Since the earliest attempts at human liver transplantation,1 research in experimental animals has had an important and intimate relationship with clinical practice.2–4 Evidence obtained in animals has been transferred to the clinics and problems encountered in the patients have been taken back to animals for clarification.5 In this review we describe examples of this flux, as well as the development of operative techniques and the significance of team construction in the laboratory as a preparatory step for clinical practice.
KINDS OF LIVER TRANSPLANTATION
There are two general approaches to transplantation of the liver. With one method, an extra (auxiliary) liver is inserted at an ectopic site, without removal of the host liver. The other is to transplant the homograft orthotopically after total removal of the native liver.
Auxiliary Liver Transplantation
The first experiments with liver transplantation were performed by Welch,2 who placed the extra canine livers in the right paravertebral gutter or pelvis (Fig. 1). The hepatic arterial supply was derived from the aorta or iliac artery. The portal inflow was via the distal iliac vein or inferior vena cava and the outflow was into the proximal iliac vein or vena cava. The gallbladder was anastomosed to the duodenum for biliary drainage. The livers produced bile for several days and then ceased to function.
FIG. 1. Auxiliary liver transplantation in dogs by a modification of Welch’s original technique.
Note that the reconstituted portal blood supply is from the distal inferior vena cava. Cholecystoduodenostomy is performed. (Reproduced with permission from Starzl et al.8)
Auxiliary liver transplantation was envisioned by Welch as a therapeutic possibility for patients with liver cirrhosis or non-neoplastic hepatic diseases. Many clinical trials with this procedure have been recorded. All such attempts failed except for two successful cases reported from New York6 and Paris.7 The results obtained in animals were inferior to those with orthotopic allografts, partly because the extra graft atrophied rapidly.8,9 One hypothesis was that the atrophy of the liver was the result of an inadequate portal flow,10,11 but experiments from our laboratory first suggested8,9 and then proved12 that the liver atrophied from interliver competition in which the extra liver failed to receive some metabolite or other substance in the portal blood.
Such atrophy could be prevented by diverting the nonhepatic splanchnic venous blood away from the host liver and through the graft.9,12 Later, endogenous insulin as well as other undefined multiple hepatotrophic factors found in splanchnic venous blood were found to play a central role in the maintenance of liver cell integrity.13–16 Thus, auxiliary liver transplantation has lost much favor in actual practice. Nevertheless, auxiliary transplantation has been a valuable tool for the investigation of hepatotrophic physiology.17
Orthotopic Liver Transplantation
The first experimental orthotopic transplantation was mentioned by Cannon18 in 1956, but with so little information that his publication is rarely cited. Two separate research programs were established in 1958, one in Boston3 and the other in Chicago,4 with a maximum survival in untreated dogs of 12 and 20.5 days, respectively. The operative technique that is now employed for dogs in our laboratory is essentially the same as described before,4 but several significant modifications have been made.19 The techniques of clinical liver transplantation also are modifications of this original laboratory procedure.20,21
ANESTHESIA FOR ORTHOTOPIC TRANSPLANTATION IN DOGS
Animals are fasted from the evening before the operation and the weights of the donor and recipient are closely matched. Pairs weighing as little as 7 kg and as much as 40 kg have been used. Under intravenous induction with 25 to 30 mg/kg thiopental sodium, a cuffed endotracheal tube is inserted into the trachea and the dogs are placed on respirators. In recipient animals ventilation is with an air-oxygen mixture (FIO2 of 0.3), and 5 cm H2O of positive expiratory pressure is applied to keep the arterial carbon dioxide tension at 30 to 35 mmHg. Maintenance of anesthesia is by the intravenous injection of 2 mg/kg ketamine every 20 to 30 minutes, and 0.5 mg pancuronium. No ketamine is given after revascularization of the graft. The arterial pressure and central venous pressure are monitored. Approximately, 2 to 3 liters of electrolyte or plasma solution plus 2 units of blood usually are given intraoperatively. Low-dose dopamine is given during and after the bypass period. Calcium chloride and sodium bicarbonate are given when necessary to correct abnormalities of ionized calcium and acid-base balance, especially just after the revascularization and unclamping of the abdominal aorta. Frequent measurements of blood gases and electrolytes are useful. External heating with blankets or lamps is applied because the body temperature usually decreases to 32 to 33°C by the end of the operation. In canine liver transplantation, perfect anesthesia is one of the most important factors if the operation is to succeed.
SURGICAL TECHNIQUE
Donor Operation
Our original technique is still used.4 The abdominal cavity is entered through a midline incision. The dissection starts from the abdominal aorta above the iliac bifurcation and advances upward by ligating and dividing the right and left renal arteries, small tributaries, and lumbar arteries. When the crura of the diaphragm are reached and divided, the celiac axis and superior mesenteric artery are easily isolated. After dividing the left gastric and splenic arteries, the common hepatic artery is skeletonized distally into the lesser sac.
Then, the duodenum and stomach are retracted downward to expose the hepatic hilum. The fundus of the gallbladder is incized and irrigated with normal saline to avoid autolysis by bile during the ischemic period. The common bile duct is ligated and divided below the entrance of the cystic and lowest hepatic ducts. The gastroduodenal artery is divided near the duodenum. The portal vein is cleaned off down to the confluence of the superior mesenteric vein and the splenic vein. A cannula is inserted into the latter for perfusion of cold fluids.
The gastrohepatic, falciform, and left and right triangular and coronary ligaments are divided. The connective tissue attaching the retrohepatic vena cava is bluntly dissected by lifting the caudate lobe gently. The liver is then retracted to the left and after dividing the right triangular ligament, the suprahepatic vena cava is dissected free circumferentially. When the infrahepatic vena cava above the entrance of the adrenal veins is encircled, the donor liver becomes completely isolated.
The distal aorta is then cannulated to collect 2 units of blood for transfusion. After ligating the superior mesenteric artery and vein, perfusion and cooling of the liver is commenced through the cannula in the splenic vein with 2 liters of cold lactated Ringer’s solution at the same time as the dog is exsanguinated into the blood collection bags. The liver is then removed by transecting the portal vein, the upper abdominal aorta, and the vena cava above and below the liver, leaving enough vessel lengths for anastomoses (Fig. 2).
FIG. 2. Orthotopic liver transplantation in dogs.
(Reproduced with permission from Kam et al.19)
Veno-Venous Bypasses in the Dog
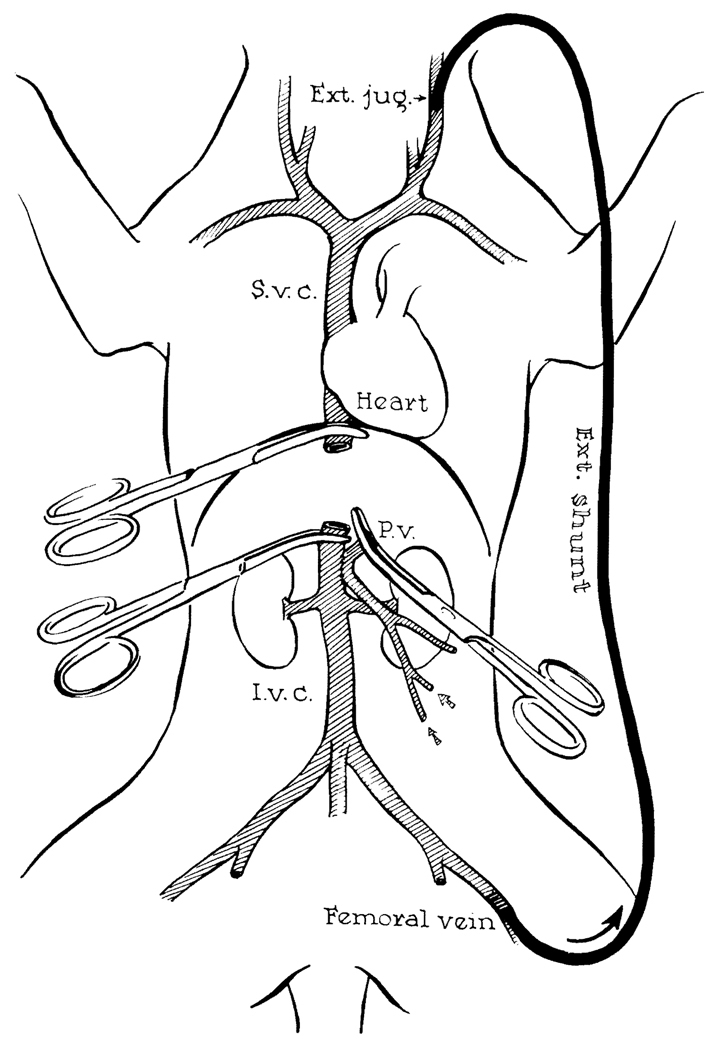
When the technique of liver transplantation was developed in dogs,3,4 operative survival required veno-venous bypasses that transmitted blood from the inferior vena cava and the portal vein to the upper part of the body while the venous systems were obstructed during the anhepatic phase of the procedure. Without bypasses, the capillary beds were ruined in dogs by acute venous hypertension, even with occlusion times as short as 30 minutes; the animals died of immediate or delayed irreversible shock. The original bypasses were used without heparinization or pumps (Fig. 3). A single bypass was used with our original technique,4 since a temporary portacaval shunt was constructed to connect the splanchnic and vena caval systems (Fig. 3).
FIG. 3. Method used in dogs for decompression of the inferior vena caval and splanchnic systems during removal of recipient liver and replacement with a homograft.
Note that a preliminary portacaval shunt has been placed. By means of this temporary anastomosis, the two venous systems are connected, allowing their decompression with a single external bypass. (Reproduced with permission from Starzl et al.4)
In 1982 and 1983, a pump-driven veno-venous bypass system without recipient heparinization was developed, tested in dogs,22 and eventually brought to the clinical operating room.23,24 Then, it became possible to modify and improve several aspects of the human recipient operation, including the technique of hepatectomy.25 The bypass system has revolutionized clinical liver transplantation, and it has opened up new horizons of potential research in dogs and other animals. Thus, a description of the techriique as used in dogs is in order.
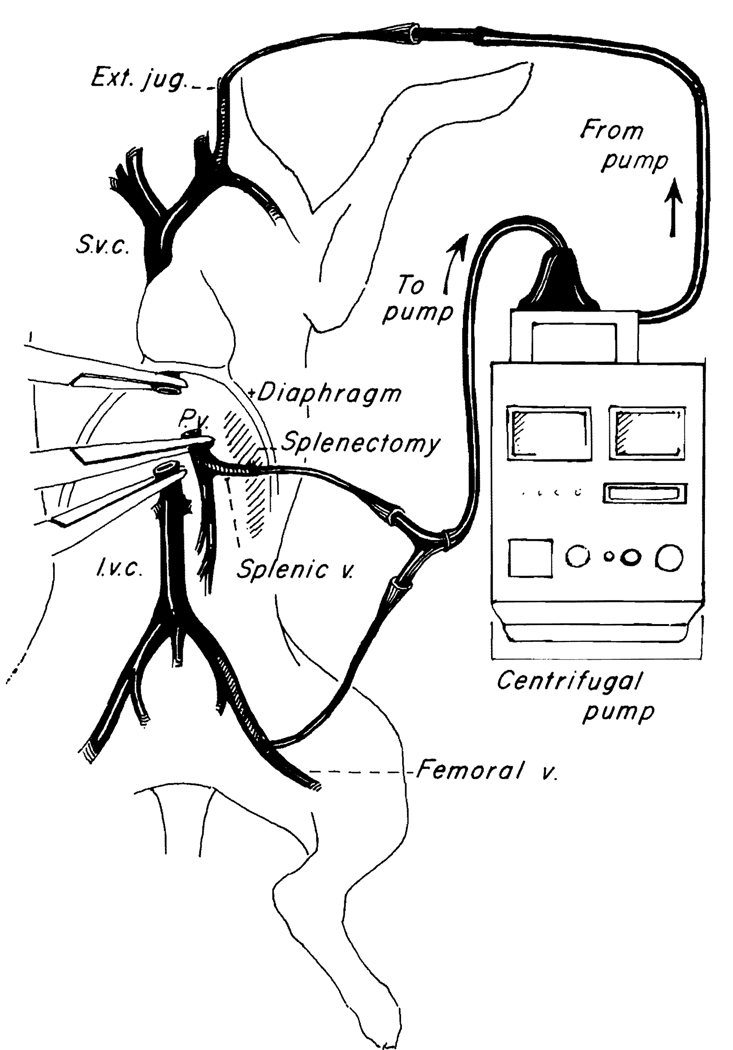
The technique is that of Denmark et al,22 as modified by Kam et al.19 The inferior vena caval system is drained via the proximal femoral vein after ligation of the distal femoral vein. Splenectomy is performed and the central splenic vein is cannulated to drain the splanchnic bed (Fig. 4). Venous reentry is via the external jugular vein (Fig. 4). All of the cannulas are standard no. 12–16 chest tubes (Argyle Division of Sherwood Medical, St. Louis, MO).
FIG. 4. Pump-driven veno-venous bypass used in dogs for decompression of inferior vena caval and splanchnic systems during anhepatic phase.
(Reproduced with permission from Kam et al.19)
The extracorporeal bypass includes a 3/8 inch (internal diameter) Tygon (Norton Industrial Plastics, Akron, OH) tubing interrupted with a centrifugal pump (Bio-Medicus, Minnetonka, MN), and primed with 250 ml Plasmalyte (Fig. 4). An electromagnetic probe on the venous return side is used to measure flow.
The venous bypass time is that required to complete the recipient hepectectomy, obtain hemostasis in the hepatic fossa, and perform the venous anastomoses. Usually, the suprahepatic caval, infrahepatic caval, and portal anastomoses are carried out while on bypass before revascularizing the portal vein (Fig. 2). In a few animals, the portal vein is revascularized after only the suprahepatic caval and portal anastomoses. This modification reduces the cold ischemia time, but it necessitates a period of low veno-venous bypass flow, since only the vena caval bed is being drained while the third anastomosis is performed. The aortic anastomosis is carried out after the bypass is terminated, after all cannulas had been removed, and not until good hemostasis has been obtained (Fig. 2).
In 40 dogs, the flow range during the bypass was 200 to 1500 ml/minute and the bypass time was 53.1 ± 12.9 (SD) minutes, ranging from 34 to 82 minutes. Two dogs died suddenly of pulmonary emboli and two others had fibrin formation in the system that caused no complications. Otherwise the bypass technique was trouble-free.
Recipient Operation
The recipient operation is performed through a midline incision. Before starting dissection of the liver, the abdominal aorta below the left renal vein is cleaned for the anastomosis. Attention is taken not to injure the cisterna chyli that runs behind the abdominal aorta. The dissection of the host liver is similar to the donor operation, the difference being that ligation and division of the hepatic arteries and biliary ducts is at a high level in the liver hilum. Having completed the dissection of the recipient liver, the animal is placed on the veno-venous bypass, as just described. The portal vein, the infrahepatic vena cava above the right adrenal vein, and the suprahepatic vena cava are cross-clamped. When the suprahepatic vena cava, the portal vein, and the infrahepatic vena cava are cut, the host liver can be removed.
The vascular anastomoses of the graft start with the suprahepatic vena cava, using an everted running suture with 5-0 polypropylene. The infrahepatic vena cava and portal vein anastomoses are done with running 6-0 polypropylene, usually in this order as described in the preceding section. After the veno-venous bypass is discontinued and bleeding from the anastomoses is completely controlled, hepatic arterial flow is reconstructed with an end-to-side aorta-to-aorta anastomosis with running 6-0 polypropylene suture (Fig. 2). The air inside of the aorta is flushed with blood and the proximal opening of the donor aorta is doubly ligated. Biliary reconstruction is with cholecystoduodenostomy (Fig. 2) with an inner layer of 4-0 polyglycolic acid suture and an external layer of silk. After controlling the bleeding, the abdominal wall is closed in two layers and the skin is approximated with a running subcutaneous suture with 2-0 polyglycolic acid.
Using a two-team approach, the donor operation usually takes 1½ hours and the recipient hepatectomy requires about 1 hour. The vascular anastomoses require another 1 to 1½ hours. Thus, the total recipient operation time is about 4 hours. In the first consecutive 30 transplantations using this method, there was a 24-hour survival of 73%, and a 5-day survival of 60%.
PROBLEMS OF LIVER TRANSPLANTATION SUSCEPTIBLE TO ANIMAL RESEARCH
Preservation
The liver is extremely sensitive to ischemia and livers removed at normal temperature become unsuitable for transplantation within 20 to 30 minutes.3,4 The first attempt to extend this permissible ischemia time by core cooling of the graft was achieved with preliminary total body hypothermia of the donor (to 30°C) followed by perfusion of the excised liver with a chilled lactated Ringer’s solution.4 Those organs could support the life of recipients dogs if revascularized as orthotopic homografts within 2 hours. Because of the extreme time limitation, further efforts to cool the entire or the lower half of the body with an extracorporeal heart-lung apparatus were attempted for 2 to 8 hours in dogs.26 All animals died within 5 days after transplantation. Subsequently, a combination of hyperbaric oxygenation and hypothermic perfusion was used.27,28 The livers thereby preserved (Fig. 5) for 8 to 12 hours always provided life-sustaining function after transplantation. Three of five recipients of livers preserved for 24 hours survived more than 8 days until they died of rejection. Although successful, the technique was too complex to be widely applicable.
FIG. 5. Preservation unit.
The perfusion pumps are located outside the hyperbaric chamber; the organ receptacle, the oxygenator, and the venous reservoir are inside. The various chamber inlets permit sampling of the perfusate, gas sterilization, and oxygen delivery and removal. The temperature is electronically controlled. (Reproduced with permission from Brettschneider et al.27)
In 1976, core cooling with Collins’ solution, which has a composition similar to intracellular fluid, was proved to allow safe preservation of the liver for up to 18 hours in dogs and 10 hours in humans.29 This method is now applied in all of the clinical cases and allows multiple organ procurement30 and the shipment of organs from city to city and between countries. Wall et al31 have been able to do the same thing with plasmalike fluids. Nevertheless, at the present time, safer and longer preservation methods that are based on the response of liver cells to cold storage and reperfusion are under investigation. Such efforts may bring further insight into mechanisms involved in liver preservation and pathophysiologic changes of the liver after transplantation.
Immunosuppression
Without immunosuppression, dogs receiving liver allografts inevitably die of rejection. In a recent study the mean survival time of such animals after both beagle-to-beagle and mongrel-to-beagle liver allografting was 11.8 ± 9.6 days.32 The longest survival was 35 days after a beagle-to-beagle transplantation. Survivals were somewhat longer than after grafting between outbred mongrel dogs.33–36
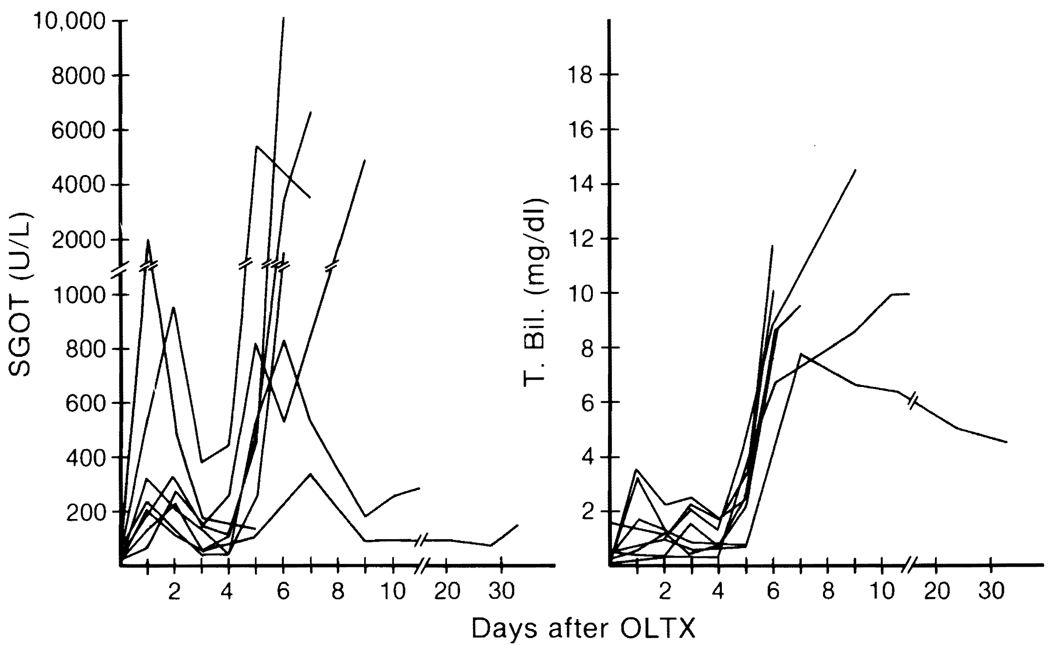
Figure 6 shows the changes of hepatic function of untreated animals in the beagle series,31 whose early postoperative course was smooth. Within 4 or 5 days, the dogs stopped eating and there was elevation of SGOT and bilirubin. Histopathology of rejecting livers has been well characterized.33 There is dense mononuclear cell infiltration, particularly around the smaller bile ducts and portal vein branches, with blast cells and mitoses. In the case of pigs, such evidence of rejection may not be prominent.33 Even without immunosuppression, two of nine animals in older experiments in our laboratory survived for more than 15 months.34
FIG. 6. Changes in SGOT and total bilirubin of untreated dogs after orthotopic liver transplantation (OLTX).
(Reproduced with permission from Todo et al.32)
The history of clinical liver transplantation has been that of multiple agent immunosuppressive regimens. These have been azathioprine and prednisone from 1963 to 1965, azathioprine, prednisone, and antilymphocyte globulin (ALG) between 1966 and 1979, thoracic duct drainage, azathioprine, and prednisone in 1978 and 1979, and finally cyclosporine (CS) and prednisone (with or without azathioprine and monoclonal ALG) after 1980.24,25 In animals, the most precise information about immunosuppression has been obtained from controlled experimentation in which the individual drugs were used alone.35,36
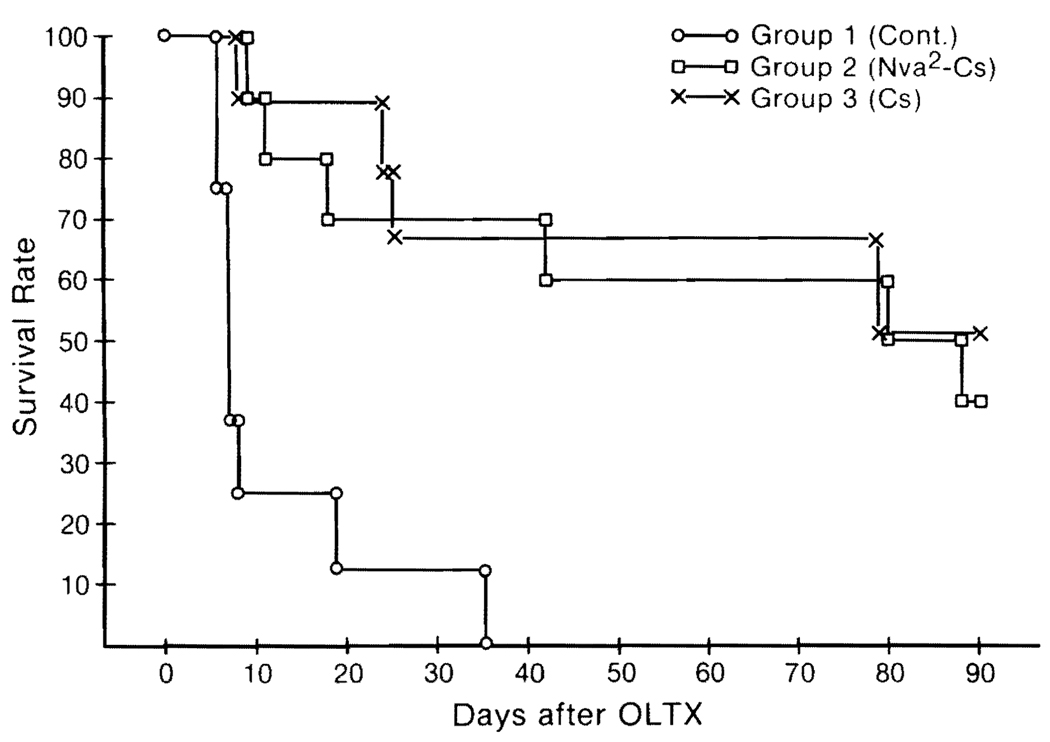
The advent of Cs was revolutionary in transplantation surgery.37,38 This drug, in combination with steroid,39 provides the highest survival rate of allografts that has ever been achieved, but the existence of nephrotoxicity and hepatotoxic side effects have been disadvantages.38,40,41 In 1984, a new Cs analogue, Nva2-Cs was shown to provide potent immunosuppression with little nephrotoxicity in rats.42 We have compared the features of the original Cs and the new analogue using 46 canine orthotopic liver transplantations.32 The mean survival time, with an arbitrary observation limit of 90 days, was 60.8 ± 34.4 with Nva2-Cs and 65.1 ± 33.9 days with Cs (Fig. 7). Thus, the immunosuppressive properties of both drugs are almost identical. Using the same oral dose, the absorption of Nva2-Cs was faster and more complete than Cs (Fig. 8). Functional abnormalities of liver and kidney were not noted in either group, but histopathologic studies showed similar changes in the straight part of the proximal renal tubules. Thus, the possibility of less nephrotoxicity of Nva2-Cs has not been proved.
FIG. 7. Changes in the survival rate of dogs among un-Nva2-Cs- and Cs-treated groups after orthotopic liver transplantation (OLTX).
(Reproduced with permission from Todo et al.32)
FIG. 8. Changes in the blood level of Nva2-Cs and Cs in dogs after orthotopic liver transplantation (OLTX).
(Reproduced with permission from Todo et al.32)
Team Construction
The application of developments in liver transplantation demands cooperation among specialists in different fields. The physical and social conditions of the recipient candidate are closely evaluated by surgeons, hepatologists, psychologists, social workers, and nurses. Putting together donor and recipient patient combinations is facilitated by coordinators. The surgery as carried out today requires separate donor and recipient teams, the activities of which must be closely knit. During operation, cooperation among surgeons, anesthesiologists, perfusionists, and nurses is essential. Even after the operation, the patient’s care in the intensive care unit, ward, and outpatient clinic needs more specialists.
Laboratory work with liver transplantation has the two objectives of performing research and of allowing the creation of harmonious teams which include surgeons, anesthesiologists, perfusionists, and scrub nurses. Both the donor team and the recipient team should have four surgeons, the team leader and three assistants. The roles of the surgeons and the steps in performing the operation are the same as for clinical transplantation. With experience in the laboratory, the team is more apt to perform perfectly in the human operating room. We wrote earlier “It is unlikely that anyone would wish to attempt clinical liver transplantation without first personally recapitulating in the laboratory at least some of the earlier experiments in dogs or alternatively in pigs.”43
CONCLUSION
The operative techniques and several problems of liver transplantation in the dog are described. The continuing laboratory activity is important not only for research, but also for the training of teams planning clinical programs.
Acknowledgments
Supported by Research Grants from the Veterans Administration and Project Grant No. AM-29961 from the National Institutes of Health, Bethesda, Maryland.
We sincerely appreciate the collaboration of Bo-Goren Ericzon, M.D., from Sweden, Frank Jakab, M.D., from Hungary, Shunichi Takaya, M.D., and Seiki Tashiro, M.D., from Japan, Benjamin Jeng, M.D., from the Republic of China, Andre DeWolf, M.D., from Belgium, and all of the other Fellows who have worked together in the laboratory.
REFERENCES
- 1.Starzl TE, Marchioro TL, von Kaulla KN, et al. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Welch CW. A note on the transplantation of the whole liver in dogs. Transplant Bull. 1955;2:54–55. [Google Scholar]
- 3.Moore FD, Wheeler HB, Demissianos HV, et al. Experimental whole-organ transplantation of the liver and of the spleen. ann Surg. 1960;152:374–387. [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Kaupp HA, Brock DR, et al. Reconstructive problems in canine liver homotransplantation with special reference to postoperative role of hepatic venous flow. Surg Gynecol Obstet. 1960;111:733–743. [PMC free article] [PubMed] [Google Scholar]
- 5.Waddell WR. Starzl TE (Ed) with the assistance of Putnam CS: Experience in Hepatic Transplantation. Philadelphia: W.B. Saunders; 1969. Foreward; p. ix. [Google Scholar]
- 6.Fortner JG, Kim DK, Shin MH, et al. Heterotopic (auxiliary) liver transplantation in man. Transplant Proc. 1977;9:217–221. [PubMed] [Google Scholar]
- 7.Houssin D, Franco D, Berthelot P, Bismuth H. Heterotopic liver transplantation in end-stage HBs Ag-positive cirrhosis. Lancet. 1980;1:990–992. doi: 10.1016/s0140-6736(80)91435-x. [DOI] [PubMed] [Google Scholar]
- 8.Starzl TE, Marchioro TL, Rowlands DT, Jr, et al. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann Surg. 1964;160:411–439. doi: 10.1097/00000658-196409000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halgrimson CG, Marchioro TL, Faris TD, et al. Auxiliary liver homotransplantation: Effect of host portacaval shunt. Arch Surg. 1966;93:107–118. doi: 10.1001/archsurg.1966.01330010109014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Child CG, Barr D, Holswade GR, Harrison CS. Liver regeneration following portacaval transposition in dogs. Ann Surg. 1953;138:600–608. doi: 10.1097/00000658-195310000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Russ C, Updegraff H, Fisher ER. Effect of increased hepatic blood flow upon liver regeneration. Arch Surg. 1954;69:263–272. doi: 10.1001/archsurg.1954.01270020129015. [DOI] [PubMed] [Google Scholar]
- 12.Marchioro TL, Porter KA, Dickinson TC, et al. Physiologic requirement for auxiliary liver homotransplantation. Surg Gynecol Obstet. 1965;121:17–31. [PMC free article] [PubMed] [Google Scholar]
- 13.Starzl TE, Francavilla A, Halgrimson CG, et al. The origin, hormonal nature, and action of hepatotrophic substances in portal blood. Surg Gynecol Obstet. 1975;137:179–199. [PMC free article] [PubMed] [Google Scholar]
- 14.Starzl TE, Porter KA, Kashiwagi N, Putnam CW. Portal hepatotrophic factors, diabetes mellitus and acute liver atrophy, hypertrophy and regeneration. Surg Gynecol Obstet. 1975;141:843–859. [PMC free article] [PubMed] [Google Scholar]
- 15.Starzl TE, Porter KA, Watanabe K, Putnam CW. The effects of insulin, glucagon, and insulin/glucagon infusions upon liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821–825. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 16.Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524–531. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Porter KA, Francavilla A. The Eck fistula in animals and humans. Curr Probl Surg. 1983;20:687–752. doi: 10.1016/s0011-3840(83)80010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannon JA. Transplant Bull. 1956;3:7. [Google Scholar]
- 19.Kam I, Lynch S, Todo S, et al. Low flow envo-venous bypass in small animals and pediatric patients undergoing liver replacement. Surg Gynecol Obstet. 1985 [PMC free article] [PubMed] [Google Scholar]
- 20.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Iwatsuki S, Shaw BW., Jr . Transplantation of the human liver. In: Schwarts SI, editor. Abdominal Operation, 8th ed. (Maingot) E. Norwalk, CT: Appleton-Century-Crofts; 1985. pp. 1687–1722. [Google Scholar]
- 22.Denmark SW, Shaw BW, Jr, Griffith BP, Starzl TE. Venous-venous bypass without systemic anticoagulation in canine and human liver transplantation. Surg Forum. 1983;34:380–382. [PMC free article] [PubMed] [Google Scholar]
- 23.Griffith BP, Shaw BW, Jr, Hardesty RL, et al. Veno-venous bypass without systemic anticoagulation for transplantation of the human liver. Surg Gynecol Obstet. 1985;160:270–272. [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw BW, Jr, Martin DJ, Marquez JM, et al. Venous bypass in clinical liver transplantation. Ann Surg. 1984;200:524–534. doi: 10.1097/00000658-198410000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Iwatsuki S, Shaw BW, Jr, et al. Factors in the development of liver transplantation. Transplant Proc. 1985 [PMC free article] [PubMed] [Google Scholar]
- 26.Marchioro TL, Huntley RY, Waddell WR, Starzl TE. The use of extracorporeal perfusion for obtaining postmortem grafts. Surgery. 1963;54:900–911. [PMC free article] [PubMed] [Google Scholar]
- 27.Brettschneider L, Daloze PM, Huguet C, et al. The use of combined preservation techniques for extended storage of orthotopic liver homografts. Surg Gynecol Obstet. 1968;126:263–274. [PMC free article] [PubMed] [Google Scholar]
- 28.Brettschneider L, Groth CG, Starzl TE. Experimental and clinical preservation of liver homografts. In: Norman J, editor. Organ Perfusion and Preservation. New York: Appleton-Century-Crofts; 1968. pp. 271–284. [Google Scholar]
- 29.Benichou J, Halgrimson CG, Weil R, III, et al. Canine and human liver preservation for 6–18 hours by cold infusion. Transplantation. 1977;24:407–411. doi: 10.1097/00007890-197712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Starzl TE, Hakala TR, Shaw BW, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 31.Wall WJ, Caine RY, Herbertson BM, et al. Simple hypothermic preservation for transporting human livers long distance for transplantation. Transplantation. 1977;23:210–216. doi: 10.1097/00007890-197703000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Todo S, Porter KA, Kam I, et al. Canine liver transplantation under Nva2-cyclosporine versus cyclosporine. Trnasplantation. 1985 doi: 10.1097/00007890-198603000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porter KA. Starzl TE, Ed with the assistance of Putnam CW: Experience in Hepatic Transplantation. Philadelphia: W.B. Saunders; 1969. Pathology of the orthotopic homograft and heterograft; pp. 422–471. [Google Scholar]
- 34.Starzl TE. Starzl TE, Ed with the assistance of Putnam CW. Philadelphia: W.B. Saunders; 1969. Rejection in unmodified animals; pp. 184–190. [Google Scholar]
- 35.Starzl TE, Marchioro TL, Porter KA, et al. Factors determining short- and long-term survival after orthotopic liver homotransplantation in the dog. Surgery. 1965;58:131–155. [PMC free article] [PubMed] [Google Scholar]
- 36.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation, and in human renal transplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 37.Borel IF, Feurer C, Gubler HV, Stahelin H. Biological effects of cyclosporin A; a new antilymphocytic agents. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 38.Calne RY, Rolles K, White DJG, et al. Cyclosporin-A initially as the only immunosuppression in 34 patients of cadaveric organs: 32 kidneys, 2 pancreases and 2 livers. Lancet. 1979;2:1033–1036. doi: 10.1016/s0140-6736(79)92440-1. [DOI] [PubMed] [Google Scholar]
- 39.Starzl TE, Weil R, III, Iwatsuki S, et al. The use of cyclosporin-A and prednisone in cadaver kidney transplantation. Surg Gynecol Obstet. 1980;151:17–26. [PMC free article] [PubMed] [Google Scholar]
- 40.Klintmalm GBG, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin-A in liver and kidney transplant patients. Lancet. 1981;1:470–471. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klintmalm GBG, Iwatsuki S, Starzl TE. Cyclosporin-A hepatotoxicity in 66 renal allograft recipients. Transplantation. 1981;32:488–489. doi: 10.1097/00007890-198112000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hiestand PC, Gunn H, Gale J, et al. The immunosuppressive profile of a new natural cyclosporine analogue: Nva2-cyclosporine. Transplant Proc. 1985;17:1362–1364. [Google Scholar]
- 43.Starzl TE. Starzl TE, Ed with the assistance of Putnam CW: Experience in Hepatic Transplantation. Philadelphia: W.B. Saunders; 1969. The recipient operation in man; p. 112. [Google Scholar]