Abstract
We have previously shown that nicotine enhances learning in a negative occasion setting task in which rats are trained to distinguish between two different trial types. During reinforced trials, a target stimulus (a tone) is presented and immediately followed by food reward. On non-reinforced trials, a feature stimulus (a light) is presented prior to the tone and indicates the absence of reward following presentation of the tone. The goal of the present study was to identify the behavioral mechanism through which nicotine affects this form of learning, and to determine which subtype(s) of nicotinic acetylcholine receptors mediate the effects of nicotine. Consistent with our prior findings, nicotine administration enhanced the ability of rats to discriminate between the two trial types. Nicotine enhanced the magnitude of the discrimination by decreasing responding to the tone on non-reinforced trials Nicotine-treated rats also learned the discrimination in fewer sessions than control rats. A significant new finding was that nicotine also increased the orienting response to the light, suggesting that nicotine may enhance learning the serial feature negative discrimination by increasing attention to the visual feature. In addition, we found that RJR-2403, a selective α4β2 nicotinic receptor agonist, also enhanced discrimination. However, RJR-2403 did not affect responding on non-reinforced trials, nor did RJR-2403 affect orienting to the light. Together these data indicate that nicotine may enhance discrimination by enhancing tone-reward associability through α4β2 nicotinic receptors and by enhancing attention to the light through non-α4β2 receptor subtypes.
Keywords: RJR-2403, nicotine, serial feature negative discrimination, inhibition
Introduction
A substantial body of research indicates that stimulation of nicotinic acetylcholine receptors modulates learning and memory (Levin, 2002). Administration of nicotine or other nicotinic receptor agonists has repeatedly been shown to improve working memory and attention in a variety of tasks in normal humans and rodents (Levin, Conners, Silva, Hinton et al., 1998; McGaughy, Decker, & Sarter, 1999; Ohno, Yamamoto, & Watanabe, 1993). Similarly, nicotine is known to enhance contextual fear memory and spatial learning (Felix & Levin, 1997; Gould & Wehner, 1999). The beneficial effects of nicotine on cognitive function have also been demonstrated in populations that exhibit cognitive dysfunction, suggesting that nicotine might be used by persons with certain forms of mental illness to alleviate cognitive impairment (Potter & Newhouse, 2004, 2008; Kumari & Postma, 2005).
Most often, the effects of nicotine on learning and memory have been demonstrated using tasks that require subjects to emit a particular learned response. Although far fewer studies have focused on the effects of nicotine on learning to inhibit a behavioral response, there is accumulating evidence that stimulation of nicotinic receptors also improves the ability to withhold behavior. In humans, nicotine has been shown to enhance performance in the Stop Signal Task, a commonly used measure of behavioral inhibition, by enhancing the ability of subjects to inhibit a learned response (Potter & Newhouse, 2004, 2008). Nicotine has also been shown to reduce premature responding in the five-choice serial reaction time task in rats (Blondel, Sanger, & Moser, 2000). Consistent with these findings, a recent study from our laboratory suggests that nicotine enhances the ability to inhibit responding during negative occasion setting (MacLeod, Potter, Simoni, & Bucci, 2006).
A typical negative occasion setting paradigm involves a serial feature negative discrimination in which rats are trained to distinguish between two different trial types. During reinforced trials, a target stimulus (e.g., a tone) is presented and immediately followed by food reward. On non-reinforced trials, a feature stimulus (a light) is presented prior to the tone and indicates the absence of reward following presentation of the tone. Rats learn to approach the food cup during presentation of the tone on reinforced trials but not when the tone is preceded by the light. In other words, rats inhibit responding to the target when the feature precedes the target (Bouton & Nelson, 1994; Bueno & Holland, 2008; Holland & Morell, 1996). Although traditional learning theory suggests that negative occasion setting does not produce conditioned inhibition (Rescorla & Wagner, 1972), the results of several experiments suggest that there is a learned inhibitory response in this form of conditioning (Bueno & Holland, 2008; Holland, 1984). We have previously found that nicotine enhanced negative occasion setting by facilitating discrimination and reducing responding on non-reinforced trials (MacLeod et al., 2006). However, the behavioral mechanisms through which nicotine affects this form of learning remain unknown. In addition, it is unclear which nicotinic receptor subtype mediates the effects of nicotine on inhibition.
Negative occasion setting is thought to involve memory processes in that rats must maintain a representation of the feature (the light) for a period of time before the target stimulus (the tone) is presented (Holland, 1984). In addition, the negative feature may “gate” the inhibitory properties of the target stimulus, thus allowing the target to obtain both excitatory and inhibitory characteristics (Bueno & Holland, 2008; Bouton, & Nelson, 1994; Holland, 1984; Figure 1). As shown in Figure 1, the light may “activate” the inhibitory association of the tone with food, thus resulting in less food cup behavior during the tone on trials in which the light precedes the tone. One mechanism through which nicotine might enhance negative occasion setting is by altering attentional processing of the light such that the feature is more likely to control responding to the target (i.e., the tone). Indeed, nicotine has been shown to enhance performance in the five-choice serial reaction time task by improving the ability to attend to and detect visual stimuli (Blondel et al., 2000), an effect that is blocked by co-administration of a selective α4β2 nicotinic receptor antagonist. The present study compared the effects of nicotine and a selective α4β2 nicotinic receptor agonist (RJR-2403; Lipello, Bencherif, Gray, Peters, et al., 1996) on negative occasion setting to test the hypothesis that α4β2 nicotinic receptors mediate the effects of nicotine on this form of inhibitory learning. Furthermore, we examined the effects of each compound on attentional processing of the light by assessing orienting behavior during the presentation of the light. The orienting response to a visual stimulus is defined as rearing up on the hind legs during presentation of the cue (“rearing behavior,” Holland, 1977, 1984) and is an often-used measure of attentional processing (Gallagher, Graham, & Holland, 1990; Kaye & Pearce, 1984; Lang, Simons, & Balaban, 1997).
Figure 1.
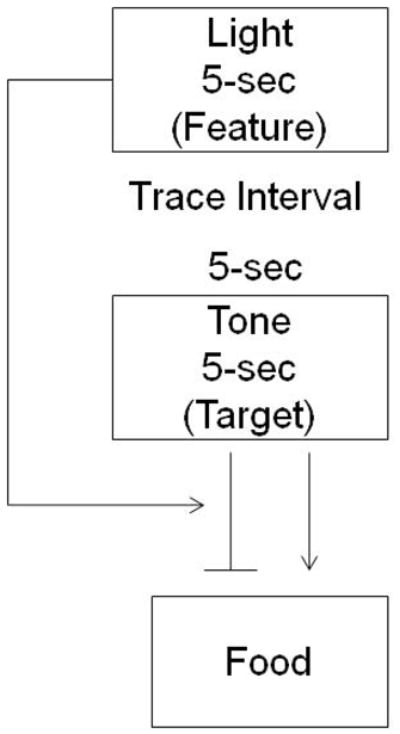
Putative behavioral mechanisms underlying performance on non-reinforced trials in the serial feature negative discrimination task. The negative feature (the light) is thought to gate the inhibitory properties of the target stimulus (tone), thus allowing the target to obtain both excitatory and inhibitory characteristics (Bueno & Holland, 2008; Bouton, & Nelson, 1994; Holland, 1984)
Materials and Methods
Subjects
A total of 48 Long Evans rats (weighing ~300g) were obtained from Harlan Laboratories (Indianapolis, IN). Rats were housed individually and allowed 7 days to acclimate to the vivarium with food available ad libitum (Purina Rat Chow; Nestle Purina, St. Louis, MO). Throughout the study, rats were maintained on a 14:10 light–dark cycle and monitored and cared for in compliance with Association for Assessment and Accreditation of Laboratory Animal Care guidelines and the Dartmouth Institutional Animal Care and Use Committee.
Drugs
RJR-2403 ((E)-N-Methyl-4-(3-pyridinyl)-3-buten-1-amine oxalate; Tocris Bioscience, Inc Ellisville, MO) or – (-) nicotine hydrogen ditartrate (Sigma-Aldrich, Inc., St. Louis, MO) was dissolved in sterile 1X phosphate buffered saline. The final concentration of RJR-2403 was 2 mg/mL total salt weight. The final concentration of nicotine was 0.17 mg/mL, calculated as free-base, and adjusted to pH 7.0 with 2N NaOH. RJR-2403 (2.0 mg/kg) or saline was administered subcutaneously between the shoulder blades using a volume of 1.0 mL/kg. Nicotine (0.35mg/kg) or saline was administered using a volume of 2.0 mL/kg. These doses of nicotine and RJR-2403 were based on previous studies (Blondel et al., 2000; Levin, 2002; Lippiello et al., 1996; MacLeod et al., 2006).
Behavioral Apparatus
Experiments were conducted in standard conditioning chambers (24 cm × 30.5 cm × 29 cm) obtained from Med Associates Inc. (St. Albans, VT) and connected to a PC-clone computer. Each chamber was enclosed in a sound-attenuating cubicle (62 cm × 56 cm × 56 cm) outfitted with an exhaust fan to provide airflow and background noise (~68 dB). The chambers consisted of aluminum front and back walls, clear acrylic sides and top, and grid floors. A dimly illuminated food cup was recessed in the center of the front wall and a white panel light (2.5cm diameter disc, illuminated by a 2.8 W bulb) was located 5 cm above the opening to the recessed food cup and served as the visual stimulus in this study. A speaker was located 15 cm above and to the right of the food cup and was used to present the auditory conditioned stimulus (CS; 1,500 Hz, 78 dB). A red house-light providing background illumination was mounted 15 cm high on the wall of the sound-attenuating cubicle. Delivery of two 45-mg food pellets (Noyes, New Brunswick, NJ) served as the unconditioned stimulus. A pair of infrared photocells was located across the entrance to the food cup to monitor entries into the cup. To detect rearing behavior (rising up on the hindlegs), additional pairs of photocells were located 15 cm above the grid floor. One pair was directly above the food cup and just below the white panel light. Two other pairs were to either side, evenly spaced 8 cm from the center pair of photocells, so that a rearing response in any part of the chamber would be detected by one of the photobeams (Keene & Bucci, 2007; Hopkins, Sharma, Evans, & Bucci, 2008).
Behavioral Procedures
Before beginning behavioral training rats were trained to eat from the food cup during a single 64-min session in which two food pellets were randomly delivered 16 times. For the next 16 days rats were weighed and placed in a plastic transporter used to carry them from the colony room to the treatment room. Rats were then injected with nicotine, RJR-2403, or equivalent volumes of saline. Rats were treated with nicotine 10 min prior to training and rats given RJR-2403 were treated 20 min prior to training. Equal numbers of saline-treated rats were injected accordingly. Daily sessions lasted for 68 min and consisted of 16 trials of two types: rats received four trials per session consisting of a 5-sec presentation of the tone followed immediately by delivery of two food pellets. For the other 12 trials, the panel light was presented for 5 sec, followed by a 5-sec empty period, and then the tone was presented for 5 sec (Holland, Lamoureux, Han, & Gallagher, 1999). No food was delivered after the tone on these trials. The two trial types occurred randomly during the session (inter-trial intervals averaged 4 min) and the order of trials differed on each day.
Data Analysis
The number of times that the photobeam located across the entry of the food cup was broken (i.e., “nose-pokes”) was monitored by the computer and provided a measure of conditioned food-cup behavior during presentation of the tone. Total nose-pokes were averaged for each trial type in each session. Difference scores were also calculated for each rat by subtracting the number of nose-pokes during presentation of the tone on non-reinforced trials from the number of nose-pokes during the tone on reinforced trials. The difference scores served as the primary dependent variable of interest. Group differences in conditioned responding were assessed by conducting logarithmic curve estimations across training sessions for each subject (MacLeod et al., 2006). The resulting beta coefficients served as a measure of the rate of discrimination and were analyzed with a univariate ANOVA with planned orthogonal contrasts between all groups. The number of training sessions required until rats began to significantly discriminate between trial types was also analyzed within each group using paired sample t-tests.
The orienting response to the visual stimulus was also monitored for a subset of rats. Data was not collected for some of the subjects due to a computer program update, resulting in sample sizes of 15, 10, and 9 for the saline, nicotine, and RJR-2403 groups, respectively). The number of times the photobeams were broken during presentation of the light was summed across the 3 beams since previous studies indicate that it is unlikely that a rearing response would simultaneously break more than one of the 3 photobeams (Keene & Bucci, 2007). Because we found evidence of a small, but statistically significant difference between groups in the amount of rearing observed during the 5-sec period prior to the onset of any stimuli (“pre-CS” period; 0.3 rears for nicotine-treated rats vs. 0.1 for the saline and RJR-2403 groups), we calculated “elevations scores” by subtracting the amount of rearing during the 5-sec pre-CS period from the amount of rearing measured during the 5-sec presentation of the light (e.g., Baxter, Holland, & Gallagher, 1997). Group differences were assessed by conducting logarithmic curve estimations for the resulting data across training sessions for each subject. The beta coefficients were used as a measure of the orienting response to the light and were analyzed with a univariate ANOVA with planned contrasts between all groups. All analyses were conducted using an alpha level of 0.05.
Results
Food cup behavior
One saline-treated rat and two RJR-2403-treated rats were eliminated from the analysis of food cup behavior because their beta coefficients were more than two standard deviations from the mean for their respective groups. Thus, there were 19 rats remaining in the saline group, 16 rats in the RJR-2403 group, and 10 rats in the nicotine group.
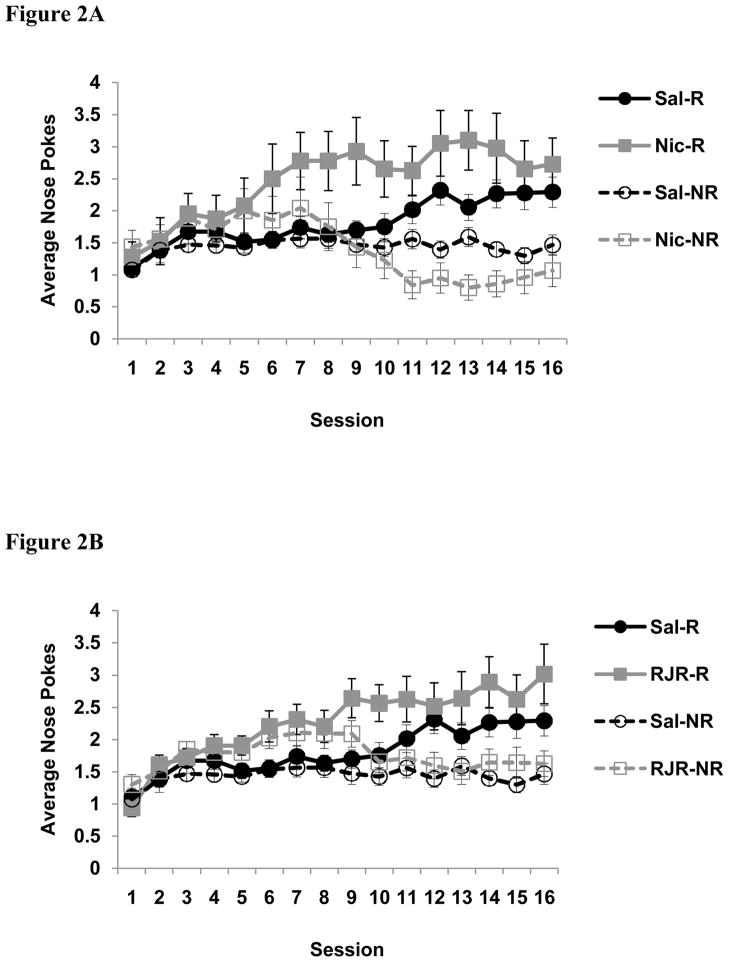
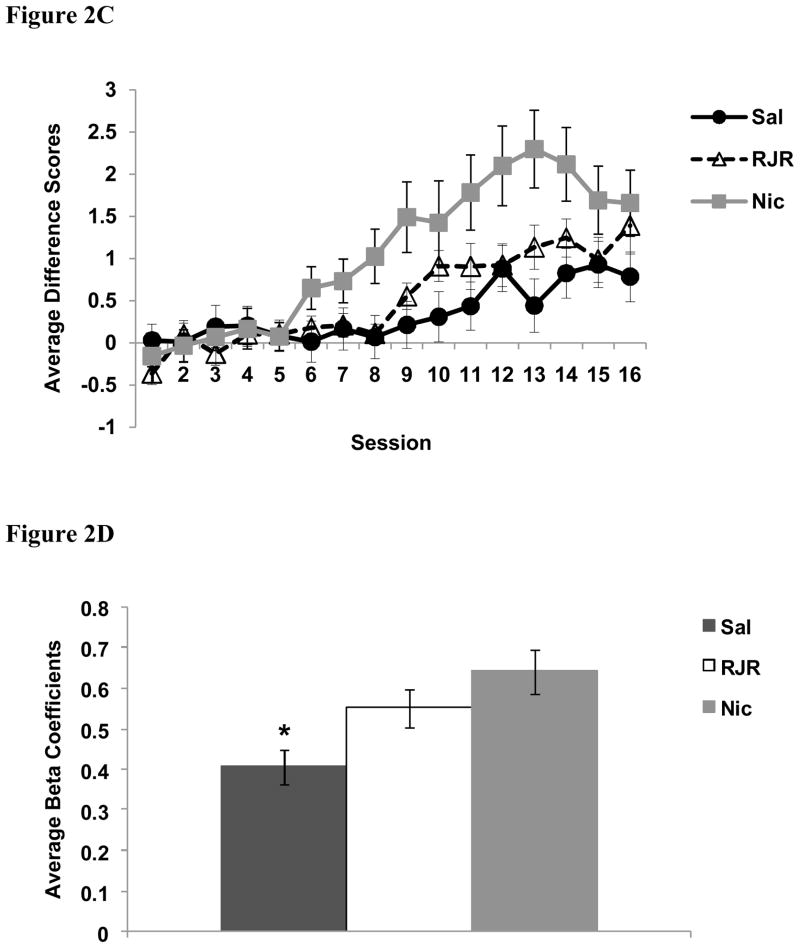
The average number of nose-pokes into the food-cup during presentation of the tone on reinforced and non-reinforced trials is illustrated in Figure 2. A univariate ANOVA of beta coefficients revealed a main effect of Group [F (2, 42) = 5.7, p < 0.01]. Planned orthogonal contrasts indicate that nicotine-treated rats discriminated between trials types better than saline-treated rats [t(42) = 3.2, p < 0.01], and that RJR-2403-treated rats also discriminated better than the saline group [t(42) = 2.3, p < 0.05]. However, there was no difference in discrimination between nicotine-treated rats and RJR-2403-treated rats [t(42) = 1.2, p = 0.24]. Analysis of food cup behavior during the 5-sec period before any stimuli were presented did not reveal any group differences [F (2, 42) = 2.1, p = 0.14; Saline-treated group, M = 0.5, SD = 0.3; RJR-2403 group, M = 0.6, SD = 0.4; nicotine group, M = 0.4, SD = 0.2)
Figure 2.
Average number of nose-pokes into the food cup during presentation of the tone on reinforced and non-reinforced trials for nicotine-treated and saline-treated rats (A) and RJR-2403-rats and saline-treated rats (B). Nicotine-treated rats learned to discriminate between trial types beginning on day 6, RJR-2403-treated rats on day 9, and saline-treated rats on day 11. C) Average difference in the number of nose pokes into the food cup during the presentation of the tone on the two trial types. D) Average beta coefficients reflecting the rate of discrimination for each treatment group. * = p < 0.05 (drug groups compared to saline); Data are means ± S.E.M. Abbreviations: Sal, saline; RJR, RJR-2403; Nic, nicotine; R, reinforced trials; NR, non-reinforced trials.
In addition, paired-sample t-tests indicate that nicotine-treated rats began to exhibit significant discrimination between trial types on day 6 [t(9) = 2.6, p < 0.05]. Rats treated with RJR-2403 discriminated significantly between the trial types beginning on day 9 [t(15) =3.5, p < 0.01], however saline-treated rats did not significantly discriminate until day 11 [t(18) =2.4, p < 0.05]. Follow up analyses were conducted to compare the level of responding on each trial type at asymptote. As indicated previously, all groups significantly discriminated between trial types by the end of training. Thus, trial types were evaluated individually. The level of responding during presentation of the tone on non-reinforced trials (averaged over the last four training sessions) was different between the groups [F (2,42) = 3.4, p < 0.05]. Planned orthogonal contrasts indicated that nicotine-treated rats responded less compared to both saline [t (42) = 2.0, p < 0.05] and RJR-2403-treated rats [t (42) = 2.6, p < 0.05], while there were no differences between the saline and RJR-2403 groups [t (42) = 0.8, p = 0.46]. In contrast, there were no group differences during presentation of the tone on reinforced trials [F (2, 42) = 1.3, p = 0.29].
Rearing to the visual stimulus
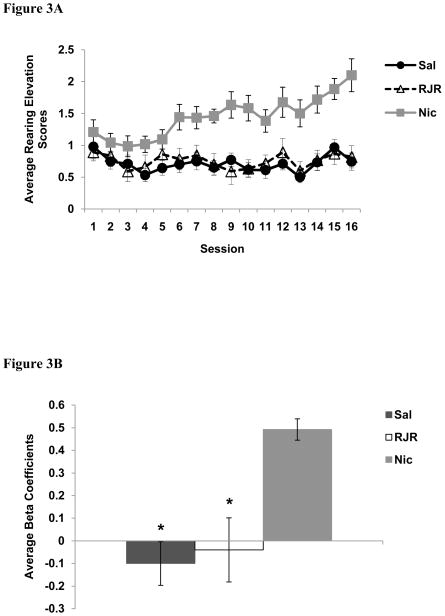
Rearing behavior observed during presentation of the light is illustrated in Figure 3. The data is presented as an elevation score, i.e., the amount of rearing observed during the light minus the amount of rearing observed prior to cue onset (pre-CS period). There was a significant main effect of Group on rearing behavior [F (2,32) =1.2, p < 0.01]. Planned orthogonal contrasts indicate that nicotine-treated rats reared significantly more during the presentation of the light compared to both saline-treated rats [t (32) = 4.1, p < 0.001] and RJR-2403-treated rats [t (32) = 3.4, p < 0.01]. There was no difference in rearing between the saline and RJR-2403 groups [t (32) = 0.4, p = 0.7].
Figure 3.
A) Average number of rears to the 5-sec presentation of the panel light minus rearing during the pre-CS period. B) Average beta coefficients for rearing for each group. * = p < 0.05 for saline vs. nicotine, and for RJR-2403 vs. nicotine; Data are means ± S.E.M.
Discussion
Consistent with the results of our previous study (MacLeod et al., 2006), nicotine administration significantly enhanced the ability of rats to discriminate between the two trial types compared to saline-treated control rats. Nicotine enhanced the magnitude of the discrimination by increasing the associability of the tone on reinforced trials and by decreasing responding to the tone on non-reinforced trials. In addition, rats treated with nicotine learned the discrimination in fewer sessions than saline controls. A significant new finding from the present study was that nicotine also increased the orienting response to the light compared to control rats, suggesting that nicotine may enhance learning the serial feature negative discrimination by increasing attention to the visual feature.
Nicotine may have enhanced inhibition of responding to the tone on non-reinforced trials due to an increase in attention to the negative occasion setter. It has been suggested that the negative feature “gates” the inhibitory properties of the target stimulus, thus allowing the target to obtain both excitatory and inhibitory characteristics (Bouton & Nelson, 1994; Holland, 1984). The increase in attention by the nicotine-treated animals observed in the current experiment could contribute to the “gating” effect of the feature (the light) over responding to the target (the tone) during non-reinforced trials as illustrated in the model depicted in Figure 1.
Interestingly, the frequency of orienting behavior increased over the course of training for rats in the nicotine group. Previous research suggests that rearing to a visual stimulus typically increases during the early phase of training when a visual stimulus is paired with a food reward, but then decreases as training continues (Kaye & Pearce, 1984). This pattern of rearing behavior has been suggested to reflect increased attention to the stimulus when it is new and the Pavlovian association between stimulus and reward is being established. Attention is then thought to decrease as the stimulus becomes an established and reliable predictor of reward (Kaye & Pearce, 1984). While this is true for a visual stimulus directly paired with a reward, our data suggest a different, perhaps non-declining attentional process is required for successfully reducing responding during the tone on non-reinforced trials in this task. Indeed, nicotine increased the orienting response to the light during the second half of training, coincident with successful discrimination between trial types.
A second goal of this study was to determine whether α4β2 nicotinic receptors mediated the effects of nicotine on occasion setting. RJR-2403, a specific α4β2 nicotinic receptor agonist, mimicked a subset of the effects produced by nicotine. Like nicotine, RJR-2403 administration enhanced discrimination and decreased the number of sessions to learn the discrimination compared to saline control rats. However, unlike nicotine, RJR-2403 did not affect asymptotic levels of responding to the tone on non-reinforced trials. Consistent with this finding, RJR-2403 also did not affect orienting to the light. Thus, although RJR-2403 enhanced discrimination in this task, this was apparently not due to an increase in attentional processing of the light or enhanced inhibition of responding to the tone on non-reinforced trials. One interpretation of these data is that nicotine may act to increase learning about the tone-food relationship through stimulation of α4β2 receptors. However, the effect of nicotine on attention to the light and decreased responding to the tone on L→T trials may be mediated by other receptor subtypes.
These interpretations are supported by the results of previous studies that have investigated the role of different nicotinic receptor subtypes in learning and attention. For example, administration of nicotine has been shown to enhance performance in the five-choice serial reaction time task by increasing correct responses and decreasing the latency to emit the response. These effects are blocked by co-administration of a selective α4β2 nicotinic receptor antagonist (Blondel, Sanger, & Moser, 2000). Similarly, within-session declines in performance exhibited by aged rats were reversed following treatment with either nicotine or α4β2 receptor agonists (Grottick & Higgins, 2002). Again, co-administration of an α4β2 antagonist with nicotine disrupted the enhancement observed in aged animals. The effect of nicotine on decreasing response time and increasing the number of correct responses in these prior studies may be akin to the enhancement of tone-food associability observed in the nicotine-treated rats and RJR-2403-treated rats in the present study. Thus, it is likely that α4β2 nicotinic receptors mediate reward associability in these tasks. Yet, although RJR-2403-treated rats were able to discriminate between trial types sooner than controls, they did not learn the discrimination as quickly as nicotine-treated rats, perhaps due to the added effect of nicotine on non-α4β2 nicotinic receptors.
Different nicotinic receptor configurations bind acetylcholine and nicotine to varying degrees (Gotti et al., 2007). Thus, drugs that target specific configurations can influence behavior differentially. Indeed, α7 nicotinic receptors have also been shown to modulate learning, memory, and attention (Rushforth, Allison, Wonnacott, & Shoaib, 2010; Young et al., 2004; Levin, 2002; Levin, Bradley, Addy, & Sigurani, 2002). While α7 nicotinic antagonists fail to reduce the enhancing effects of nicotine in the five-choice serial reaction time task (Blondel et al., 2000), agonists of this receptor subtype do enhance working memory in radial arm maze tasks (Levin, 2002) and both α7 and α4β2 receptor agonists improve olfactory working memory (Rushforth, Allison, Wonnacott, & Shoaib, 2010). Like these tasks, the negative occasion setting paradigm includes a component of working memory. Due to the ability of the negative feature to modulate behavior to the target stimulus, learning the negative occasion setting paradigm relies on encoding the meaning of the feature and maintaining the memory trace throughout the empty period until presentation of the target in order to correctly discriminate between trial types (Holland, 1984). Thus, nicotine in the negative occasion setting paradigm may enhance discrimination by both enhancing tone-reward associability through α4β2 receptors and by enhancing working memory, possibly through another nicotinic receptor subtype. Indeed, systemic pretreatment with an α4β2 antagonist before nicotine administration attenuated the response-enhancing effects on nicotine in a lever-press task, whereas pretreatment with an α7 receptor antagonist did not (Grottick & Higgins, 2002).
The current findings add to a small but growing literature regarding the role of nicotinic acetylcholine receptors in modulating various aspects of inhibitory behavior in rats. In addition, these findings also have implications for understanding the effects of nicotine in recent studies in humans. For example, the negative occasion setting paradigm shares several procedural similarities with go/no-go paradigms and the Stop Signal Task used to assess inhibition in normal humans as well as clinical populations (Rubia, Russell, Bullmore, Soni et al., 2001; Vaidya, Austin, Kirkorian, Ridlehuber et al., 1998). Nicotine has been shown to enhance behavioral inhibition in persons with Attention-Deficit/Hyperactivity Disorder, as measured with the Stop signal Task (Potter & Newhouse, 2004, 2008). Furthermore, other recent studies support the use of learned inhibition procedures to assess inhibitory behavior in persons with psychopathology (Migo, Corbett, Graham, Smith et al., 2006). The use of translational models such as these may aid in the further description of the neural substrates underlying nicotine abuse as well as potential treatments for an array of cognitive disorders.
Acknowledgments
Research funding was provided by a NARSAD Young Investigator Award and NIH Grant MH069670. The authors thank Michael Hopkins for valuable discussion of the data.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
References
- Baxter MG, Holland PC, Gallagher M. Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J Neurosci. 1997;17(13):5230–6. doi: 10.1523/JNEUROSCI.17-13-05230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel A, Sanger DJ, Moser PC. Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology. 2000;149(3):293–305. doi: 10.1007/s002130000378. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Nelson JB. Context-specificity of target versus feature inhibition in a feature-negative discrimination. Journal of Experimental Psychology: Animal Behavior Process. 1994;20(1):51–65. [PubMed] [Google Scholar]
- Bueno JLO, Holland PC. Occasion setting in Pavlovian ambiguous target discriminations. Behavioural Processes. 2008;79(3):132–147. doi: 10.1016/j.beproc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Felix R, Levin ED. Nicotinic antagonist administration into ventral hippocampus and spatial working memory in rats. Neuroscience. 1997;81(4):1009–1017. doi: 10.1016/s0306-4522(97)00224-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. Journal of Neuroscience. 1990;10(6):1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner J. Nicotine enhancement of contextual fear conditioning. Behavioral Brain Research. 1999;102(1–2):31–39. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Higgins GA. Assessing vigalance decrement in aged rats: effects of pre- feeding, task manipulation, and psychostimulants. Psychopharmacology. 2002;164(1):33–41. doi: 10.1007/s00213-002-1174-3. [DOI] [PubMed] [Google Scholar]
- Holland PC. Conditioned stimulus as a determinant of the form of the Pavlovian conditioned response. Journal of Experimental Psychology Animal Behavior Processes. 1977;3(1):77–104. doi: 10.1037//0097-7403.3.1.77. [DOI] [PubMed] [Google Scholar]
- Holland PC. Differential effects of reinforcement of an inhibitory feature after serial and simultaneous feature negative discrimination training. Journal of Experimental Psychology: Animal Behavior Process. 1984;10(4):461–475. [PubMed] [Google Scholar]
- Holland PC, Lamoureux JA, Han JS, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9(2):143–157. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Holland PC, Morell JR. The effects of intertrial and feature-target intervals on operant serial feature negative discrimination learning. Learning and Motivation. 1996;27:21–42. [Google Scholar]
- Hopkins ME, Sharma M, Evans GC, Bucci DJ. Voluntary pysical exercise alters attentional orienting and social behavior in a rat model of attention-deficit/hyperactivity disorder. Behavioral Neuroscience. 2009;123(3):599–606. doi: 10.1037/a0015632. [DOI] [PubMed] [Google Scholar]
- Kaye H, Pearce JM. The strength of the orienting response during Pavlovian conditioning. Journal of Experimental Psychology: Animal Behavior Process. 1984;10(1):90–109. [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Automated measure of conditioned orienting behavior in rats. Behavioral Research Methods. 2007;39(2):303–308. doi: 10.3758/bf03193161. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neuroscience and Biobehavioral Reviews. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Simons RF, Balaban MT. Attention and Orienting: Sensory and Motivational Processes. Mahwah: NJ: Lawrence Erlbaum Associates; 1997. [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology. 2002;53(4):633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Bradley A, Addy N, Sigurani N. Hippocampal [alpha]7 and [alpha]4[beta]2 nicotinic receptors and working memory. Neuroscience. 2002;109(4):757–765. doi: 10.1016/s0306-4522(01)00538-3. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140(2):135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Bencherif M, Gray JA, Peters S, Grigoryan G, Hodges H, Collins AC. RJR-2403: a nicotinic agonist with CNS selectivity II. In vivo characterization. Journal of Pharmacology and Experimental Therapeutics. 1996;279(3):1422–1429. [PubMed] [Google Scholar]
- McGaughy J, Decker MW, Sarter M. Enhancement of sustained attention performance by the nicotinic acetylcholine receptor agonist ABT-418 in intact but not basal forebrain-lesioned rats. Psycholpharmacology. 1999;144(2):175–182. doi: 10.1007/s002130050991. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Potter AS, Simoni MK, Bucci DJ. Nicotine administration enhances conditioned inhibition in rats. European Journal of Pharmacology. 2006;551(1–3):76–79. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migo EM, Corbett K, Graham J, Smith S, Tate S, Moran PM, Cassaday HJ. A novel test of conditioned inhibition correlates with personality measures of schizotypy and reward sensitivity. Behavioural Brain Research. 2006;168(2):299–306. doi: 10.1016/j.bbr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Ohno M, Yamamoto T, Watanabe S. Blockade of hippocampal nicotinic receptors impairs working memory but not reference memory in rats. Pharmacology, Biochemistry, and Behavior. 1993;45(1):89–93. doi: 10.1016/0091-3057(93)90091-7. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhous PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl) 2004;176(2):182–94. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology Biochemistry and Behavior. 2008;88(4):407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AHP, WF, editors. Classical Conditioning II. New York: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- Rubia K, Russell T, Bullmore ET, Soni W, Brammer MJ, Simmons A, Taylor E, Andrew C, Giampietro V, Sharma T. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophrenia Research. 2001;52(1–2):47–55. doi: 10.1016/s0920-9964(00)00173-0. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: A novel use of the odour span task. Neuroscience Letters. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JDE. Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(24):14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youg JW, Finlayson K, Spratt C, Marston HM, Crawford N, Kelly JS, Sharkey J. Nicotine improves sustained attention in mice: Evidence for involvement of the α7 nicotinic acetylcholine receptor. Neuropsychopharmacology. 2004;29:891–900. doi: 10.1038/sj.npp.1300393. [DOI] [PubMed] [Google Scholar]