzymes. The reduced expression of mitochondrial proteins results in decreased mitochondrial respiration efficiency and limited ATP synthesis capacity and myocardial energy production.28 The decreased oxidative capacity of the failing myocardium therefore limits the ability of the heart to meet hemodynamic demands and leads to symptoms of heart failure. Mitochondria are essential for cell survival because of their roles as metabolic energy producers and regulators of programmed cell death.29 Mitochondria rely on an intrinsic genome that is replicated and transcribed semiautonomously but whose maintenance requires nuclear factors such as NRF-1. Recently, the promoter region of NRF-1 was found to contain 4 antioxidant responsive elements that, when bound by Nrf2, led to an increase in NRF-1 protein levels and an increase in gene activation responsible for mitochondrial biogenesis.14 Additionally, NRF-1 transcriptional activity was reported to be regulated by Akt.15 Despite increasing the phosphorylation of Akt and the nuclear accumulation of both Nrf2 and NRF-1, H2S therapy failed to increase mitochondrial biogenesis 4 weeks after myocardial infarction. H2S therapy also failed to provide significant improvements in mitochondrial function, although slight improvements in ATP synthesis were noted. These slight improvements suggest that the effects of H2S on the mitochondria were not direct. Rather, the slight improvements are more likely attributed to the ability of H2S to reduce oxidative stress, suggesting that, in this model of heart failure, the antioxidant effects of H2S may play a more prominent role in mediating its cardioprotective actions.
We live in the midst of the proclaimed epidemic of heart failure, as evidenced by a rise in the number of hospitalizations for heart failure, the number of deaths attributed to heart failure, and the costs associated with care.30,31 Couple this with the ever-increasing prevalence of diabetes mellitus and obesity, 2 of the main risk factors for the development of coronary artery disease, and it is readily apparent that treatment strategies aimed at combating the development and progression of heart failure are important and severely needed. The findings of the present study provide the first evidence that either the modulation of endogenous H2S production or direct H2S administration significantly attenuates the severity of ischemia-induced heart failure in mice by reducing oxidative stress and attenuating mitochondrial dysfunction. Therefore, these findings further support the emerging concept that H2S therapy may be of clinical importance in the treatment of cardiovascular disease and may have a practical clinical use after myocardial infarction to reduce the morbidity and mortality associated with ischemia-induced heart failure.
CLINICAL PERSPECTIVE.
Heart failure continues to be a major health problem as evidenced by a rise in the number of hospitalizations for heart failure, the number of deaths attributed to heart failure, and the ever-increasing costs associated with care. Therapeutic strategies designed to coincide with the standard means of care are, therefore, needed to combat the development and progression of heart failure. Hydrogen sulfide (H2S) is an endogenous gaseous signaling molecule with a diverse physiological profile that has recently been shown to be cardioprotective in various models of cardiac injury. In the present study, we found that either the modulation of endogenous H2S production or direct pharmacologic H2S administration significantly reduced mortality and attenuated the severity of ischemia-induced heart failure in mice. Importantly, the present study demonstrates that although a single administration of H2S at the time of reperfusion is beneficial in attenuating infarct size, this alone is not sufficient to improve cardiac function significantly. On the other hand, daily H2S therapy for the first 7 days of reperfusion or increased endogenous H2S production provided significant improvements in cardiac function, suggesting that multiple therapeutic interventions are paramount for improvements in outcome. Together, these findings further support the emerging concept that H2S therapy may be of clinical importance in the treatment of cardiovascular disease and may have a practical clinical use after myocardial infarction to reduce the morbidity and mortality associated with ischemia-induced heart failure.
Supplementary Material
Acknowledgments
We thank P. Hill (Ikaria Holdings, Inc) for the formulation of the Na2S and thank D.B. Grinsfelder and J.P. Aragon for their invaluable assistance in the completion of this study.
Sources of Funding
This work was supported by grants from the American Diabetes Association (7-09-BS-26 to Dr Calvert) and the National Heart, Lung, and Blood Institute (National Institutes of Health; 2R01HL-060849-09, 5R01HL-092141-01, and 1R01HL093579-01 to Dr Lefer, 1R01HL098481-01 to Dr Calvert, and F32HL092737 to Dr Elrod). This work was also supported by funding from the Carlyle Fraser Heart Center of Emory University Hospital Midtown.
Footnotes
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/cgi/content/full/CIRCULATIONAHA.109.920991/DC1.
Disclosures
Ikaria Holdings, Inc provided the Na2S. The authors report no other conflicts.
References
- 1.Adabag AS, Therneau TM, Gersh BJ, Weston SA, Roger VL. Sudden death after myocardial infarction. JAMA. 2008;300:2022–2029. doi: 10.1001/jama.2008.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen-Solal A, Beauvais F, Logeart D. Heart failure and diabetes mellitus: epidemiology and management of an alarming association. J Card Fail. 2008;14:615–625. doi: 10.1016/j.cardfail.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 5.Bian JS, Yong QC, Pan TT, Feng ZN, Ali MY, Zhou S, Moore PK. Role of hydrogen sulfide in the cardioprotection caused by ischemic preconditioning in the rat heart and cardiac myocytes. J Pharmacol Exp Ther. 2006;316:670–678. doi: 10.1124/jpet.105.092023. [DOI] [PubMed] [Google Scholar]
- 6.Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, Jiao X, Scalia R, Kiss L, Szabo C, Kimura H, Chow CW, Lefer DJ. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A. 2007;104:15560–15565. doi: 10.1073/pnas.0705891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, Kevil CG, Lefer DJ. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ Res. 2009;105:365–374. doi: 10.1161/CIRCRESAHA.109.199919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M, Tian R, Lefer DJ. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvert JW, Gundewar S, Yamakuchi M, Park PC, Baldwin WM, III, Lefer DJ, Lowenstein CJ. Inhibition of N-ethylmaleimide-sensitive factor protects against myocardial ischemia/reperfusion injury. Circ Res. 2007;101:1247–1254. doi: 10.1161/CIRCRESAHA.107.162610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher CD, Augustine LM, Maher JM, Nelson DM, Slitt AL, Klaassen CD, Lehman-McKeeman LD, Cherrington NJ. Induction of drug-metabolizing enzymes by garlic and allyl sulfide compounds via activation of constitutive androstane receptor and nuclear factor E2-related factor 2. Drug Metab Dispos. 2007;35:995–1000. doi: 10.1124/dmd.106.014340. [DOI] [PubMed] [Google Scholar]
- 12.Tanito M, Agbaga MP, Anderson RE. Upregulation of thioredoxin system via Nrf2-antioxidant responsive element pathway in adaptive-retinal neuroprotection in vivo and in vitro. Free Radic Biol Med. 2007;42:1838–1850. doi: 10.1016/j.freeradbiomed.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 13.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 14.Piantadosi CA, Carraway MS, Babiker A, Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ago T, Yeh I, Yamamoto M, Schinke-Braun M, Brown JA, Tian B, Sadoshima J. Thioredoxin1 upregulates mitochondrial proteins related to oxidative phosphorylation and TCA cycle in the heart. Antioxid Redox Signal. 2006;8:1635–1650. doi: 10.1089/ars.2006.8.1635. [DOI] [PubMed] [Google Scholar]
- 16.Scarpulla RC. Nuclear control of respiratory chain expression in mammalian cells. J Bioenerg Biomembr. 1997;29:109–119. doi: 10.1023/a:1022681828846. [DOI] [PubMed] [Google Scholar]
- 17.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 18.Sodha NR, Clements RT, Feng J, Liu Y, Bianchi C, Horvath EM, Szabo C, Sellke FW. The effects of therapeutic sulfide on myocardial apoptosis in response to ischemia-reperfusion injury. Eur J Cardiothorac Surg. 2008;33:906–913. doi: 10.1016/j.ejcts.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci U S A. 2007;104:17907–17908. doi: 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 21.Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, Halliwell B, Moore PK. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”? J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 23.Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol. 2005;203:529–537. doi: 10.1002/jcp.20246. [DOI] [PubMed] [Google Scholar]
- 24.Jones CI, III, Zhu H, Martin SF, Han Z, Li Y, Alevriadou BR. Regulation of antioxidants and phase 2 enzymes by shear-induced reactive oxygen species in endothelial cells. Ann Biomed Eng. 2007;35:683–693. doi: 10.1007/s10439-007-9279-9. [DOI] [PubMed] [Google Scholar]
- 25.Maack C, Kartes T, Kilter H, Schafers HJ, Nickenig G, Bohm M, Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Siu B, Ho YS, Vincent R, Chua CC, Hamdy RC, Chua BH. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 27.Roth MB, Nystul T. Buying time in suspended animation. Sci Am. 2005;292:48–55. doi: 10.1038/scientificamerican0605-48. [DOI] [PubMed] [Google Scholar]
- 28.Ning XH, Zhang J, Liu J, Ye Y, Chen SH, From AH, Bache RJ, Portman MA. Signaling and expression for mitochondrial membrane proteins during left ventricular remodeling and contractile failure after myocardial infarction. J Am Coll Cardiol. 2000;36:282–287. doi: 10.1016/s0735-1097(00)00689-6. [DOI] [PubMed] [Google Scholar]
- 29.Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- 30.Redfield MM. Heart failure: an epidemic of uncertain proportions. N Engl J Med. 2002;347:1442–1444. doi: 10.1056/NEJMe020115. [DOI] [PubMed] [Google Scholar]
- 31.Braunwald E. Shattuck lecture: cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.