Abstract
Background
Celecoxib and other non-steroidal anti-inflammatory drugs (NSAIDs) are being evaluated in the prevention of bladder and other cancers. Here we investigate molecular effects of celecoxib independent of cyclooxygenase (COX)-2 expression levels in urothelial carcinoma of the bladder.
Materials and Methods
Low-grade RT-4 and high-grade UM-UC-3 bladder cancer cells were treated with 0–50 μM celecoxib. Growth, cell cycle and apoptosis were measured by crystal violet elution and flow cytometry. Western analysis was performed for COX-2, Rb, cyclin B1/D1, and phosphocyclin B1/D1. COX-2 induction was achieved with phorbol ester.
Results
Celecoxib inhibited growth of RT-4 and UM-UC-3, with G1 cell cycle arrest and altered cyclin B1/D1 expression in RT-4, whereas Rb up-regulation occurred in UM-UC-3. Apoptosis occurred in both cell lines.
Conclusion
Celecoxib induces G1 cell cycle arrest in low- and high-grade bladder cancer by different pathways. This heterogeneous molecular response supports combination approaches to prevention and treatment.
Keywords: Cyclooxygenase (COX)-2, celecoxib, bladder cancer, chemoprevention
Aberrant expression of cyclooxygenase (COX)-2 has been identified in multiple tumor types including bladder cancer. This enzyme is thought to contribute to the malignant phenotype and has been implicated in increased growth, invasion, angiogenesis, and resistance to apoptosis. Non-steroidal anti-inflammatory drugs (NSAIDs) are known to inhibit this enzyme, and selective COX-2 inhibitors have been developed in an attempt to limit toxicity associated with the inhibition of COX-1 by non-selective COX inhibitors (1). More recently, COX-2 selective inhibitors have been the focus of much scrutiny and publicity in terms of cardiovascular risk such that COX-2 inhibitors and NSAIDs in general are now being critically evaluated. Of such inhibitors, celecoxib is thought to have a better safety profile than other COX-2 selective inhibitors, and has been evaluated in an NCI-sponsored study in the secondary prevention of high-risk bladder cancer (2, 3). Furthermore, recent clinical trials of celecoxib in the prevention of adenomatous intestinal polyps have demonstrated the efficacy of celecoxib as a preventive agent but found a trend toward elevated risk primarily in those patients with a cardiac history and when celecoxib was given in a b.i.d. dosing schedule. In contrast, a recent meta-analysis of muliple recent large clinical trials failed to identify elevated cardiovascular risk with celecoxib (4), supporting further study of this agent in cancer prevention for select patients at lower risk (5). Recent studies have also supported the use of celecoxib as an adjunct to chemotherapy in patients with advanced cancers (6–8). Its efficacy in growth inhibition and potency as an inducer of apoptosis has been found to be better than that of other COX-2 selective drugs and to be independent of COX-2 (9–13). The COX-2–independent activity of celecoxib and other NSAIDs has also been described in multiple tumor types, although the specific pathways involved have been largely unexplored in bladder cancer. We have previously demonstrated that celecoxib inhibits growth and induces apoptosis in the UM-UC-3 cell line, which lacks COX-2 expression (14). Given the finding of heterogeneous expression of COX-2 in bladder cancer (15), an agent such as celecoxib that features both COX-2-dependent and -independent activity may be more effective than other NSAIDs. This study focuses on the mechanism by which celecoxib inhibits growth in bladder cancer. The effects of celecoxib in bladder cancer cells that express COX-2 or lack COX-2 expression will be evaluated.
Materials and Methods
Cell culture
Two human bladder cancer cell lines representing either low or high grade malignant phenotypes are the RT-4 urothelial cell line derived from low-grade papillary urothelial carcinoma as well as the poorly differentiated urothelial cell line UM-UC-3 (ATCC). RT-4 and UM-UC-3 were maintained respectively in either MEM containing 10% fetal calf serum along with penicillin-streptomycin or McCoy’s medium supplemented with 1% fetal bovine serum, insulin, transferrin, hydrocortisone, L-glutamine, dextrose, and non-essential amino acids.
Cell proliferation
Cells were plated on 96-well plates (2×103 cells per well). After 24 h, the cells were treated with 0, 6.25, 12.5, 25, and 50 μM celecoxib (LKT Labs). The cells were then fixed in 1% glutaraldehyde in phosphate-buffered saline (PBS) at 24-h intervals and stained with crystal violet. The dye was eluted with Sorenson’s solution (0.03 M sodium citrate, 0.02 N HCl, and 45% ethanol), and absorbance was read at 540 nm.
Induction of COX-2 expression
RT-4 and UM-UC-3 were grown to semi-confluence in 6-well plates and treated with phorbol ester 0.1 μM 6 h prior to protein lysis. Lysates were then evaluated for COX-2 expression by Western analysis.
Flow cytometry and apoptosis
Cells were grown in 10-cm dishes to 70% confluence and treated with celecoxib (0–50 μM) for a range of fixed time points (30 min, 1 h, 3 h, 5 h, and 24 h). The cells were then harvested by trypsinization. Floating cells were recovered from the supernatant. The cells were then washed twice with PBS and fixed in 1% paraformaldehyde in PBS at 4°C for 20 min. They were again washed twice with PBS, resuspended in 70% ethanol and stored at −20°C overnight. Prior to flow cytometry, the cells were washed twice with PBS, resuspended in a 1-ml solution containing 50 μg/ml propidium iodide and 20 μg/ml RNAse A in PBS, and incubated for 30 min at 37°C. Apoptosis was determined with the APO-BRDU kit (Phoenix Flow Systems, San Diego, CA, USA). All experiments were repeated in triplicate.
Western blotting
RT-4 and UM-UC-3 cells were grown to near confluence in 6-well dishes prior to treatment with celecoxib (0–50 μM) for 24 h or phorbol ester (0.1 μM) for 6 h. Cells were then washed with PBS and extracted by scraping the cells from each well into 100 μl of lysis buffer (PBS with 1% Triton X-100, 1 mM PMSF, 0.1 mM leupeptin and 1% aprotinin). After incubation on ice for 30 min, these lysates were centrifuged at 11,000 xg at 4°C. For Western blotting, the extracts were mixed with non-reducing 6× Laemmli sample buffer, and 20 μg of sample was applied to a 10% polyacrylamide gel. Following electrophoresis at 140 V, protein was transferred to a nitrocellulose membrane. Membranes were then probed with specific antibodies. Samples were run in parallel with prestained molecular weight standards (Bio-Rad Prestained Standards). The nitrocellulose was blocked overnight at 4°C while shaking in PBS containing 0.05% Tween 20 and 10% nonfat dry milk. The nitrocellulose sheet was incubated while shaking at room temperature for 1 h with primary antibodies to either COX-2 (Cayman), Cyclin D1 and Cyclin B1 (Upstate), phospho-Cyclin D1 [Thr 286] and phospho-Cyclin B1 [Ser 147] (Cell Signaling Technology), or Rb (Abcam), washed three times with PBS, and incubated 1 h with peroxidase-conjugated goat anti-mouse immunoglobulin (Santa Cruz Biotechnology) diluted in blocking solution. The nitrocellulose sheet was then washed in PBS and developed using chemiluminescence with the SuperSignal West Femto Maximum Sensitivity Substrate kit (Pierce Chemical, Rockford, IL, USA).
Statistical methods
For cellular proliferation, regression analysis was used to test whether celecoxib inhibited the growth of UM-UC-3 and RT-4 in a dose-dependent fashion. For cyclin and Rb expression, the relationships of celecoxib dose to protein expression in the different cell lines were determined as follows. The log of the ratio of the densitometry value divided by the vehicle control values was calculated for each dose level. This was regressed on the log of dose. Regression equations were fit so the intercept was forced to be 0 –that is, at dose 0, the log of the dose ratios was assumed to be (and is by definition) 0. Computations were performed with R software (R Development Core Team, Vienna Austria; http://www.R-project.org).
Results
COX-2 expression
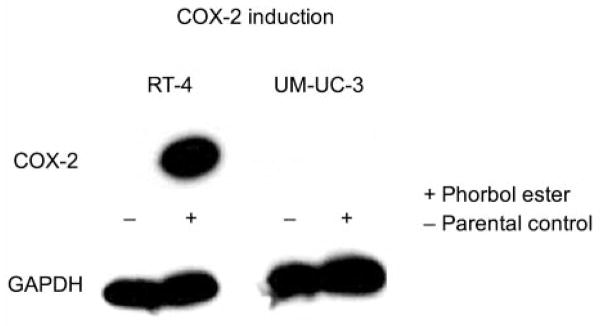
Low levels of COX-2 expression were identified in the RT-4 cell line by Western blotting, as compared with the UM-UC-3 cell line, which lacks COX-2 expression. Induction of COX-2 in RT-4 was achieved by treatment with phorbol ester for 6 h, whereas no induction of COX-2 was detected in UM-UC-3 (Figure 1).
Figure 1.
COX-2 induction by 6-h exposure to phorbol ester. COX-2 expression is induced in RT-4 (which normally expresses low basal levels of COX-2), whereas COX-2 up-regulation does not occur in the UM-UC-3 cell line (in which COX-2 expression is undetectable under control conditions).
Cellular proliferation
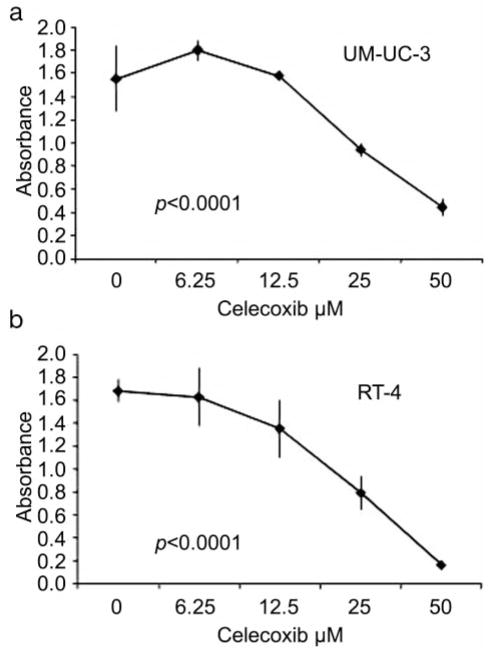
Cellular proliferation was measured at 24, 48, and 72 h in the presence and absence of celecoxib. Celecoxib inhibited the growth of RT-4 and UM-UC-3 in a dose-dependent fashion (p<0.0001). The IC50 for celecoxib was 12.5 μM and 25 μM for RT-4 and UM-UC-3, respectively (Figure 2).
Figure 2.
Celecoxib effectively inhibits growth in a dose-dependent fashion in the UM-UC-3 and RT-4 cell lines independent of COX-2 expression levels.
Cell cycle and apoptosis
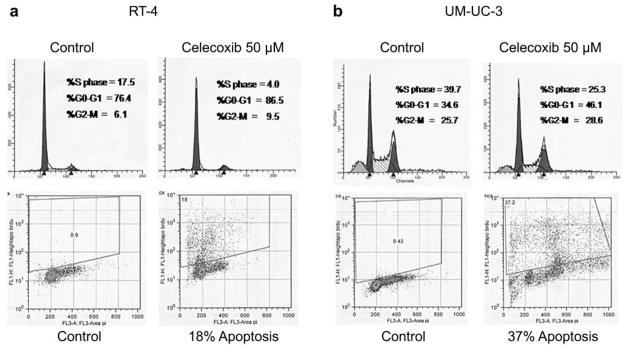
To determine the mechanism by which growth inhibition occurs independent of COX-2 expression, we then evaluated the effects of celecoxib on cell cycle distribution. We found that for celecoxib concentrations ranging from 0 to 50 μM, a dose-dependent increase was observed in the G0/G1 cell population in the UM-UC-3 and RT-4 cell lines. Apoptosis was also observed in response to celecoxib for both cell lines (Figure 3).
Figure 3.
An increase in the G0/G1 cell cycle population is observed in the presence of celecoxib 50 μM in the UM-UC-3 cell line. G1 arrest is also observed in the COX-2–expressing cell line RT-4. Apoptosis is also observed for UM-UC-3 and RT-4 treated for 48 h with celecoxib 50 μM.
Cyclin and Rb expression
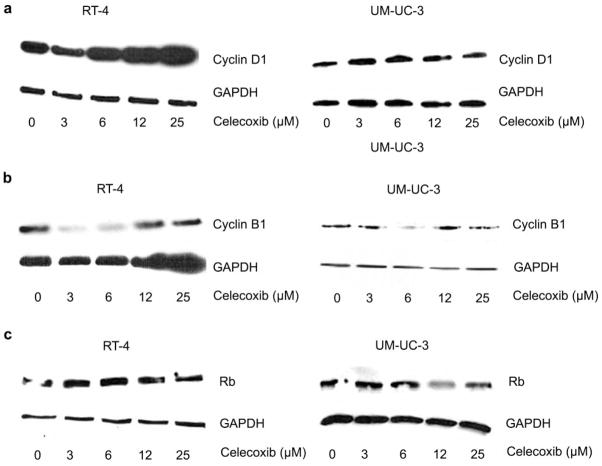
In evaluating cell cycle regulatory checkpoints, we determined whether cyclin expression was altered in the presence of celecoxib for these cell lines by western analysis (Figure 4). Cyclin and Rb expression was measured with doses of 0 (vehicle), 3.125, 6.25, 12.5, 25, and 50 μM celecoxib. The highest dose, 50 μM, was considered likely to cause apoptosis, so was excluded from this analysis.
Figure 4.
Expression of cyclin D1 (A) and cyclin B1 (B), and Rb (C) in the presence of celecoxib (0–25 μM). Minimal variation in cyclin expression in the high-grade, COX-2–null cell line UM-UC-3 is observed as compared with the low-grade, COX-2 expressor RT-4.
For each set of data points, the densitometry values are normalized to vehicle control values. Therefore, control densitometry values are by definition 1, and regression analysis was performed for these values. Table I gives the slopes for change in expression of the cyclins and Rb as well as p-values indicating statistical significance. Expression of cyclin and Rb was measured for doses of 0, 3.125, 6.25, 12.5, 25, and 50 μM celecoxib. However, for the above analysis, the highest dose (50 μM) was considered likely to cause apoptosis, and therefore was excluded from analysis.
Table I.
Western blot densitometry.
| Target | Cell type | Slope | Standard error | R2 | P-value |
|---|---|---|---|---|---|
| Cyclin D | RT-4 | 0.05483 | 0.01324 | 0.71 | 0.004 |
| Cyclin B | RT-4 | −0.10126 | 0.01558 | 0.858 | <0.001 |
| Phospho-cyclin D | RT-4 | 0.04152 | 0.01953 | 0.392 | 0.071 |
| Phospho-cyclin B | RT-4 | −0.00782 | 0.01287 | 0.05 | 0.562 |
| Rb | RT-4 | −0.03815 | 0.03688 | 0.133 | 0.335 |
| Cyclin D | UM-UC-3 | 0.00633 | 0.00483 | 0.223 | 0.238 |
| Cyclin B | UM-UC-3 | −0.00235 | 0.00909 | 0.011 | 0.805 |
| Phospho-cyclin D | UM-UC-3 | 0.01989 | 0.01755 | 0.155 | 0.294 |
| Phospho-cyclin B | UM-UC-3 | −0.04111 | 0.02283 | 0.316 | 0.115 |
| Rb | UM-UC-3 | 0.0254 | 0.00756 | 0.617 | 0.012 |
The comparisons that are statistically significant are highlighted in bold.
For the cell line RT-4, cyclin D1 (p=0.004) and cyclin B1 (p<0.001) expression show a significant relationship with dose, the first having a positive relationship, and the second having a negative relationship.
For the cell line UM-UC-3, cyclin expression was not significantly altered, although Rb shows a significant result (p=0.012) with a positive relationship. In terms of phosphorylated cyclins, phospho-cyclin D1 shows a positive but also only marginally significant relationship in RT-4 (p=0.071). Overall, phosphorylated cyclins (data not shown) did not change significantly in relationship to celecoxib dose, implying that inhibition of phosphorylation is not a major mechanism of growth inhibition in these bladder cancer cell lines.
Discussion
The cyclooxygenase enzyme mediates prostaglandin production and consists of two isoforms, COX-1 and COX-2. COX-1 is constitutively expressed in normal tissues. COX-2 is inducible in the setting of neoplasia and inflammation. COX-2 expression is generally absent in normal tissue, but up-regulation of this enzyme has been reported in multiple tumor types. These include colon, breast, and pancreatic tumors as well as transitional cell carcinoma (16, 17). COX-2 facilitates production of prostaglandins and downstream mediators that enhance growth, angiogenesis, invasion, and resistance to apoptosis. For instance, vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP) have been identified with the prostaglandin pathway (18–20). Furthermore, increased expression of the anti-apoptotic factor Bcl-2 has been observed with COX-2 up-regulation (21). It is also known that in vitro treatment of colon cancer cells with prostaglandin (PGE2) causes activation of the phosphatidyl 3-kinase (PI3K)/Akt pathway (22) with resulting increased cellular proliferation and motility.
We have also explored the biologic potential of COX-2 in urothelium. Utilizing a premalignant urothelial model, we have demonstrated that forced COX-2 overexpression in SV-HUC cells increases PGE2 production with associated enhanced cellular growth and invasion (23). COX-2 activity may be counterbalanced by metabolism of prostaglandins by the prostaglandin dehydrogenase (PGDH) enzyme (24). More recently, we have also reported the loss of PGDH in TCC cell lines and in bladder tumors, which may contribute to higher prostaglandin levels (25, 26).
Celecoxib is a selective inhibitor of COX-2. COX-2 inhibitors decrease growth and promote apoptosis in multiple tumor types in vitro. We have previously shown that celecoxib reduces PGE2 levels, inhibits growth, and induces apoptosis in a panel of bladder cancer cell lines (14). Celecoxib is often found to be more effective than other COX-2 inhibitors such as rofecoxib (9–11) and NS-398 (13). This would suggest additional COX-2–independent mechanisms of activity. Celecoxib has been shown to inhibit growth independently of COX-2 expression levels with a G0/G1 cell cycle arrest and decreased levels of cyclins A and B1 or cyclin D1 depending on tumor type (27, 28). Furthermore, both celecoxib and NS-398 can induce apoptosis independently of COX-2 with release of cytochrome C, poly (ADP-ribose) polymerase (PARP) cleavage, and activation of caspases 3 and 9 (27, 29). Other possible COX-2–independent effects have also been identified with NSAIDs, involving the 3-phosphoinositide-dependent kinase 1 (PKB1) and the serine/threonine protein kinase B (PKB)/Akt pathways in colon and prostate cancer cell lines (30–32). The mechanism by which celecoxib inhibits growth of bladder cancer cell lines remains uncertain and is the focus of this study.
We previously identified activity of celecoxib in the UM-UC-3 cell line, which lacks detectable COX-2 expression (14). Whereas COX-2 expression was undetectable for UM-UC-3 by Western analysis or by RT-PCR, we sought to demonstrate whether COX-2 enzyme expression could be induced in these cells by exposure to phorbol ester. The mechanism by which this occurs is thought to involve activation of protein kinase C with subsequent up-regulation of COX-2. Whereas significant up-regulation of COX-2 was observed for RT-4, COX-2 expression remained undetectable for UM-UC-3 following exposure to phorbol ester. This suggests either a COX-2 promoter mutation or other defect of this pathway that interferes with COX-2 gene expression, such that even low, undetectable basal levels of COX-2 in this cell line probably do not exist. Ironically, this cell line, which lacks COX-2 expression, is the most aggressive of the two, having been derived from an invasive urothelial carcinoma (33). Whereas this may represent an aberration of cell culture, certainly it is known that heterogeneous expression of COX-2 occurs in urothelial tumors (15).
It is conceivable that inhibition of low basal levels of COX-2 in UM-UC-3 could account for the activity of celecoxib. However, COX-2 expression is undetectable by Western analysis for UM-UC-3 (14), and induction of COX-2 does not occur with UM-UC-3 in this study, further confirming that low basal levels of COX-2 are not expressed by UM-UC-3. Therefore, it is most likely an actual COX-2–independent mechanism of action that we observe with celecoxib in UM-UC-3. COX-2–independent activity of NSAIDs could play an important role in which COX-2 is not expressed homogeneously throughout the tumor.
Given the increase in G0/G1 cell population implying G1 cell cycle arrest, we chose to evaluate cyclin and Rb expression levels in response to celecoxib. An interesting observation for the RT-4 cell line is that up-regulation of cyclin D1 accompanied G1 arrest in a dose-dependent fashion with celecoxib, whereas cyclin B1 levels decreased, suggesting that modulation of cyclin expression occurs in response to celecoxib in this cell line. Whereas down-regulation of cyclin B has been observed previously in association with G1 arrest with celecoxib (27), we also identified paradoxical up-regulation of cyclin D1. This would be inconsistent with prior findings of cyclin D down-regulation with celecoxib in other tumor types, which may be anticipated with G1 arrest (28, 34). One possible explanation for this is the reported induction of the cell cycle inhibitor p27 with celecoxib (27), which may, in turn, trigger up-regulation of cyclin D as part of a positive feedback mechanism (35). In this sense, induction of cyclin D may represent a homeostatic feedback response to celecoxib in bladder cancer. While a trend revealing up-regulation of phospho-cyclin D1 also exists, neither levels of phospho-cyclin D1 nor phospho-cyclin B1 change significantly in response to celecoxib, implying that modulation in cyclin expression plays a greater role than inhibition of cyclin phosphorylation in the RT-4 cell line.
In this study, we also found that celecoxib inhibits growth independently of COX-2 expression in UM-UC-3 with an increase in the G0/G1 cell population accompanied by intense induction of apoptosis. In contrast to RT-4, no significant change in cyclin or phospho-cyclin levels occurs in the COX-2–null UM-UC-3 cell line, implying that cyclin dependent regulation may not be intact in these high grade cells. Nevertheless, up-regulation of Rb was observed in UM-UC-3, indicating that cell cycle arrest may be achieved with celecoxib through alterations in the Rb pathway as a COX-2 independent mechanism. One explanation for this discrepancy is that RT-4, derived from a low-grade tumor, may harbor intact cell cycle machinery and therefore displays changes in cyclin cell cycle mediator expression, whereas UM-UC-3 is a poorly differentiated cell line. In a study by Delia et al., dissociation between cell cycle arrest and apoptosis has been reported for cells of the same tumor type, which differ in whether or not they have an intact G1/S checkpoint (36). Alternatively, there may be a necessary role for the COX-2 pathway in modulation of the cyclins by celecoxib in bladder cancer. Further studies are necessary to explore these hypotheses, as these observations appear to be cell line-dependent.
While differences exist in cell cycle regulation in low-versus high-grade bladder cancer cells, apoptosis occurs in response to celecoxib for both cell lines and may be a more reliable and consistent indicator of biologic activity of this agent in bladder cancer. We have previously reported that apoptosis occurs independent of COX-2 in association with Bcl-2 down-regulation in bladder cancer (14). In a recent study by Song et al., celecoxib induced apoptosis more effectively than other COX-2 agents independent of COX-2 inhibition (12). What we can conclude from this study is that celecoxib inhibits growth of both low- and high-grade bladder cancer, by cell cycle arrest and apoptosis, and that the primary molecular mechanism of growth inhibition and cellular susceptibility may be ultimately dependent on the degree of cellular differentiation.
Of note, the concentration at which these events occur in vitro is significantly higher than the physiologic concentration reported for celecoxib with oral administration. Similarly, Williams et al. (37) reported that in vitro concentrations of celecoxib as well as other NSAIDs ranging from 25 to 100 μM were necessary to produce growth inhibition and apoptosis in colon carcinoma cells. Furthermore, serum concentrations of only 2–3 μM celecoxib were required to inhibit the growth of colon carcinoma xenografts in nude mice, illustrating the importance of in vivo factors in this process (37). An antiangiogenic effect may also account for increased efficacy of these agents in vivo (38). Indeed, celecoxib has been shown to be effective in reducing bladder cancer incidence and growth in an in vivo model of bladder carcinogenesis (39). An effect on multiple molecular pathways other than COX-2 is suggested as antitumor effects including apoptosis, cell cycle inhibition, and inhibition of angiogenesis occur at NSAID concentrations 100- to 1,000-fold higher than that necessary to inhibit PGE2 synthesis (40). For instance, celecoxib has been reported to inhibit the COX-2 enzyme with an IC50 of less than 1 μM (40). Whether the tissue concentration of celecoxib in in vivo models of bladder cancer is sufficient to activate this COX-2–independent pathway remains to be determined.
In summary, celecoxib inhibits the growth of both low-grade and high-grade bladder cancer cells with an accompanying increase in the G0/G1 cell population. Whereby modulation of cyclin cell cycle mediators occurs in the low-grade, COX-2–expressing RT-4 cell line, essentially no change in these cyclins is observed for the poorly differentiated UM-UC-3 cell line, in which cell cycle regulatory pathways are less likely to be intact. However, apoptosis occurs for both RT-4 and UM-UC-3, implying that apoptotic mediators may be more important and universal downstream mediators of celecoxib in terms of prevention and treatment for patients with low- and high-grade bladder cancer. In this sense, markers of apoptosis may most reliably indicate biologic activity in clinical trials of celecoxib in the prevention and treatment of patients with bladder cancer.
As we continue to study the role of NSAIDs and molecular mechanisms of COX-2 and inhibition of this enzyme, hopefully these studies will permit us to better select patients who may benefit from preventive or treatment strategies utilizing these agents. In balancing risks associated with NSAIDs, these findings support the further study of celecoxib and other NSAIDs in the prevention and treatment of bladder cancer.
Acknowledgments
Terry Oberley, MD, Ph.D., assisted with editing of this manuscript.
This work is supported by the Office of Research and Development, Biomedical Laboratory R&D Service, Department of Veterans Affairs (J.R. Gee) and by P30 CA 14520 (T. Havighurst & K. Kim).
Abbreviations
- NSAIDs
Non-steroidal anti-inflammatory agents
- COX
Cyclooxygenase
- PI
Propidium Iodide
References
- 1.Thun MJ, Henley SJ, Patrono C. Nonsteroidal anti-inflammatory drugs as anticancer agents: mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–266. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 2.Okie S. Raising the safety bar – the FDA’s coxib meeting. N Engl J Med. 2005;352(13):1283–1285. doi: 10.1056/NEJMp058055. [DOI] [PubMed] [Google Scholar]
- 3.Gee J, Sabichi AL, Grossman HB. Chemoprevention of superficial bladder cancer. Crit Rev Oncol/Hematol. 2002;43:277–288. doi: 10.1016/s1040-8428(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 4.White WB, West CR, Borer JS, et al. Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol. 2007;99(1):91–98. doi: 10.1016/j.amjcard.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 5.Bertagnolli MM, Eagle CJ, Zauber AG, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355(9):873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 6.Dandekar DS, Lopez M, Carey RI, Lokeshwar BL. Cyclooxygenase-2 inhibitor celecoxib augments chemotherapeutic drug-induced apoptosis by enhancing activation of caspase-3 and -9 in prostate cancer cells. Int J Cancer. 2005;115(3):484–492. doi: 10.1002/ijc.20878. [DOI] [PubMed] [Google Scholar]
- 7.Altorki NK, Port JL, Zhang F, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res. 2005;11(11):4191–4197. doi: 10.1158/1078-0432.CCR-05-0108. [DOI] [PubMed] [Google Scholar]
- 8.Nugent FW, Mertens WC, Graziano S, et al. Docetaxel and cyclooxygenase-2 inhibition with celecoxib for advanced non-small cell lung cancer progressing after platinum-based chemotherapy: a multicenter phase II trial. Lung Cancer. 2005;48(2):267–273. doi: 10.1016/j.lungcan.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kazanov D, Dvory-Sobol H, Pick M, et al. Celecoxib but not rofecoxib inhibits the growth of transformed cells in vitro. Clin Cancer Res. 2004;10(1 Pt 1):267–271. doi: 10.1158/1078-0432.ccr-0412-3. [DOI] [PubMed] [Google Scholar]
- 10.Yamazaki R, Kusunoki N, Matsuzaki T, Hashimoto S, Kawai S. Selective cyclooxygenase-2 inhibitors show a differential ability to inhibit proliferation and induce apoptosis of colon adenocarcinoma cells. FEBS Lett. 2002;531(2):278–284. doi: 10.1016/s0014-5793(02)03535-4. [DOI] [PubMed] [Google Scholar]
- 11.Waskewich C, Blumenthal RD, Li H, et al. Celecoxib exhibits the greatest potency amongst cyclooxygenase (COX) inhibitors for growth inhibition of COX-2-negative hematopoietic and epithelial cell lines. Cancer Res. 2002;62(7):2029–2033. [PubMed] [Google Scholar]
- 12.Song X, Lin H-P, Johnson AJ, et al. Cyclooxygenase-2, player or spectator in cyclooxygenase-2 inhibitor-induced apoptosis in prostate cancer cells. J Natl Cancer Inst. 2002;94:585–591. doi: 10.1093/jnci/94.8.585. [DOI] [PubMed] [Google Scholar]
- 13.Johnson AJ, Song X, Hsu A-L, Chen C-S. Apoptosis signaling pathways mediated by cyclooxygenase-2 inhibitors in prostate cancer cells. Adv Enzyme Regul. 2001;41:221–235. doi: 10.1016/s0065-2571(00)00015-7. [DOI] [PubMed] [Google Scholar]
- 14.Gee J, Lee I-L, Jendiroba D, et al. Selective cyclooxygenase-2 inhibitors inhibit growth and induce apoptosis of bladder cancer. Oncol Rep. 2006;15(2):471–477. [PubMed] [Google Scholar]
- 15.Gee JR, Montoya RG, Khaled HM, Sabichi AL, Grossman HB. Cytokeratin 20, AN43, PGDH, and COX-2 expression in transitional and squamous cell carcinoma of the bladder. Urol Oncol. 2003;21(4):266–270. doi: 10.1016/s1078-1439(02)00271-5. [DOI] [PubMed] [Google Scholar]
- 16.DuBois RN, Arbramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 17.Mohammed SI, Knapp DW, Bostwick DG, et al. Expression of cyclooxygenase-2 (COX-2) in human invasive transitional cell carcinoma (TCC) of the urinary bladder. Cancer Res. 1999;59:5647–5650. [PubMed] [Google Scholar]
- 18.Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci. 2000;30:3–21. [PubMed] [Google Scholar]
- 19.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 20.Tsujii M, Kawano S, Dubois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336–3340. doi: 10.1073/pnas.94.7.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 22.Sheng H, Shao J, Washington MK, et al. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 23.Gee J, Lee I-L, Grossman HB, Sabichi AL. Effects of COX-2 overexpression on the immortalized, nontumorigenic urothelial cell line SV-HUC. Urol Oncol. 2008;26(6):641–645. doi: 10.1016/j.urolonc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Tong M, Tai HH. Induction of NAD+-linked 15-hydroxyprostaglandin dehydrogenase expression by androgens in human prostate cancer cells. Biochem Biophys Res Commun. 2000;276:77–81. doi: 10.1006/bbrc.2000.3437. [DOI] [PubMed] [Google Scholar]
- 25.Liebert M, Chen I-L, Gebhardt D, et al. Loss of expression of prostaglandin dehydrogenase in human bladder cancers. Proc Am Assoc Cancer Res. 1998;39:118. [Google Scholar]
- 26.Gee JR, Chen I-L, Montoya RG, et al. Down-regulation of prostaglandin dehydrogenase in invasive transitional cell carcinoma. Proc Am Assoc Cancer Res. 2001;42:264. [Google Scholar]
- 27.Maier TJ, Schilling K, Schmidt R, Geisslinger G, Grosch S. Cyclooxygenase-2 (COX-2)-dependent and -independent anticarcinogenic effects of celecoxib in human colon carcinoma cells. Biochem Pharmacol. 2004;67(8):1469–1478. doi: 10.1016/j.bcp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Narayanan BA, Condon MS, Bosland MC, Narayanan NK, Reddy BS. Suppression of N-methyl-N-nitrosourea/testosterone-induced rat prostate cancer growth by celecoxib: effects on cyclooxygenase-2, cell cycle regulation, and apoptosis mechanism(s) Clin Cancer Res. 2003;9(9):3503–3513. [PubMed] [Google Scholar]
- 29.Li M, Wu X, Xu XC. Induction of apoptosis by cyclooxygenase-2 inhibitor NS398 through a cytochrome c-dependent pathway in esophageal cancer cells. Int J Cancer. 2001;93(2):218–223. doi: 10.1002/ijc.1322. [DOI] [PubMed] [Google Scholar]
- 30.Leng J, Han C, Demetris AJ, Michalopoulos GK, Wu T. Cyclooxygenase-2 promotes hepatocellular carcinoma cell growth through Akt activation: evidence for Akt inhibition in celecoxib-induced apoptosis. Hepatology. 2003;8(3):756–768. doi: 10.1053/jhep.2003.50380. [DOI] [PubMed] [Google Scholar]
- 31.Hsu AL, Ching TT, Wang DS, et al. The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem. 2000;275(15):11397–11403. doi: 10.1074/jbc.275.15.11397. [DOI] [PubMed] [Google Scholar]
- 32.Arico S, Pattingre S, Bauvy C, et al. Celecoxib induces apoptosis by inhibiting 3-phosphoinositide-dependent protein kinase-1 activity in the human colon cancer HT-29 cell line. J Biol Chem. 2002;277(31):27613–27621. doi: 10.1074/jbc.M201119200. [DOI] [PubMed] [Google Scholar]
- 33.Grossman HB, Wedemeyer G, Ren L, Wilson GN, Cox B. Improved growth of human urothelial carcinoma cell cultures. J Urol. 1986;136:953–959. doi: 10.1016/s0022-5347(17)45139-1. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein IB. Disorders in cell circuitry during multistage carcinogenesis: the role of homeostasis. Carcinogenesis. 2000;21(5):857–864. doi: 10.1093/carcin/21.5.857. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto H, Soh JW, Shirin H, et al. Comparative effects of overexpression of p27Kip1 and p21Cip1/Waf1 on growth and differentiation in human colon carcinoma cells. Oncogene. 1999;18(1):103–115. doi: 10.1038/sj.onc.1202269. [DOI] [PubMed] [Google Scholar]
- 36.Delia D, Goi K, Mizutani S, et al. Dissociation between cell cycle arrest and apoptosis can occur in Li-Fraumeni cells heterozygous for p53 gene mutations. Oncogene. 1997;14(18):2137–2147. doi: 10.1038/sj.onc.1201050. [DOI] [PubMed] [Google Scholar]
- 37.Williams CS, Watson AJM, Sheng H, et al. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: Lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60:6045–6051. [PubMed] [Google Scholar]
- 38.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 39.Grubbs CJ, Lubet RA, Koki AT, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60(20):5599–5602. [PubMed] [Google Scholar]
- 40.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2058–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]