Abstract
Studies have shown that molecules in an extract made from bodies of the ectoparasitic mite, Sarcoptes scabiei De Geer, modulate cytokine secretion from cultured human keratinocytes and fibroblasts. In vivo, in the parasitized skin, these cells interact with each other by contact and cytokine mediators and with the matrix in which they reside. Therefore, these cell types may function differently together than they do separately. In this study, we used a human skin equivalent (HSE) model to investigate the influence of cellular interactions between keratinocytes and fibroblasts when the cells were exposed to active/burrowing scabies mites, mite products, and mite extracts. The HSE consisted of an epidermis of stratified stratum corneum, living keratinocytes, and basal cells above a dermis of fibroblasts in a collagen matrix. HSEs were inoculated on the surface or in the culture medium, and their cytokine secretions on the skin surface and into the culture medium were determined by enzyme-linked immunosorbent assay. Active mites on the surface of the HSE induced secretion of cutaneous T cell-attracting chemokine, thymic stromal lymphopoietin, interleukin (IL)-1α, IL-1β, IL-1 receptor antagonist (IL-1ra), IL-6, IL-8, monocyte chemoattractant protein-1, granulocyte/macrophage colony-stimulating factor, and macrophage colony-stimulating factor. The main difference between HSEs and monocultured cells was that the HSEs produced the proinflammatory cytokines IL-1α and IL-1β and their competitive inhibitor IL-1ra, whereas very little of these mediators was previously found for cultured keratinocytes and fibroblasts. It is not clear how the balance between these cytokines influences the overall host response. However, IL-1ra may contribute to the depression of an early cutaneous inflammatory response to scabies in humans. These contrasting results illustrate that cell interactions are important in the host’s response to burrowing scabies mites.
Keywords: scabies mites, human skin equivalents, interleukin-1 receptor antagonist, immuno-modulation, skin cell cytokine secretion
The ectoparasitic mite, Sarcoptes scabiei De Geer, is the source of molecules that modulate the function of many cell types involved in the host’s innate and immune responses to them. These cells include human dermal keratinocytes and fibroblasts (Arlian et al. 2003, Mullins et al. 2009), peripheral blood mononuclear cells and dendritic cells derived from them (Arlian et al. 2004), T-regulatory lymphocytes (Arlian et al. 2006), and skin endothelial cells of the microvasculature (Elder et al. 2006, 2009). This adaptation may allow mites to circumvent the inflammatory and immune responses that eliminate them during an early infestation and favor the parasites becoming established in the host skin. Evasion of the specific components of the host’s inflammatory and immune responses is a common strategy shared by many endo- and ectoparasites. For example, scabies mite-inactivated protease paralogs have been demonstrated to inhibit the human complement system (Bergstrom et al. 2009). Anti-inflammatory, anti-immune, and anti-complement strategies have been identified in ticks and other invertebrate parasites (Ramachandra and Wikel 1992, Diaz et al. 1997, Wikel 1999, Maxwell et al. 2005, Nunn et al. 2005, Daix et al. 2007, Hepburn et al. 2007, Schroeder et al. 2007, Zipfel et al. 2007).
Previous in vitro studies showed that monocultures of normal human keratinocytes and fibroblasts stimulated with S. scabiei extracts modulated their secretion of specific cytokines and chemokines (Arlian et al. 2003, Mullins et al. 2009). In vivo, keratinocytes and fibroblasts interact with each other by contact and cytokine mediators and with the collagen matrix in which they reside, and therefore, these cells may not respond in the same way as they do in isolation (monoculture). Also, substances present in mite extracts may not accurately represent what these cells are exposed to in vivo during an active infestation with this parasite. Burrowing mites secrete salivary solution that aids in penetration of the epidermis (Arlian et al. 1984). The live mite salivary secretions may contain more, less, or different bioactive molecules than are present in extracts made from mite bodies. Physical stimulation of the skin such as scratching is also known to induce keratinocytes and fibroblasts to produce proinflammatory cytokines (Homey et al. 2006). Therefore, actively burrowing mites may physically stimulate epidermal keratinocytes and even fibroblasts in the dermis. We previously found that mites burrowing into human skin equivalents (HSE; keratinocytes grown over fibroblasts in a collagen matrix) produced interleukin (IL)-1α and IL-1β (Arlian et al. 1996b), but cultured cells alone did not produce appreciable amounts of these cytokines in response to stimulation with mite extract (Arlian et al. 2003).
The purpose of this research was to investigate the effect of living scabies mites and mite extracts on cytokine and chemokine secretion by keratinocytes and fibroblasts in a HSE model. The HSE model (Epiderm EFT-400) is structurally much like normal human skin in vivo and consists of an epidermis of stratified stratum corneum, living keratinocytes, and basal cells above a dermis of fibroblasts in a collagen matrix.
Materials and Methods
Scabies Mites and Extract
S. scabiei variety canis De Geer were collected by aspiration onto a 400-mesh (38-μm) stainless steel screen after they had migrated from crusts collected from infested rabbits. For extract preparation, mites were killed by freezing and later were homogenized in endotoxin-free water, as previously described (Elder et al. 2009). The soluble material was collected after centrifugation, and the supernatant was sterile filtered into sterile vials. Protein concentrations were determined by the Bradford protein assay using bovine serum albumin as the standard (Bradford 1976). For inoculation onto HSEs, mites were collected onto the screen and washed by aspiration of sequential 4-ml volumes of phosphate-buffered saline with 0.05% Tween-20, endotoxin-free water, and 70% ethanol.
HSE Challenge
EpiDerm EFT-400 full-thickness HSEs and medium were purchased from MatTek (Ashland, MA). Upon arrival, HSEs on their supports were transferred to new 6-well culture plates containing 3.0 ml of fresh medium/well, and plates were placed in a 37°C incubator with 5–7% CO2. The next day, HSEs were transferred to new plates containing 5.0 ml of fresh medium or of medium containing scabies extract at a final protein concentration of 100 μg/ml (n = 5). Several hundred live mites were then transferred to the surface of one set (n = 5) of HSEs or into the lower well of another set (n = 5). Another set (n = 5) was inoculated on the surface with 100 μg of scabies extract protein, whereas the final set (n = 4) remained as untreated controls. HSEs were then returned to the incubator.
At 6, 12, 18, and 24 h after inoculation, each HSE on its support was lifted from the well, the medium was mixed, and a 550-μl aliquot was removed from the sample well and frozen at −80°C. At the conclusion of the experiment (48 h), all medium was collected from the well and the surface of each HSE was washed with 500 μl of medium that was collected and also frozen at −80°C.
Cytokine Measurements
The concentrations of various cytokines in the culture medium aliquots and surface washes were measured using enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN). Data are from one representative experiment of several replicates and are presented as mean ± SEM. Statistical significance was determined using a single-factor analysis of variance analysis with differences from control with P < 0.05 judged as significant.
Results
Cutaneous T Cell-Attracting Chemokine (CTACK/CC Chemokine Ligand [CCL] 27)
Burrowing mites on the surface of the HSE induced significant secretion of CTACK onto the surface (stratum corneum) of the HSE (Table 1). Scabies extract on the surface of HSEs induced secretion of CTACK into the culture medium, whereas neither extract in the medium nor live mites on the HSE surface or in the culture medium increased CTACK secretion above constitutive levels (Fig. 1). Interestingly, mite extract added to the culture medium did induce increased CTACK secretion at 6 h compared with the control, but this significant difference disappeared by 18 h (Fig. 1).
Table 1. Cytokine secretion at 48 h (pg/ml) onto the surface of HSEs inoculated with live S. scabiei mites on the HSE surface or in the lower well, or with S. scabiei extract on the surface or in the lower well.
| Inoculum | None | Live mites | Live mites |
S. scabiei extract |
S. scabiei extract |
|---|---|---|---|---|---|
| Route | Control | HSE surface | Lower well | HSE surface | Lower well |
| CTACK | 1 ± 1 | 23 ± 6** | 3 ± 2 | 9 ± 6 | 36 ± 17 |
| TSLP | 1 ± 1 | 29 ± 3** | 1 ± 0 | 9 ± 1** | 2 ± 1 |
| GROα | 6,440 ± 390 | 10,950 ± 1,700 | 5,750 ± 140 | 10,360 ± 2,170 | 8,300 ± 1,180 |
| TGFα | N.D. | N.D. | N.D. | N.D. | N.D. |
| IL-1α | 260 ± 60 | 850 ± 20** | 240 ± 70 | 430 ± 40** | 340 ± 80 |
| IL-1β | 9 ± 8 | 39 ± 3** | 1 ± 1 | 6 ± 6 | 1 ± 1 |
| IL-1ra | 8,900 ± 860 | 20,410 ± 1,520** | 10,580 ± 1,640 | 12,410 ± 770 | 10,140 ± 1,480 |
| IL-6 | 4,870 ± 1,740 | 34,060 ± 3,610** | 3,980 ± 2,000 | 16,340 ± 6,080 | 13,550 ± 3,460 |
| IL-8 | 8,080 ± 2,400 | 24,700 ± 3,650** | 11,000 ± 4,220 | 19,360 ± 7,210 | 15,950 ± 4,950 |
| MCP-1 | 1,580 ± 350 | 3,100 ± 370** | 1,360 ± 340 | 3,180 ± 1,100 | 3,450 ± 860 |
| G-CSF | 1,730 ± 720 | 4,080 ± 730 | 1,200 ± 700 | 5,130 ± 2,220 | 3,060 ± 980 |
| GM-CSF | 220 ± 90 | 890 ± 120** | 290 ± 190 | 950 ± 370 | 320 ± 100 |
| M-CSF | 150 ± 70 | 750 ± 80** | 230 ± 90 | 220 ± 80 | 260 ± 90 |
| VEGF | 880 ± 320 | 1,540 ± 150 | 490 ± 200 | 1,370 ± 440 | 1,300 ± 330 |
Uninoculated HSEs served as controls. Data are from one representative experiment of several replicates and are presented as mean ± SEM. N.D., None detected.
Indicates significant (P < 0.05) difference from control.
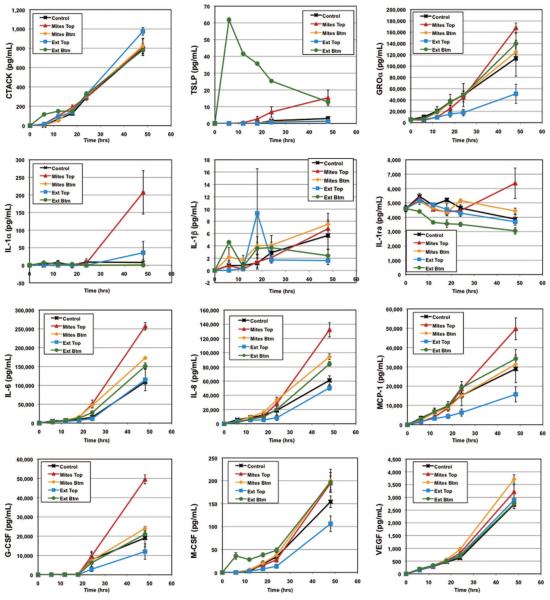
Fig. 1.
Cytokine secretion over time into the well below HSE inoculated with live S. scabiei mites on the HSE surface (Mites Top), mites in the lower well (Mites Btm), S. scabiei extract on the surface (Ext Top), or extract in the lower well (Ext Btm). Uninoculated HSEs served as controls. Data are from one representative experiment of several replicates and are presented as mean ± SEM. (Online figure in color.)
Thymic Stromal Lymphopoietin (TSLP)
Live mites burrowing into the surface of the HSEs induced TSLP secretion onto the skin surface and into the culture medium (Table 1; Fig. 1). Scabies extract inoculated into the medium induced a substantial secretion of TSLP into the culture medium at 6 h, but the concentration waned with time (Fig. 1).
Growth-Related Oncogene α (GROα/CXC Chemokine Ligand 1)
HSEs constitutively secreted GROα (Table 1; Fig. 1). Mites inoculated onto the HSE surface induced the most GROα secretion both onto the HSE surface (Table 1) and into the culture medium (Fig. 1) compared with the other stimuli. Scabies extract inoculated onto the HSE surface also induced a 1.6-fold increase in GROα secretion onto the HSE surface (Table 1), but it down-regulated the constitutive secretion of GROα into the well by >50% (Fig. 1).
Transforming Growth Factor α (TGFα)
There was no constitutive secretion of TGFα. None of the stimuli induced TGFα secretion onto the HSE surface (Table 1), but small amounts of TGFα were detected in the wells at 48 h in response to the burrowing of live mites into the HSE surface (data not shown).
Interleukin-1α (IL-1α), IL-1β, and IL-1 Receptor Antagonist (IL-1ra)
Mites burrowing into the epidermis on the surface of HSEs induced secretion of significantly increased amounts of IL-1α, IL-1β, and IL-1ra onto the skin-equivalent surface (Table 1). The burrowing of mites into the HSE surface also induced secretion of significant amounts of IL-1α into the culture medium (Fig. 1).
IL-6 and IL-8 (CXC Chemokine Ligand 8)
Live mites burrowing into the surface also induced the secretion of significantly increased levels of IL-6 and IL-8 onto the surface of HSEs (Table 1) and into the culture medium (Fig. 1). Live mites or scabies extract present in the culture medium in the wells below the HSEs also induced significantly increased secretion of IL-6 and IL-8 into the medium (Fig. 1). Scabies extract applied either onto the surface of the HSE or in the culture medium induced a 2- to 3-fold increase in IL-6 and IL-8 secretion onto the HSE surface (Table 1).
Monocyte Chemoattractant Protein-1 (MCP-1/CCL2)
This chemokine was constitutively produced by HSEs (Table 1; Fig. 1). Surface secretion of MCP-1 was significantly increased by burrowing live mites (Table 1), whereas no other treatment significantly altered MCP-1 secretion either onto the HSE surface (Table 1) or into the culture medium in the lower well (Fig. 1).
Granulocyte and Granulocyte/Macrophage Colony-Stimulating Factors (G-CSF and GM-CSF)
These growth factors were constitutively secreted by HSEs (Table 1; Fig. 1). By 48 h, the level in medium of mite-infested HSEs was significantly higher than all other levels that were essentially identical (Fig. 1). Mite-infested HSEs also secreted increased levels of GM-CSF and G-CSF onto their surfaces (Table 1).
Macrophage Colony-Stimulating Factor (M-CSF)
Mites burrowing into the HSE induced the secretion of large amounts of M-CSF onto the surface, and these levels far exceeded the amounts produced in response to any of the other test conditions (Table 1).
Vascular Endothelial Growth Factor (VEGF)
HSEs constitutively secreted VEGF. At 48 h, only those HSEs exposed to mites in the well secreted significantly more VEGF into the culture medium than the controls (Fig. 1), although most other stimuli also increased VEGF secretion on the surface (Table 1) and into the culture medium (Fig. 1).
IL-3 and Thymus- and Activation-Regulated Cytokine (TARC/CCL17)
In a preliminary experiment using a different set of HSEs, we assayed the medium for IL-3 and TARC secretion. Neither IL-3 nor TARC was detected at any time in response to any stimulus (Table 2).
Table 2. Immune modulation activities of Sarcoptes scabiei on normal human skin keratinocytes and fibroblasts in monocultures and skin equivalents.
| Our previous studiesa-e |
This studyf |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell type: | Keratinocytes (monoculture) |
Fibroblasts (monoculture) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
Skin equivalents (keratinocytes + fibroblasts) |
| Inoculum: |
S. scabiei extract (in media) |
S. scabiei extract (in media) |
S. scabiei mites (on surface) |
S. scabiei mites (on surface) |
S. scabiei mites (in media) |
S. scabiei extract (on surface) |
S. scabiei extract (in media) |
S. scabiei mites (on surface) |
S. scabiei mites (in media) |
S. scabiei extract (on surface) |
S. scabiei extract (in media) |
| Collection site: |
Media in well |
Media in well |
Media in well |
Media in well |
Media in well |
Media in well |
Media in well |
Surface wash |
Surface wash |
Surface wash |
Surface wash |
| CTACK | >a | 0a | x | > | = | = | = | > | = | = | = |
| TSLP | >a | 0a | x | > | = | > | = | > | = | > | = |
| GROα | >b | x | x | = | = | = | = | = | = | = | = |
| TGFα | >b | x | x | = | = | = | = | x | x | x | x |
| IL-1α | =c | 0c | >e | > | = | > | = | > | = | > | = |
| IL-1β | >c | 0c | >e | > | = | = | = | > | = | = | = |
| IL-1ra | <c | 0c | x | > | = | = | = | > | = | = | = |
| IL-3 | >d | x | x | = | = | = | = | x | x | x | x |
| IL-6 | >c | ⪢ c | x | > | = | = | = | ⪢ | = | = | = |
| IL-7 | 0d | x | x | x | x | x | x | x | x | x | x |
| IL-8 | <c | ⪢ c | x | > | = | = | = | ⪢ | = | = | = |
| IL-10 | >d | x | x | x | x | x | x | x | x | x | x |
| IL-12p7 | 0d | x | x | x | x | x | x | x | x | x | x |
| IL-15 | 0d | x | x | x | x | x | x | x | x | x | x |
| MCP-1 | >d | ⪢ d | x | > | = | = | = | > | = | = | = |
| G-CSF | >c | ⪢ c | x | = | = | = | = | = | = | = | = |
| GM-CSF | >d | 0b | x | > | = | = | = | > | = | = | = |
| M-CSF | x | >d | x | > | = | = | = | > | = | = | = |
| VEGF | >c | >c | x | = | = | = | = | = | = | = | = |
| Eotaxin | 0c | 0c | x | x | x | x | x | x | x | x | x |
| SCF | 0c | 0c | x | x | x | x | x | x | x | x | x |
| TARC | >a | 0a | x | = | = | = | = | x | x | x | x |
| TNFα | 0c | 0c | x | x | x | x | x | x | x | x | x |
Data are compared with paired constitutive controls and are scored as equivalent to (=), down-modulated (<), or up-modulated by a moderate (>), or large (>>) amount. Some cytokines were not detected (0), whereas others were not measured (x). SCF, stem cell factor.
Data taken from Mullins et al. (2009) at 48 h.
Data taken from Mullins et al. (2009) at 24 h.
Data taken from Arlian et al. (2003) at 24 h.
Data taken from L.G.A., Peterson, and M.S.M. (unpublished) at 24 h.
Data taken from Arlian et al. (1996b) at 16 h.
Discussion
Chemical and physical cell-cell interactions in an extracellular matrix framework and between the cells and the matrix are important aspects that determine how cells respond to stimuli in vivo in the skin. Previous studies showed that monocultured keratinocytes and fibroblasts increase constitutive levels of secretion or decrease proinflammatory-induced secretion levels of specific cytokines and chemokines in response to unidentified molecules in S. scabiei extracts (Table 2). A limitation of the monoculture system is that there is an absence of direct communication between the keratinocytes and fibroblasts, and between these cells and the matrix, and with other cells that also may be present in the skin (e.g., Langerhans cells, other dendritic cells, leukocytes). Thus, one cell type cannot influence the behavior of another in response to scabies molecules because the cells lack intercellular interactions and associations with the collagen matrix of the normal skin. We have attempted to model the in vivo situation to some extent by costimulation of these cells with scabies extract and proinflammatory cytokines from other cells that are most likely present in the scabietic lesion (Elder et al. 2009, Mullins et al. 2009).
In this study, we used HSEs to more fully investigate cellular interactions that occur between keratinocytes and fibroblasts when the cells are exposed to infesting mites, mite products, and mite extracts. Keratinocytes and fibroblasts are the two primary structural cells of the skin. One shortcoming of this model system is that the cytokine secretions measured represent the total contribution of the two interacting cells, and thus, the contribution of each cell type alone cannot be identified.
Our previous studies found that normal human keratinocytes up-regulated secretion of IL-6, VEGF, GROα, TGFα, G-CSF, and CTACK, and down-regulated constitutive and IL-1α, IL-1β, tumor necrosis factor-α (TNFα) plus IL-17, and lipopolysaccharide-induced secretion of IL-8, and the constitutive secretion of IL-1ra when cultured in the presence of S. scabiei extract (Table 2; Arlian et al. 2003, Mullins et al. 2009). Scabies extract did not appreciably stimulate secretion of IL-1α or IL-1β by keratinocytes, but it did reduce their constitutive secretion of IL-1ra (Arlian et al. 2003). Likewise, normal human fibroblasts cultured in the presence of S. scabiei extract up-regulated secretion of IL-6, G-CSF, and VEGF, and down-regulated secretion of GM-CSF and IL-8 (after 8-h stimulation) that was induced by stimulation of the cells with IL-1α, IL-1β, and TNFα plus IL-17 (Mullins et al. 2009). In addition, IL-8 secretion induced by TNFα plus IL-17 and IL-1β was decreased after 24 h of stimulation (Mullins et al. 2009).
In this study, we found that active mites on the surface of the skin equivalents, composed of epidermal keratin-ocytes and dermal fibroblasts together, resulted in secretion of CTACK, TSLP, IL-1α, IL-1β, IL-1ra, IL-6, IL-8, MCP-1, GM-CSF, and M-CSF onto the surface of and, in most cases, into the culture medium in the well below the HSEs (Table 2). Most of these cytokines were measured for the first time in HSEs challenged with scabies mites or extracts. In contrast, mite body extract placed on the surface of the HSEs only induced up-regulated secretion of TSLP and IL-1α onto the surface and of CTACK into the wells. Therefore, the greatest secretion of cytokines by these HSEs was induced by the burrowing and secretions of live mites.
The notable difference between our HSE data and our previous monoculture experiments is that HSEs secreted IL-1α, IL-1β, and IL-1ra in response to live scabies mites on the surface of and burrowing into the HSE, whereas monocultured keratinocytes and fibroblasts secreted little or none of these in response to scabies extract (Table 2). Similarly, we had previously found that HSEs made from cells from two different human skin donors also produced IL-1α and IL-1β in response to live scabies mites burrowing on the surface (Table 2; Arlian et al. 1996b). That study only measured IL-1α and IL-1β secretion into the medium, whereas in the current study 14 additional cytokines were monitored. Most importantly, IL-1ra was not measured, nor were surface samples collected during that study. These contrasting results between monocultures and HSEs clearly illustrate the importance of cell interactions and the matrix in understanding the influence of a parasite on the host’s inflammatory and immune responses to the parasite. However, in the HSE containing both cell types together and a matrix, live scabies mites induced IL-1α and IL-1β secretion that was most likely from keratinocytes based on our previous research (Arlian et al. 2003).
The cytokines IL-1α and IL-1β in vivo would normally activate cells with IL-1 receptors and therefore promote inflammation. Cells with IL-1 receptors include vascular endothelial cells, T cells, B cells, natural killer cells, macrophages, and neutrophils. IL-1ra is a competitive inhibitor of IL-1. IL-1ra binds to the IL-1 receptor on the cell, and thus blocks IL-1 from binding to the receptor, and this prevents the initiation of the inflammatory reaction. Up-regulating the production of IL-1ra may be a key first step in the scabies mite’s ability to delay the inflammatory reaction that is typically observed during an early scabies infestation. However, it is not clear how the balance between the increased secretion of the proinflammatory IL-1 cytokines and their inhibitor IL-1ra along with the influence of scabies mites and their products on the secretion of other cytokines from these cells and the secretion of cytokines and expression of cellular adhesion molecules from endothelial cells have on the overall response. Because of their proximity to the mite in the skin, keratinocytes and fibroblasts are probably the early responders and play an important part in the host response to scabies mites in the skin. However, scabies extract also down-regulates the expression of the cell adhesion molecules vascular cell adhesion molecule-1 and E-selectin and the secretion of IL-8 by vascular endothelial cells (Elder et al. 2006), and this, together with the increased secretion of IL-1ra by skin cells, may down-regulate the inflammatory response to this parasite.
The scabies molecules that are able to modulate the function of human skin cells and their source are still unknown. The stimulatory molecules that induced the cell responses we observed for the HSEs could have come from mite salivary secretions, fecal material, physical activity of the mite (leg and mouth part digging/crawling motion), molecules released by decomposing mite bodies, and molting secretions released during development. Modulation of the host defenses in blood-feeding insects and mites that feed from the surface of the skin is common and usually associated with pharmacological properties of the saliva (Wikel et al. 1996). Scabies mites are different from these arthropod ectoparasites because they actually burrow into the epidermis to feed and reproduce. However, it is reasonable to assume that selection would have favored a similar adaptation in these mites. Our results suggest that the most likely source of the molecules that modulate fibroblast and keratinocyte function is the salivary secretions or physical activity because the experiments were short-term (48 h) and did not allow sufficient time for significant molting or decomposition of mites that died. One would not expect down-regulation from just physical activity. Evidence for this is that extracts placed on the surface of the HSE, in most cases, did not induce up-regulation of most of the cytokines that were induced by active mites on the surface. Similarly, lack of increased secretion of all cytokines was observed when the extract was placed in the medium that bathed the underside of the HSE. This suggests that salivary secretions or the digging (leg trashing and mouth part movement) that damages the epidermis was primarily responsible for the response.
It appears that early in an infestation, molecules in mite salivary secretions contribute most to modulation of the local keratinocyte and fibroblast responses in the skin. There are generally few mites present early in an infestation. As the population grows, mite numbers increase and mites begin to die as their life expectance (longevity) is reached or the host defenses kill them. The mite breakdown in the burrow would release many different mite molecules compared with that secreted by live mites. This mite breakdown material is more analogous to the composition of the extract, which contains all soluble body material that was used to stimulate cells in culture. However, the mite body material also contains antigenic molecules that trigger the progressive inflammatory and immune reactions. Evidence for this is that hosts treated for scabies begin to develop strong antibody titers after acaricide treatment when mites die and decompose in the skin. As the amount of antigenic material increases over time, the antigenic stimulus over-whelms the inhibitory influence. In human and animal scabies, significant inflammation in the skin is delayed for weeks and the early scabies-specific antibody titer is low (Arlian et al. 1994, 1995, 1996a; Morgan et al. 1997; Arlian and Morgan 2000).
This research identifies key aspects of understanding the host-parasite interaction in the skin and the delay in clinical manifestation that is characteristic of an early primary scabies infestation. There is increasing interest in developing drugs based on immune modulation to treat many diseases. This research provides some data that are a prerequisite to the possible development of novel approaches to preventing or treating scabies.
Acknowledgments
This work was supported by a grant to L.G.A. from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI-017252).
References Cited
- Arlian LG, Morgan MS. Serum antibody to Sarcoptes scabiei and house dust mite prior to and during infestation with S. scabiei. Vet. Parasitol. 2000;90:315–326. doi: 10.1016/s0304-4017(00)00251-x. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Runyan RA, Achar S, Estes SA. Survival and infectivity of Sarcoptes scabiei var. canis and var. hominis. J. Am. Acad. Dermatol. 1984;11:210–215. doi: 10.1016/s0190-9622(84)70151-4. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Vyszenski-Moher DL, Stemmer BL. Sarcoptes scabiei: the circulating antibody response and induced immunity to scabies. Exp. Parasitol. 1994;78:37–50. doi: 10.1006/expr.1994.1004. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Rapp CM, Morgan MS. Resistance and immune response in scabies-infested hosts immunized with Dermatophagoides mites. Am. J. Trop. Med. Hyg. 1995;52:539–545. doi: 10.4269/ajtmh.1995.52.539. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Rapp CM, Vyszenski-Moher DL. The development of protective immunity in canine scabies. Vet. Parasitol. 1996a;62:133–142. doi: 10.1016/0304-4017(95)00854-3. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Vyszenski-Moher DL, Rapp CM, Hull BE. Production of IL-1α and IL-1β by human skin equivalents parasitized by Sarcoptes scabiei. J. Parasitol. 1996b;82:719–723. [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Neal JS. Modulation of cytokine expression in human keratinocytes and fibroblasts by extracts of scabies mites. Am. J. Trop. Med. Hyg. 2003;69:652–656. [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Neal JS. Extracts of scabies mites (Sarcoptidae: Sarcoptes scabiei) modulate cytokine expression by human peripheral blood mononuclear cells and dendritic cells. J. Med. Entomol. 2004;41:69–73. doi: 10.1603/0022-2585-41.1.69. [DOI] [PubMed] [Google Scholar]
- Arlian LG, Morgan MS, Paul CC. Evidence that scabies mites (Acari: Sarcoptidae) influence production of interleukin-10 and the function of T-regulatory cells (Tr1) in humans. J. Med. Entomol. 2006;43:283–287. doi: 10.1603/0022-2585(2006)043[0283:etsmas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bergstrom FC, Reynolds S, Johnstone M, Pike RN, Buckle AM, Kemp DJ, Fischer K, Blom AM. Scabies mite inactivated serine protease paralogs inhibit the human complement system. J. Immunol. 2009;182:7809–7817. doi: 10.4049/jimmunol.0804205. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Daix V, Schroeder H, Praet N, Georgin JP, Chiappino I, Gillet L, de Fays K, Decrem Y, Leboulle G, Godfroid E, Bollen A, Pastoret PP, Gern L, Sharp PM, Vanderplasschen A. Ixodes ticks belonging to the Ixodes ricinus complex encode a family of anticomplement proteins. Insect Mol. Biol. 2007;16:155–166. doi: 10.1111/j.1365-2583.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- Diaz A, Ferreira A, Sim RB. Complement evasion by Echinococcus granulosus: sequestration of host factor H in the hydatid cyst wall. J. Immunol. 1997;158:3779–3786. [PubMed] [Google Scholar]
- Elder BL, Arlian LG, Morgan MS. Sarcoptes scabiei (Acari: Sarcoptidae) mite extract modulates expression of cytokines and adhesion molecules by human dermal microvascular endothelial cells. J. Med. Entomol. 2006;43:910–915. doi: 10.1603/0022-2585(2006)43[910:ssasme]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder BL, Arlian LG, Morgan MS. Modulation of human dermal microvascular endothelial cells by Sarcoptes scabiei in combination with proinflammatory cytokines, histamine, and lipid-derived biologic mediators. Cytokine. 2009;47:103–111. doi: 10.1016/j.cyto.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn NJ, Williams AS, Nunn MA, Chamberlain-Banoub JC, Hamer J, Morgan BP, Harris CL. In vivo characterization and therapeutic efficacy of a C5-specific inhibitor from the soft tick Ornithodoros moubata. J. Biol. Chem. 2007;282:8292–8299. doi: 10.1074/jbc.M609858200. [DOI] [PubMed] [Google Scholar]
- Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J. Allergy Clin. Immunol. 2006;118:178–189. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- Maxwell SS, Stoklasek TA, Dash Y, Macaluso KR, Wikel SK. Tick modulation of the in-vitro expression of adhesion molecules by skin-derived endothelial cells. Ann. Trop. Med. Parasitol. 2005;99:661–672. doi: 10.1179/136485905X51490. [DOI] [PubMed] [Google Scholar]
- Morgan MS, Arlian LG, Estes SA. Skin test and radioallergosorbent test characteristics of scabietic patients. Am. J. Trop. Med. Hyg. 1997;57:190–196. doi: 10.4269/ajtmh.1997.57.190. [DOI] [PubMed] [Google Scholar]
- Mullins JS, Arlian LG, Morgan MS. Extracts of Sarcoptes scabiei De Geer downmodulate secretion of IL-8 by skin keratinocytes and fibroblasts and of GM-CSF by fibroblasts in the presence of proinflammatory cytokines. J. Med. Entomol. 2009;46:845–851. doi: 10.1603/033.046.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn MA, Sharma A, Paesen GC, Adamson S, Lissina O, Willis AC, Nuttall PA. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata. J. Immunol. 2005;174:2084–2091. doi: 10.4049/jimmunol.174.4.2084. [DOI] [PubMed] [Google Scholar]
- Ramachandra RN, Wikel SK. Modulation of host-immune responses by ticks (Acari: Ixodidae): effect of salivary gland extracts on host macrophages and lymphocyte cytokine production. J. Med. Entomol. 1992;29:818–826. doi: 10.1093/jmedent/29.5.818. [DOI] [PubMed] [Google Scholar]
- Schroeder H, Daix V, Gillet L, Renauld JC, Vanderplasschen A. The paralogous salivary anticomplement proteins IRAC I and IRAC II encoded by Ixodes ricinus ticks have broad and complementary inhibitory activities against the complement of different host species. Microbes Infect. 2007;9:247–250. doi: 10.1016/j.micinf.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Wikel SK. Tick modulation of host immunity: an important factor in pathogen transmission. Int. J. Parasitol. 1999;29:851–859. doi: 10.1016/s0020-7519(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Wikel SK, Ramachandra RN, Bergman DK. Arthropod modulation of host immune responses. In: Wikel SK, editor. The Immunology of Host-Ectoparasitic Arthropod Relationships. CAB International; Wallingford, United Kingdom: 1996. pp. 107–130. [Google Scholar]
- Zipfel PF, Wurzner R, Skerka C. Complement evasion of pathogens: common strategies are shared by diverse organisms. Mol. Immunol. 2007;44:3850–3857. doi: 10.1016/j.molimm.2007.06.149. [DOI] [PubMed] [Google Scholar]