Abstract
Chronic exposure to low doses of arsenite causes transformation of human osteogenic sarcoma (HOS) cells. Although oxidative stress is considered important in arsenite-induced cell transformation, the molecular and cellular mechanisms by which arsenite transforms human cells are still unknown. In the present study, we investigated whether altered iron homeostasis, known to affect cellular oxidative stress, can contribute to the arsenite-mediated cell transformation. Using arsenite-induced HOS cell transformation as a model, it was found that total iron levels are significantly higher in transformed HOS cells in comparison to parental control HOS cells. Under normal iron metabolism conditions, iron homeostasis is tightly controlled by inverse regulation of ferritin and transferrin receptor (TfR) through iron regulatory proteins (IRP). Increased iron levels in arsenite transformed cells should theoretically lead to higher ferritin and lower TfR in these cells than in controls. However, the results showed that both ferritin and TfR are decreased, apparently through two different mechanisms. A lower ferritin level in cytoplasm was due to the decreased mRNA in the arsenite-transformed HOS cells, while the decline in TfR was due to a lowered IRP-binding activity. By challenging cells with iron, it was further established that arsenite-transformed HOS cells are less responsive to iron treatment than control HOS cells, which allows accumulation of iron in the transformed cells, as exemplified by significantly lower ferritin induction. On the other hand, caffeic acid phenethyl ester (CAPE), an antioxidant previously shown to suppress As-mediated cell transformation, prevents As-mediated ferritin depletion. In conclusion, our results suggest that altered iron homeostasis contributes to arsenite-induced oxidative stress and, thus, may be involved in arsenite-mediated cell transformation.
Keywords: Arsenic, Cell transformation, Iron homeostasis, Ferritin, Transferrin receptor, Iron regulatory proteins, Free radicals
Introduction
Arsenic, a human carcinogen, has been widely used in various professions such as in wood preservation [1]. For example, the demand for arsenic in the United States. will continue to correlate closely with demands for new housing and growth in the renovation or replacement of existing structures using pressure-treated lumber [2,3]. Arsenic compounds were the only compounds that IARC considered to have sufficient evidence for human carcinogenicity. Some studies confirm that arsenite is not significantly mutagenic in bacterial or mammalian cell detection systems, so it is sometimes considered a tumor promoter and, more recently, was shown to be a co-carcinogen in mouse models [4,5]. Other studies show that arsenite is a complete transplacental carcinogen in mice [6], while dimethylarsinic acid, a major metabolite of arsenic in most mammals, including humans, causes bladder cancer in F344 rats [7]. In contrast to its weak mutagenicity, arsenite induces cell transformation of various types of cells to a more malignant phenotype, such as Syrian hamster embryo cells [8], mouse embryo fibroblasts [9], mouse epidermal JB6 C141 cells [10], rat liver-derived TRL 1215 cells [11], normal human immortalized prostate epithelium RWPE-1 cells [12], as well as human osteogenic sarcoma (HOS) cells [13,14]. Many mechanisms involving arsenic-mediated cell transformation have been proposed. These include genomic instability [9], changes in cell signaling [10], and DNA hypomethylation [11,15]. Arsenite exposure was found to induce oxidative stress in cells [16–18]. However, there is not enough cellular evidence linking arsenic directly to oxidative changes in arsenite-induced cell transformation.
Iron is an essential element, which plays an important role in survival, replication, and differentiation of cells in animals, plants, and almost all microorganisms [19]. Iron is easily cycled between two redox states, ferrous ions (Fe2+) and ferric ions (Fe3+), which provide electrons for enzymatic and free radical reactions [20,21]. Although iron deficiency can cause anemia leading to decreased oxygen transport [22], its excess can also cause damage leading to adverse health effects, such as inflammation and cancer [23–32]. The damage caused by excess iron is thought to be mediated by redox cycling between ferrous and ferric states, via Haber-Weiss, Fenton, or autoxidation reactions, producing powerful oxygen free radicals [33–35]. In order to maintain this delicate balance of iron states, organisms must have a timely way to deliver iron to cells and in necessary amounts, while storing excess iron for times of need.
Transferrin receptor (TfR) and ferritin are two major proteins that assure homeostasis of cellular iron [36]. TfR is a dimeric membrane-bound protein that binds iron-containing transferrin for cellular uptake. Ferritin is an iron-storage protein with a capacity to bind up to 4500 atoms of iron per molecule of ferritin. These two proteins are inversely regulated by iron-regulatory proteins (IRP) [37–39]. The IRP exhibits posttranscriptional control by binding to iron-responsive elements (IRE), present in ferritin and TfR mRNA. IRP binding responds to changes in the intracellular iron level in a coordinate manner by differentially regulating ferritin mRNA’s translational efficiency and TfR mRNA’s stability [37–40]. The intracellular iron homeostasis is maintained by the IRP-IRE interaction that up-regulates ferritin synthesis and down-regulates TfR when cellular iron is high or causing opposite effects when cellular iron is low [37].
The goal of the present study was to investigate whether arsenite alters iron homeostasis in cells during the transformation process. Using a previously developed model of a low-dose and long-term exposure to arsenite [14], we obtained transformed human osteogenic sarcoma (As-T-HOS) cells, which have exhibited anchorage independence [13]. We found that arsenite can affect iron homeostasis through an abnormal iron regulation mechanism. Our study provides a plausible explanation of how altered iron homeostasis can contribute to arsenite-induced oxidative stress and cell transformation.
Materials and methods
Materials
Sodium arsenite (NaAsO2, As), ferric ammonium citrate (FAC), SITE liquid media supplement (1.0 mg/ml insulin from bovine pancreas, 0.55 mg/ml human transferrin, 0.5 µg/ml sodium selenite, and 0.2 mg/µl ethanolamine), 2-mercaptoethanol, monoclonal mouse-anti-β-tubulin antibodies, tetramethylbenzidine (TMB), bicinchoninic acid kit, caffeic acid phenethyl ester (CAPE), and minimum essential medium alpha (α-MEM) were obtained from Sigma Chemical Company (St. Louis, MO). Fetal bovine serum (FBS) was from Atlanta Biologicals (Norcross, GA). Ferritin standard, anti-ferritin antibodies, and mouse-anti-TfR antibodies were purchased from Research Diagnostics Inc. (Flanders, NJ). Other products were as follows: peroxidase-conjugated anti-mouse antibody (Cell Signaling Technology, Inc., Beverly, MA), radioimmunoprecipitation assay (RIPA) lysis buffer (Upstate Inc., Lake Placid, NY), complete proteinase inhibitor cocktail (Roche Molecular Biochemicals Inc., Indianapolis, IN).
Cell culture
HOS cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Control parental HOS cells were cultured in α-MEM medium containing 5 or 10% FBS. Two types of As-treated cells were generated by incubating cells with a low dose of arsenite (0.1 µM), as previously described [13,14]. Cells designated here as As-8w-HOS were cells that were chronically exposed to NaAsO2 for 8 weeks. These cells exhibit higher growth density and altered morphology and grow in soft agar. Cells designated as As-T-HOS were As-8w-HOS cells that were cloned in soft agar and then propagated in normal medium. In this study, parental HOS cells, As-8w-HOS cells, and As-T-HOS cells were cultured in arsenite-free α-MEM for over 2 weeks prior to the assessment of the endpoints. After reaching 80–90% confluence, cells were washed with ice-cold phosphate-buffered saline (PBS) and lysed in various buffers containing complete protease inhibitor cocktail. After centrifugation, the supernatants were used for the assays. Protein concentration was determined by the bicinchoninic acid (BCA) method using bovine serum albumin as the standard.
Investigation of alterations in iron homeostasis
Total iron and arsenite
To determine intrinsic levels of iron and arsenic in parental HOS and As-8w-HOS cells, cells were lysed with 3.0 ml 0.2% nitric acid (HNO3, optima grade). Total iron or arsenic present in the supernatants was analyzed by atomic absorption spectroscopy, using a Graphite Furnace atomic absorption analyzer (Model GF95, Thermo Electron Corp, Madison, WI) in the NIEHS and Superfund Resource at NYU School of Medicine. Iron and arsenic were measured at 248.3 and 193.7 nm, respectively.
TfR and ferritin gene expression and their regulation
To investigate chronic effects of arsenite on iron homeostasis, protein and mRNA levels of TfR and ferritin, as well as IRP binding, were studied among the three types of HOS cells. Parental HOS, As-8w-HOS, and As-T-HOS were cultured in α-MEM and then lysed in RIPA cell lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.25% deoxycholic acid, 1% NP-40, 1 mM EDTA).
TfR levels were determined by Western blotting as follows: cell lysates (30 µg protein) were subjected to 12% SDS-polyacrylamide gel electrophoresis. After transferring onto nitrocellulose membranes, the membranes were blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST), and then probed with antibodies against TfR (1:500) and β-tubulin (1:3000) together. Antibody banding was visualized with peroxidase-conjugated anti-mouse antibody using Western Lightning Plus Chemiluminescence Reagent (Perkin-Elmer).
Levels of ferritin in cell lysates were determined according to previously published protocol [41]. In brief, antibody to a mixture of human spleen and liver ferritin was used as the capture antibody to coat an ELISA plate, followed by incubation with cell lysates. The conjugate of peroxidase and antibody to human spleen and liver ferritin was then added to serve as the detector to determine the amount of ferritin bound to the capture antibody. TMB was then added as the substrate for the peroxidase, and the absorbance of the oxidation product of TMB was determined at 450 nm using a microplate reader (SpectroMax Plus, Molecular Devices, Sunnyvale, CA). Total protein in the cell lysates was determined using bicinchoninic acid, and the results were expressed as naogram of ferritin per milligram of protein.
Levels of TfR and ferritin mRNA were measured as follows: Total RNA was isolated using TRIzol extraction reagent (Invitrogen Life Technologies, Inc., Carlsbad, CA), and reverse transcription was carried out following the manufacturer’s instructions. The resultant cDNA was amplified by semiquantitative RT-PCR as well as quantitative real-time RT-PCR for H-chain ferritin, TfR, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the following gene-specific primers: H-ferritin, 5′-CGC CAG AAC TAC CAC CAG GAC-3′ and 5′-GGA AGT CAC CCC ACG GCT ATG-3′; TfR, 5′-CAG CCC AGC AGA AGC ATT ATC-3′; and 5′-GGA AGT AGC ACG GAA GAA GTC-3′; GAPDH, 5′-CGG AGT CAA CGC ATT TGG TCG TAT-3′ and 5′-AGC CTT CTC CAT GGT TGG TGA AGA C-3′.
Semiquantitative PCR conditions were previously described [42], while quantitative real-time PCR was performed using LightCycler – FastStart DNA Master SYBR Green kit and LightCycler PCR instrument (Roche), according to the manufacturer’s instructions. GAPDH was an internal control.
The binding activities of IRP to IRE were examined by RNA gel-shift assay. Cells grown in different medium (5% FBS or serum-free α-MEM) were separately evaluated. The cells were lysed in a lysis buffer containing10 mM Hepes, pH 7.5, 3 mM MgCl2, 40 mM KCl, 5% glycerol, 0.3% NP-40, and a cocktail of proteinase inhibitors. The 32P-labeled IRE probe was synthesized by in vitro transcription using the linearized pSPT-fer plasmid as the template (a kind gift from Dr. L.C. Kuühn, ISREC, Switzerland), which contains human ferritin H chain IRE. RNA gel-shift assay was performed as previously described [43]. Briefly, samples were equally divided into two parts; 2-mercaptoethanol (2-ME) was added to one part of the sample at a 2% final concentration. The addition of 2-ME creates a reducing environment in the sample, which facilitates maximal binding of all present IRP proteins. The sample with 2-ME (+ME) was used as the internal control for IRP-binding normalization of the same sample but without 2-ME (−ME). Two parts of the same sample (2 µg proteins each) were incubated with an excess amount of 32P-labeled IRE probe (4 × 104 cpm) in 20 µl reaction buffer (10 mM Hepes, pH 7.5, 3 mM MgCl2, 40 mM KCl, 5% glycerol, 0.07% NP-40, and Complete Proteinase Inhibitor Cocktail). After 20 min incubation at room temperature, 1 unit of RNase and 100 µg of heparin were added, and the mixture was incubated for an additional 10 min. The reaction mixture was separated on a 5% nondenaturing polyacrylamide gel (Bio-Rad). The gel was dried and the radioactivity assessed by exposure to X- ray film. The IRP binding was calculated by comparing the band density of the sample without 2-ME to the same sample with 2-ME added.
Measurement of ferritin in cytoplasm and nuclei of As-treated cells with or without CAPE
To determine the protect effect of CAPE on As-mediated alteration of iron homeostasis as well as the distribution of ferritin in the cytosolic and nuclear fractions, HOS cells were treated with 0.1 µM arsenite in the presence or absence of CAPE (1 µM) for 8 weeks. After treatment, cells were collected and fractioned with a nuclear extraction kit (Panomics, Inc., Redwood City, CA). Ferritin levels in the cytoplasm and nuclei were measured as described above.
Ferritin induction assay
To verify the decreased mRNA levels of ferritin in As-8w-HOS and As-T-HOS cells, the three types of HOS cells were further challenged with iron to determine whether ferritin induction varies among the cell types. Parental HOS cells, As-8w-HOS, and As-T-HOS cells were seeded in 6-well plates at 2 × 106 cells /well in 2 ml complete α-MEM. After attachment, medium was changed to serum-free SITE liquid medium (Sigma). Cells were then treated with freshly prepared FAC at final concentrations of 0, 75, and 300 µM. After incubation for 6 and 18 h, cell lysates in RIPA cell lysis buffer were used to detect ferritin by ELISA [41].
Time course of As-induced ferritin degradation
Ferritin content in HOS cells was also measured as a function of time of a low dose of arsenite treatment (0.1 µM). Cells were collected at 2, 4, 6, and 8 week exposure time points and lysed in RIPA buffer for ferritin measurements.
Statistical analysis
The experimental differences were determined by two-tailed Student’s t test. To assure reproducibility, the experiments were repeated at least three times (except the IRP-binding assay). Graphed data represent the means ± SE of three or four experiments. A confidence level of P < 0.05 was taken to represent a significant difference in all cases.
Results
Chronic arsenite treatment increases total iron content in HOS cells
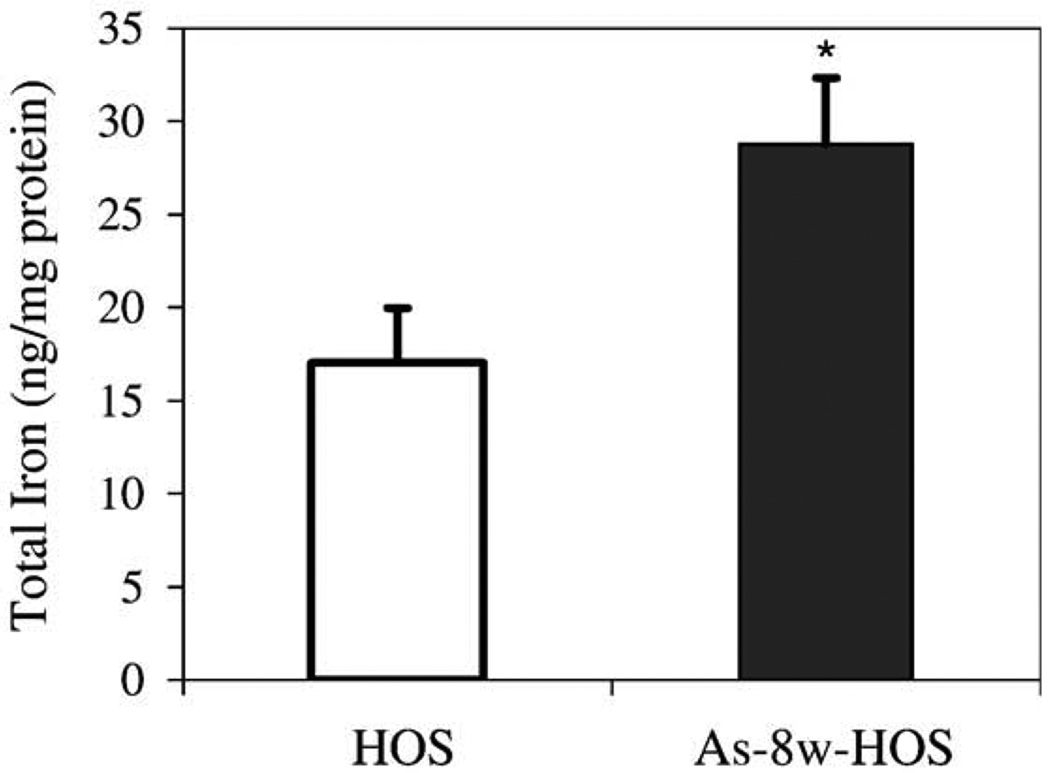
The effects of arsenite treatment on the total iron content in HOS cells are shown in Fig. 1. The results showed that 8-week arsenite treatment significantly increases the total iron content in As-8w-HOS cells (28.8 ± 3.6 ng/mg protein) as compared to parental control HOS cells (17.0 ± 2.9 ng/mg protein, P < 0.05). Because As-8w-HOS cells are capable of growth in soft agar, a significant increase in the total iron content suggests that iron homeostasis has been altered during the arsenite-induced HOS cell transformation. Since arsenic was undetectable by atomic absorption in both cell types (As-8w-HOS and As-T-HOS), the increase in total iron content is an intrinsic property of the arsenite-transformed cells.
Fig. 1.
Difference in the total iron content between parental HOS and As-8w-HOS cells. HOS cells were continuously cultured and passaged for 8 weeks in the absence (HOS controls) or presence of 0.1 µM sodium arsenite (chronic As exposure) in α-MEM completed with 10% FBS. Both parental HOS and As-8w-HOS cells were subsequently cultured in α-MEM with 5% FBS arsenite-free media for over 2 weeks prior to the measurement of total iron by atomic absorption. Results are presented as means ± SE from four independent experiments. *Significantly different from parental HOS cells (P < 0.05).
Decreased TfR protein levels in As-8w-HOS and As-T-HOS cells
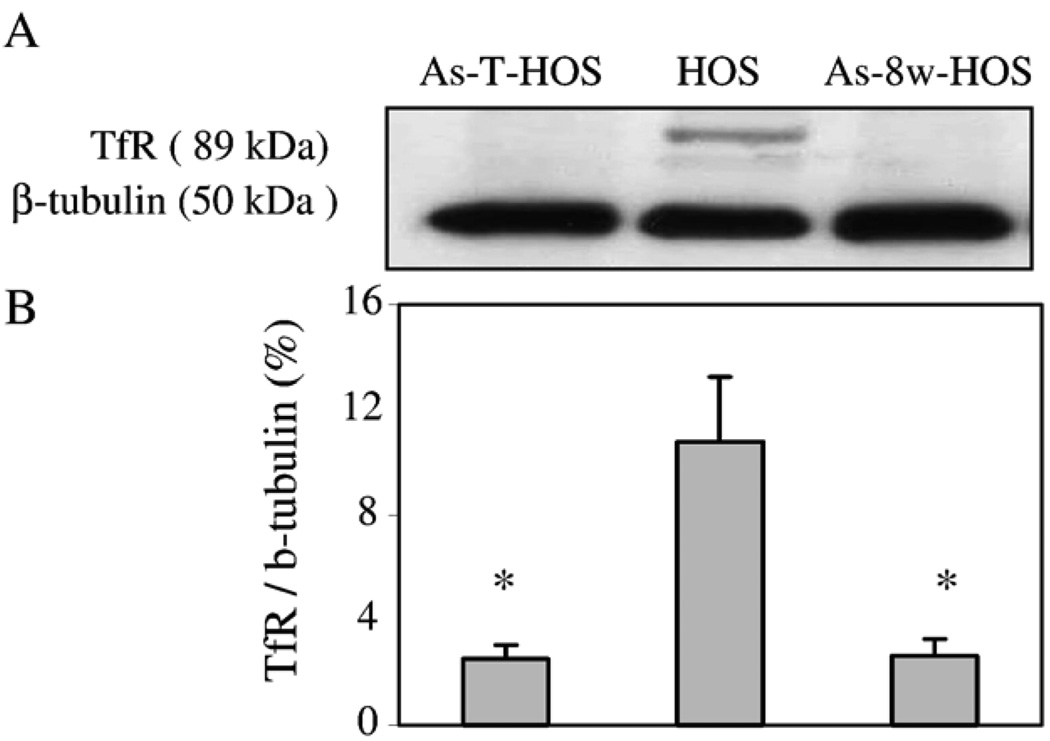
Because of the observed changes in the total iron content, we have assessed whether these changes are related to iron metabolism proteins. TfR is a protein controlling cellular iron uptake. Western blotting showed that control HOS cells express TfR (Fig. 2). However, after 8 weeks of treatment with arsenite, the expression of TfR in As-8w-HOS cells was dramatically decreased (P < 0.05). This decreased TfR expression was stably passed onto As-T-HOS cells (Fig. 2).
Fig. 2.
Effects of chronic arsenite exposure on TfR expression. Western blotting analysis was performed on cellular lysates prepared from the parental HOS, As-8w-HOS, and As-T-HOS cells using specific TfR and β-tubulin antibodies (A). The densities of TfR and β-tubulin signals were analyzed by the UN-SCAN-IT software version 4.1 (Silk Scientific Corp. Orem, UT) and the ratio of TfR/β-tubulin was calculated (B). Data represent the means ± SE from three independent experiments. *Significantly different from parental HOS cells (P < 0.05).
Decreased ferritin and TfR mRNA levels in As-8w-HOS cells
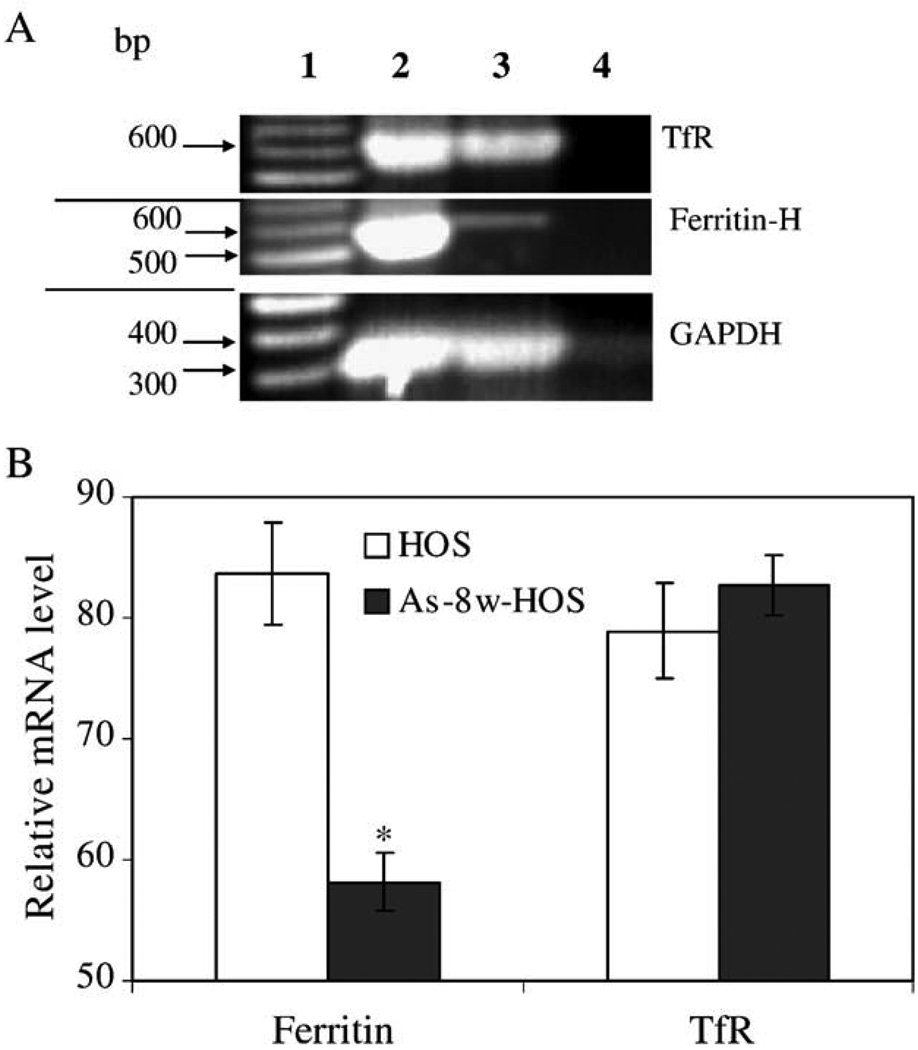
To determine whether decreased TfR in As-8w-HOS cells are the result of decreased mRNA levels, semiquantitative RT-PCR and quantitative real-time PCR were utilized to determine TfR mRNA and ferritin mRNA. The results of semiquantitative PCR showed that ferritin mRNA was dramatically decreased in As-8w-HOS cells (Fig. 3A). However, the TfR mRNA in As-8w-HOS cells was almost the same as in the parental HOS cells. Real-time PCR quantitative results showed that ferritin mRNAwas 30% lower in As-8w-HOS cells than in the parental HOS cells. In contrast, TfR mRNA expression did not significantly differ between the parental and the As-8w-HOS cells (Fig. 3B).
Fig. 3.
Ferritin and TfR mRNA levels in parental HOS and As-8w-HOS cells. Panel A is a representative gel photograph of semiquantitative RT-PCR. Lane 1, DNA markers; Lane 2, parental HOS cells; Lane 3, As-8w-HOS cells; and Lane 4, non-reverse-transcribed DNA template. Panel B depicts quantitative results from the real-time RT-PCR using GAPDH as an internal control. Samples used for the real-time RT-PCR are different from samples used in semiquantitative RT-PCR. The patterns of ferritin and TfR mRNA alterations were similar in the two types of RT-PCR. Note: Relative mRNA level (Y axis) crosses X axis at 50%.
Decreased IRP-binding activity in As-8w-HOS cells
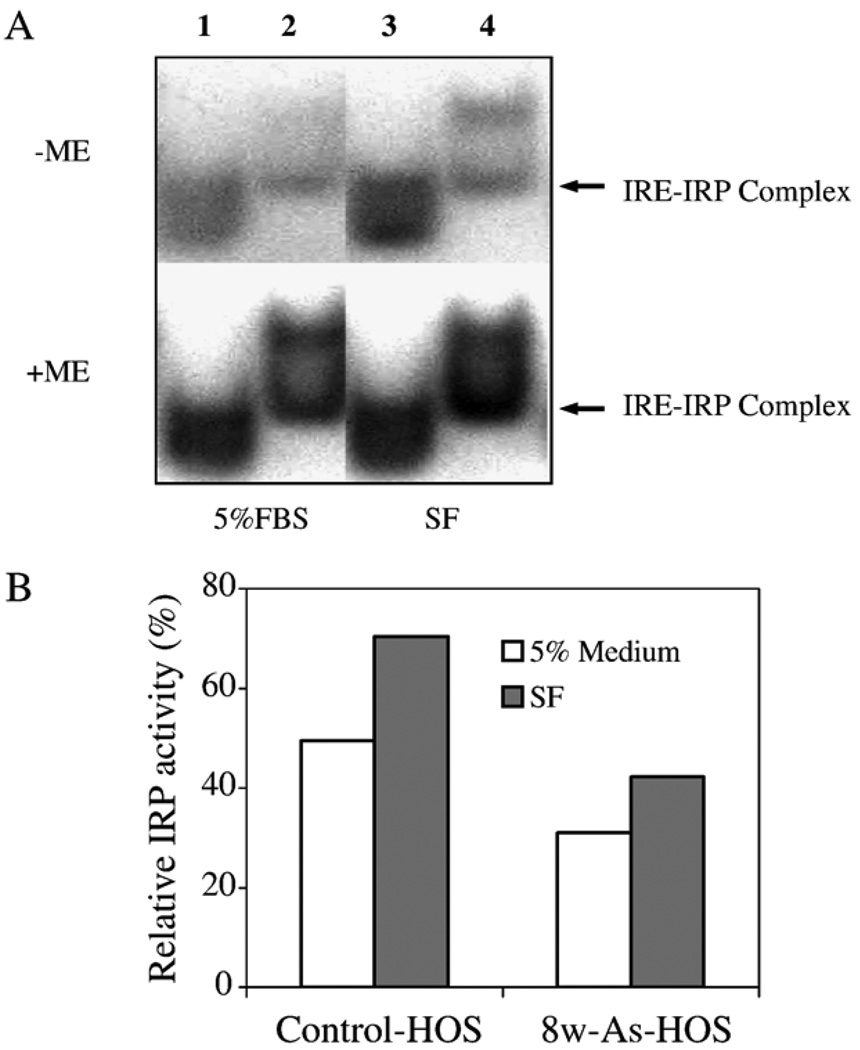
The insignificant differences in TfR mRNA levels led us to assess IRP-binding activities in the parental HOS and As-8w-HOS cells. Fig. 4A shows that IRP-binding activities are very different in the two cell types grown in either 5% FBS or serum-free medium. In general, the two human IRPs do not separate during gel-shift analyses. Hence, the detected effect was on the total IRP (IRP1 plus IRP2)-binding activity (Lanes 1 and 3 of the parental HOS cells). It is interesting to note that there are two bands in As-8w-HOS cells (Lanes 2 and 4). Based on the IRE–IRP complex position in the control parental HOS cell, it is thought that the lower band is an IRE–IRP complex of As-8w-HOS.
Fig. 4.
Effects of chronic arsenite exposure on IRP-binding activity. Cytoplasmic lysates were incubated with an excess of 32P-labeled IRE probe in the presence (+) or absence (−) of 2% 2-mercaptoethanol (ME). RNA–protein complexes were separated on nondenaturing 6% polyacrylamide gels and visualized by autoradiography (A). Lane 1, the parental HOS cells, and Lane 2, As-8w- HOS cells, both in 5% FBS medium; Lane 3, parental HOS cells, and Lane 4, As-8w-HOS cells, both maintained in serum-free (SF) medium. The quantification of IRP-binding activity is shown in Panel B. The data in Panel B represents mean value of two independent determinations.
The IRP activity in different samples was quantified by normalizing the IRP activities measured in the absence of 2-ME to the total IRP that was determined in the presence of 2-ME. Fig. 4B demonstrates that regardless of whether control HOS cells or As-8w-HOS cells were used, IRP activities were higher in serum-free (SF) medium than in 5% FBS supplemented medium. Since SF mediums represent an iron deprivation state, the increased IRP activity is expected. Fig. 4B also demonstrates that IRP activity was markedly repressed in As-8w-HOS when compared to control HOS cells in both types of media (SF and 5% FBS).
Ferritin in cytoplasm and nuclei of As-treated cells with or without CAPE
Table 1 shows that arsenite treatment depletes ferritin in both cytoplasm and nuclei as compared to the control HOS cells. Interestingly, the ferritin levels were much more significantly depleted by As in cytoplasm than in nuclei, suggesting that the defense system against oxidative stress in cytoplasm is impaired by a long-term low-dose As treatment. CAPE, a naturally occurring antioxidant, which was previously shown to prevent As-mediated HOS cell transformation [14], also partially prevented As-mediated ferritin depletion in cytoplasm and completely restored ferritin levels in nuclei. In the absence of As, CAPE had no effect on ferritin levels in HOS cells as compared to the untreated control cells.
Table 1.
Distribution of ferritin in cytoplasm and nuclei with or without arsenite and CAPE
| Treatment | Ferritin (ng/mg protein) | |
|---|---|---|
| Cytoplasm | Nuclei | |
| Control HOS | 240.0 ± 22.7 | 29.4 ± 3.1 |
| HOS + As | 1.0 ± 0.3a | 13.6 ± 1.3a |
| HOS + As + CAPE | 35.4 ± 5.8a,b | 28.6 ± 7.0 |
| HOS + CAPE | 248.3 ± 28.1 | 34.2 ± 2.6 |
Parental HOS cells were continuously treated with arsenite (0.1 µM) and/or CAPE (1 µM) for 8 weeks. After collection, cells were fractionated and ferritin levels determined as described under Materials and methods. Results are presented as the means ± SE from three independent experiments.
Significantly different from control HOS cells (P < 0.05).
Significantly different from As-treated HOS cells (P < 0.05).
Ferritin induction under iron challenge
Ferritin levels induced by iron were measured in the three types of HOS cells. Table 2 shows that parental and As-8w-HOS cells can produce ferritin in response to iron after 6 and 18 h of treatment. However, the degree of that induction was much lower in As-8w-HOS cells than in parental control HOS cells. Further, it appears that As-T-HOS cells lost the ability to produce ferritin under iron challenge. In parental HOS cells, the ferritin induction by iron was time dependent. For example, ferritin levels induced by 75 µM iron after 18 h were up to 10-fold higher than after a 6-h exposure. Moreover, parental HOS cells produced much more ferritin than the As-8w-HOS or As-T-HOS cells at the same time point and iron concentration.
Table 2.
Differences in ferritin induction among three types of HOS cells
| Cell type | Time (h) | Dose of iron (µM) | ||
|---|---|---|---|---|
| 0 | 75 | 300 | ||
| HOS | 6 | 6.69 ± 0.69c | 29.74 ± 1.07a,c | 34.95 ± 3.25a,c |
| 18 | 6.57 ± 0.15c | 289.77 ± 50.69a,b,c | 175.11 ± 34.42a,b,c | |
| As-8w-HOS | 6 | 2.52 ± 0.20 | 5.20 ± 0.67a | 5.61 ± 0.58a |
| 18 | 2.18 ± 0.08 | 7.46 ± 0.53a | 6.64 ± 0.40a | |
| As-T-HOS | 6 | 1.05 ± 0.53 | 0.87 ± 0.44 | 0.77 ± 0.47 |
| 18 | 0.82 ± 0.43 | 0.92 ± 0.46 | 1.25 ± 0.65 | |
Parental HOS cells, As-8w-HOS, and As-T-HOS cells were treated with different concentrations of FAC in serum-free media. Cells were lysed in RIPA buffer and ferritin was measured by ELISA. Results are presented as the means ± SE from three independent experiments and shown as ng ferritin per mg protein.
Significantly different from control HOS cells (P < 0.05).
Significantly different from 6-h iron treatment (P < 0.05).
Significantly different from the As-8w-HOS and As-T-HOS cells under the same experimental conditions (P < 0.05).
Changes in ferritin levels during the process of As-mediated cell transformation
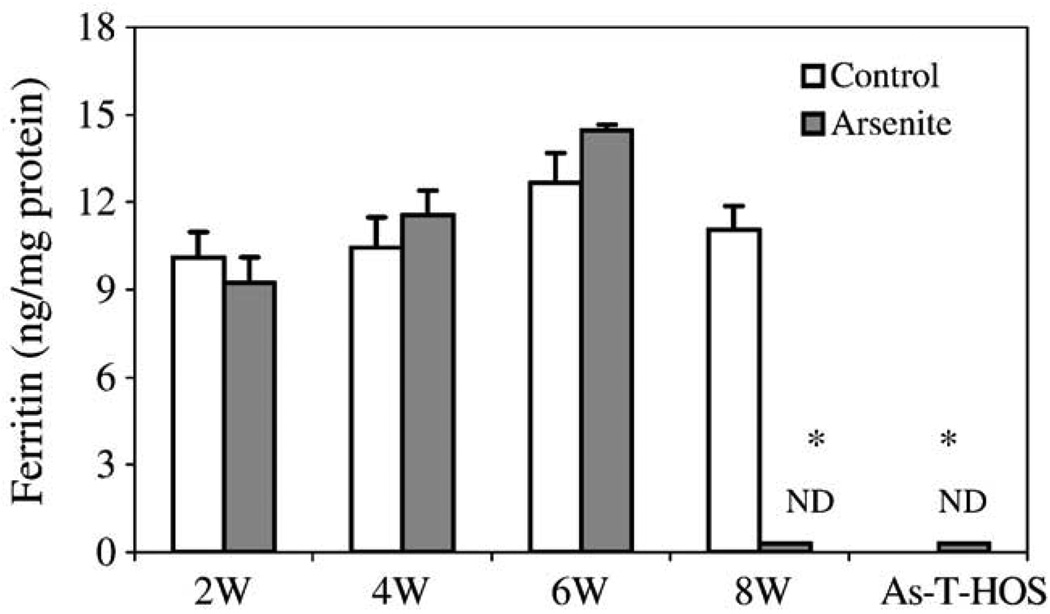
As previously described, HOS cell transformation occurred only after 8 weeks of low-dose arsenite treatment [13,14]. Ferritin levels in the parental HOS cells were measured as a function of time of exposure to 0.1 µM arsenite. The results showed that ferritin levels did not change at 2, 4, and 6 weeks (Fig. 5). However, after 8 weeks of exposure ferritin levels were dramatically decreased in comparison to the parental HOS cells grown in culture for the same amount of time but without arsenite treatment. The characteristic of low ferritin in As-8w-HOS cells was heritable because ferritin was also nondetectable in the As-T-HOS cells (HOS cells stably transformed by arsenite).
Fig. 5.
Changes in ferritin levels as a function of time of arsenite exposure. HOS cells were cultured and passaged continuously in the absence or presence of 0.1 µM arsenite. Total proteins were extracted at the end of weeks 2, 4, 6, and 8 and ferritin content was measured. For comparison, ferritin was also determined in As-T-HOS cells. Data are presented as means ± SE from three independent experiments. ND: nondetectable. *Significantly different from the 8-week cultured parental HOS cells (P < 0.05).
Discussion
Arsenic’s health effects, especially its carcinogenicity, have been a public concern for decades. Although arsenite does not react directly with DNA, it induces oxidant production and oxidative DNA damage [44], which is considered to be a contributor to arsenic-mediated carcinogenesis [4]. The chemical structure of iron and its capacity to drive one-electron reactions make iron a major component in the production and metabolism of free radicals in biological systems [45]. Our results suggest that iron homeostasis is altered by arsenite and could be involved in arsenite-mediated human cell transformation.
Intracellular iron homeostasis is maintained primarily by TfR and ferritin. Proliferating cells acquire iron through the endocytosis of iron carrier protein, transferrin bound to TfR. Generally speaking, cellular iron uptake is controlled by TfR, while excess iron is stored in ferritin in order to prevent iron-induced oxidative stress [37]. As shown under Results, the total iron was increased in As-8w-HOS cells as compared to parental HOS cells (Fig. 1). Because As-8w-HOS cells exhibit malignant characteristics, such as growth in soft agar that is lacking in parental HOS cells, these results indicate that transformed HOS cells need more iron to continue their growth [24]. Based on these initial findings, we postulated that the increased iron levels in As-8w-HOS cells are accompanied by an increase in ferritin and a decrease in TfR, a normal mechanism controlling iron homeostasis. Surprisingly, ELISA and Western blotting results showed that both ferritin and TfR are lower in As-8w-HOS as well as in As-T-HOS cells than in parental HOS cells. Combining the results of increased total iron and decreased ferritin, one could speculate that levels of “free” iron are higher in As-8w-HOS cells than in the parental HOS cells. Therefore, oxidative damage also could be higher in As-8w-HOS cells. Indeed, our preliminary data showed that levels of lipid peroxidation as well as 8-oxo-dG are higher in the As-8w-HOS cells (data not shown). These results suggest that altered iron homeostasis contributes to the observed arsenite-induced oxidative stress [4]. Considering that the chemical properties of arsenite do not favor direct oxidant formation, our finding of altered iron homeostasis provides a plausible explanation of one of the mechanisms by which arsenite increases oxidative stress. This point of view is strengthened by the fact that the significant decrease in ferritin levels, particularly in the cytoplasm (Table 1), the first line against oxidative stress, was accompanied by their growth in soft agar. CAPE, a product of the propolis of honeybee hives, possesses antioxidant and anti-inflammatory capabilities and was previously shown to prevent As-mediated HOS cell transformation [14]. Interestingly, CAPE is also able to completely prevent As-mediated ferritin depletion in the nuclei and partially in the cytoplasm (Table 1). Moreover, cells exposed to arsenite for less than 8 weeks (2–6 weeks) exhibited normal ferritin levels and did not grow in agar (Fig. 5). Overall, these results indicate that altered iron homeostasis contributes to As-induced oxidative stress and cell transformation.
The syntheses of ferritin and TfR usually are inversely controlled at the translational level by binding of the iron regulatory proteins to the iron-responsive elements located at the mRNA stem loop regulatory control sites [37–39]. At low cellular iron levels, IRP interaction with an IRE in the 5′-untranslated region (UTR) of ferritin’s subunit mRNA prevents full assembly of the ribosomal apparatus and blocks ferritin synthesis [46]. On the other hand, IRP interaction with multiple IREs in the 3′-UTR of the TfR mRNA protects that mRNA from degradation and allows continued translation of the message and synthesis of TfR protein [38]. The intracellular iron homeostasis is maintained by the iron-mediated IRP–IRE interaction that inversely regulates ferritin and TfR [47]. When iron levels are high, an iron–sulfur cluster (4Fe–4S) forms in the core of IRP-1 and prevents IRE binding [48,49], whereas IRP2 is rapidly degraded [8,48,49]. This results in a decreased IRP–IRE interaction, leading to an increased ferritin synthesis and TfR mRNA degradation.
In the present study, we found that levels of both ferritin and TfR were decreased in As-8w-HOS and As-T-HOS cells. These results suggest that iron uptake in these two cell types is abnormal. Ferritin functions as an iron-storage protein; its molecule consists of a spherical protein shell made of 24 subunits with a variable amount of iron at its core. H-chain ferritin accepts and releases iron more readily than L-chain ferritin, and H-rich ferritin shells turn over more rapidly than L-rich ferritin shells. This difference led to the suggestion that the H subunit of ferritin plays a key role in the intracellular traffic of iron [50]. Our RT-PCR results show that the H-ferritin mRNA level is lower in As-8w-HOS cells than in the control HOS cells, whereas TfR mRNA levels are virtually the same in both cell types (Fig. 3). The decreased H-chain ferritin mRNA level in As-8w-HOS cells is likely the reason for the low level of ferritin protein in As-8w-HOS cells.
Since TfR mRNA levels were not altered by the chronic low dose arsenite treatment, we then compared the IRP-binding activities between the parental and As-8w-HOS cells (Fig. 4). It can be seen that As-8w-HOS cells exhibit a greater decline in IRP binding than the parental HOS cells (Fig. 4). Although we do not understand yet the detailed mechanisms of why IRP binding is decreased or why the binding pattern is changed in As-8w-HOS cells, it is reasonable to assume that the decreased IRP binding is responsible for the observed decline in TfR protein in As-8w-HOS cells. The clearly defined function of TfR is to mediate cellular uptake of iron from transferrin. As compared to normal cells, neoplastic cells have significantly higher levels of TfR [51] and, thus, take up iron from transferrin rapidly [52,53]. Several cell lines lacking TfR have been produced [54,55] and they grow as fast as the parental cells [56]. Similarity, our results suggest that As-8w-HOS and As-T-HOS cells are capable of incorporating iron through non-TfR pathway(s). Divalent metal transporter (DMT), a protein that can transport as many as eight metals [57], may participate in the iron incorporation into the cells. This awaits further investigation.
It appears that iron homeostasis was altered at the same time as the arsenite-mediated HOS cell transformation that occurred at the end of 8 weeks of exposure. Our results suggest that the arsenite-induced decline in ferritin is one of the key factors involved in its cell-transforming potential. In fact, it was reported that the down-regulation of H-chain ferritin expression is required for cell transformation [58]. In order to confirm the role of ferritin, parental, As-8w-HOS, and As-T-HOS cells were challenged with ferric ammonium citrate. We found that HOS cells have a higher potential to produce ferritin than As-8w-HOS and As-T-HOS cells. As noted earlier, ferritin is an iron-storage protein, which can sequester “free” iron and, thus, protect cells from oxidative DNA damage [59,60]. It is likely that the decrease in the ability to produce ferritin in As-8w-HOS and As-T-HOS cells makes these cells more vulnerable to the free radical formation and consequently to DNA damage, which cumulatively contribute to arsenite-induced carcinogenesis.
Based on all the results, altered iron homeostasis is most likely involved in arsenite-mediated human cell transformation, which provides a novel view of arsenic’s carcinogenesis. These results also suggest that iron supplementation of high arsenite exposure populations should be reexamined.
Acknowledgments
This research was supported in part by NIH Grants ES10344, ES00260. We thank Juliana Powell for assistance with the preparation of the manuscript. Also, we acknowledge John Gorczynski for the Atomic Absorption measurements of iron and arsenic.
Abbreviations
- CAPE
caffeic acid phenethyl ester
- HOS
human osteogenic sarcoma
- As-T-HOS
arsenic-transformed HOS
- As-8w-HOS
HOS cells treated for 8 weeks with arsenite
- DMT
divalent metal transporter
- FAC
ferric ammonium citrate
- FBS
fetal bovine serum
- IRE
iron-responsive element
- IRP
iron regulatory protein
- ME
mercaptoethanol
- MEM
minimum essential medium
- RIPA
radioimmunoprecipitation assay
- SF
serum free
- TfR
transferrin receptor
- TMB
tetramethylbenzidine
- UTR
untranslated region
References
- 1.IARC. IARC Monograph on evaluation of carcinogenic risk to man, vol. 23. Some metal and metallic compound. Lyon, France: World Health Organization; 1980:438.
- 2.ATSDR. Agency for Toxic Substance and Disease Registry. Toxicological profile for arsenic. update (final report) Atlanta, GA: Department of Health and Human Services; 2000:466.
- 3.USGS, U. S. G. S. U.S Geological Survey. Minerals Information. Arsenic. 2000 Http://minerals.usgs.gov/minerals/pubs/commodity.myb/,2000.
- 4.Rossman TG. Mechanism of arsenic carcinogenesis: an integrated approach. Mutat. Res. 2003;533:37–65. doi: 10.1016/j.mrfmmm.2003.07.009. [Review] [299 refs.]. [DOI] [PubMed] [Google Scholar]
- 5.Rossman TG, Uddin AN, Burns FJ. Evidence that arsenite acts as a cocarcinogen in skin cancer. Toxicol. Appl. Pharmacol. 2004;198:394–404. doi: 10.1016/j.taap.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Waalkes MP, Liu J, Ward JM, Diwan BA. Animal models for arsenic carcinogenesis: inorganic arsenic is a transplacental carcinogen in mice. Toxicol. Appl. Pharmacol. 2004;198:377–384. doi: 10.1016/j.taap.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Wei M, Wanibuchi H, Morimura K, Iwai S, Yoshida K, Endo G, Nakae D, Fukushima S. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis. 2002;23:1387–1397. doi: 10.1093/carcin/23.8.1387. [DOI] [PubMed] [Google Scholar]
- 8.Barrett JC, Lamb PW, Wang TC, Lee TC. Mechanisms of arsenic-induced cell transformation. [Review] [33 refs.] Biol. Trace Elem. Res. 1989;21:421–429. doi: 10.1007/BF02917284. [DOI] [PubMed] [Google Scholar]
- 9.Landolph JR. Molecular mechanisms of transformation of C3H/10T1/2 C1 8 mouse embryo cells and diploid human fibroblasts by carcinogenic metal compounds. [Review] [58 refs] Environ. Health Perspect. 1994;3:119–125. doi: 10.1289/ehp.94102s3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Ma WY, Li J, Goranson A, Dong Z. Requirement of Erk, but not JNK, for arsenite-induced cell transformation. J. Biol. Chem. 1999;274:14595–14601. doi: 10.1074/jbc.274.21.14595. [DOI] [PubMed] [Google Scholar]
- 11.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc. Natl. Acad. Sci. USA. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J. Natl. Cancer Inst. 1888;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- 13.Mure K, Uddin AN, Lopez LC, Styblo M, Rossman TG. Arsenite induces delayed mutagenesis and transformation in human osteosarcoma cells at extremely low concentrations. Environ. Mol. Mutagen. 2003;41:322–331. doi: 10.1002/em.10164. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Wu J, Zhang R, Zhang P, Eckard J, Yusuf R, Huang X, Rossman TG, Frenkel K. Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. Toxicology. 2005;213:81–96. doi: 10.1016/j.tox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Barrett JC, Tsutsui T. Transformation by inorganic arsenic compounds of normal Syrian hamster embryo cells into a neoplastic state in which they become anchorage-independent and cause tumors in newborn hamsters. Int. J. Cancer. 2002;99:629–634. doi: 10.1002/ijc.10407. [DOI] [PubMed] [Google Scholar]; 15 Takahashi M, Barrett JC, Tsutsui T. Transformation by inorganic arsenic compounds of normal Syrian hamster embryo cells into a neoplastic state in which they become anchorage-independent and cause tumors in newborn hamsters. Int. J. Cancer. 2002;99:629–634. doi: 10.1002/ijc.10407. [DOI] [PubMed] [Google Scholar]
- 16.Pi J, Qu W, Reece JM, Kumagai Y, Waalkes MP. Transcription factor Nrf2 activation by inorganic arsenic in cultured keratinocytes: involvement of hydrogen peroxide. Exp. Cell Res. 2003;290:234–245. doi: 10.1016/s0014-4827(03)00341-0. [DOI] [PubMed] [Google Scholar]
- 17.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol. Appl. Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Shi X. Intracellular signal transduction of cells in response to carcinogenic metals. Crit. Rev. Oncol. Hematol. 2002;42:105–121. doi: 10.1016/s1040-8428(01)00211-6. [DOI] [PubMed] [Google Scholar]
- 19.Crichton RR. Inorganic biochemistry of iron metabolism from molecular mechanisms to clinical consequences. second ed. New York: Wiley, Chichester; 2001. [Google Scholar]
- 20.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic. Biol. Med. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 21.Bouton C, Hirling H, Drapier JC. Redox modulation of iron regulatory proteins by peroxynitrite. J. Biol. Chem. 1997;272:19969–19975. doi: 10.1074/jbc.272.32.19969. [DOI] [PubMed] [Google Scholar]
- 22.Ponka P, Lok CN. The transferrin receptor: role in health and disease. Int. J. Biochem. Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat. Res. 2003;533:153–171. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 24.Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim. Biophys. Acta. 2002;1603:31–46. doi: 10.1016/s0304-419x(02)00068-9. [DOI] [PubMed] [Google Scholar]
- 25.Rouault TA. Iron on the brain. Nat. Genet. 2001;28:299–300. doi: 10.1038/91036. [DOI] [PubMed] [Google Scholar]
- 26.Weinberg ED. Cellular iron metabolism in health and disease. Drug Metab. Rev. 1990;22:531–579. doi: 10.3109/03602539008991450. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg ED. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Liehr JG, Jones JS. Role of iron in estrogen-induced cancer. Curr. Med. Chem. 2001;8:839–849. doi: 10.2174/0929867013372931. [DOI] [PubMed] [Google Scholar]
- 29.Deugnier Y, Turlin B. Iron and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2001;16:491–494. doi: 10.1046/j.1440-1746.2001.02430.x. [DOI] [PubMed] [Google Scholar]
- 30.Dai J, Huang C, Wu J, Yang C, Frenkel K, Huang X. Iron-induced interleukin-6 gene expression: possible mediation through the extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways. Toxicology. 2004;203:199–209. doi: 10.1016/j.tox.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986 Sep.68(3):726–731. [PubMed] [Google Scholar]
- 32.Shang T, Kotamraju S, Kalivendi SV, Hillard CJ, Kalyanaraman B. 1-Methyl-4-phenylpyridinium-induced apoptosis in cerebellar granule neurons is mediated by transferrin receptor iron-dependent depletion of tetrahydrobiopterin and neuronal nitric-oxide synthase-derived superoxide. J. Biol. Chem. 2004;279:19099–19112. doi: 10.1074/jbc.M400101200. [DOI] [PubMed] [Google Scholar]
- 33.Genius J, Fandrey J. Nitric oxide affects the production of reactive oxygen species in hepatoma cells: implications for the process of oxygen sensing. Free Radic. Biol. Med. 2000;29:515–521. doi: 10.1016/s0891-5849(00)00343-9. [DOI] [PubMed] [Google Scholar]
- 34.Droge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 35.Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol. Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 36.Theil EC. Regulation of ferritin and transferrin receptor mRNAs. J. Biol. Chem. 1990;265:4771–4774. [PubMed] [Google Scholar]
- 37.Eisenstein RS. Iron regulatory proteins and the molecular control of mammalian iron metabolism. Annu. Rev. Nutr. 2000;20:627–662. doi: 10.1146/annurev.nutr.20.1.627. [DOI] [PubMed] [Google Scholar]
- 38.Thomson AM, Rogers JT, Leedman PJ. Iron-regulatory proteins, iron-responsive elements and ferritin mRNA translation. Int. J. Biochem. Cell Biol. 1999;31:1139–1152. doi: 10.1016/s1357-2725(99)00080-1. [DOI] [PubMed] [Google Scholar]
- 39.Cairo G, Pietrangelo A. Iron regulatory proteins in pathobiology. Biochem. J. 2000;352(Pt 2):241–250. [PMC free article] [PubMed] [Google Scholar]
- 40.Cairo G, Tacchini L, Pogliaghi G, Anzon E, Tomasi A, Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J. Biol. Chem. 1995;270:700–703. doi: 10.1074/jbc.270.2.700. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Q, Huang X. Induction of ferritin and lipid peroxidation by coal samples with different prevalence of coal workers’ pneumoconiosis: role of iron in the coals. Am. J. Ind. Med. 2002;42:171–179. doi: 10.1002/ajim.10101. [DOI] [PubMed] [Google Scholar]
- 42.Yang DC, Wang F, Elliott RL, Head JF. Expression of transferrin receptor and ferritin H-chain mRNA are associated with clinical and histopathological prognostic indicators in breast cancer. Anticancer Res. 2001;21:541–549. [PubMed] [Google Scholar]
- 43.Rothenberger S, Mullner EW, Kuhn LC. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990;18:1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li JH, Rossman TG. Mechanism of comutagenesis of sodium arsenite with N-methyl-N-nitrosourea. Biol. Trace Elem. Res. 1989;21:373–381. doi: 10.1007/BF02917278. [DOI] [PubMed] [Google Scholar]
- 45.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 46.Muckenthaler M, Gray NK, Hentze MW. IRP-1 binding to ferritin mRNA prevents the recruitment of the small ribosomal subunit by the cap-binding complex eIF4F. Mol. Cell. 1998;2:383–388. doi: 10.1016/s1097-2765(00)80282-8. [DOI] [PubMed] [Google Scholar]
- 47.Eisenstein RS, Ross KL. Novel roles for iron regulatory proteins in the adaptive response to iron deficiency. J. Nutr. 2003;133:1510S–1516S. doi: 10.1093/jn/133.5.1510S. [DOI] [PubMed] [Google Scholar]
- 48.Beinert H, Kennedy MC, Stout CD. Aconitase as iron minus sign sulfur protein, enzyme, and iron-regulatory protein. Chem. Rev. 1996;96:2335–2374. doi: 10.1021/cr950040z. [DOI] [PubMed] [Google Scholar]
- 49.Guo B, Phillips JD, Yu Y, Leibold EA. Iron regulates the intracellular degradation of iron regulatory protein 2 by the proteasome. J. Biol. Chem. 1995;270:21645–21651. doi: 10.1074/jbc.270.37.21645. [DOI] [PubMed] [Google Scholar]
- 50.McClarty GA, Chan AK, Choy BK, Wright JA. Increased ferritin gene expression is associated with increased ribonucleotide reductase gene expression and the establishment of hydroxyurea resistance in mammalian cells. J. Biol. Chem. 1990;265:7539–7547. [PubMed] [Google Scholar]
- 51.Larrick JW, Cresswell P. Modulation of cell surface iron transferrin receptors by cellular density and state of activation. J. Supramol. Struct. 1979;11:579–586. doi: 10.1002/jss.400110415. [DOI] [PubMed] [Google Scholar]
- 52.Richardson DR, Baker E. The uptake of iron and transferrin by the human malignant melanoma cell. Biochim. Biophys. Acta. 1990;1053:1–12. doi: 10.1016/0167-4889(90)90018-9. [DOI] [PubMed] [Google Scholar]
- 53.Trinder D, Zak O, Aisen P. Transferrin receptor-independent uptake of differic transferrin by human hepatoma cells with antisense inhibition of receptor expression. Hepatology. 1996;23:1512–1520. doi: 10.1053/jhep.1996.v23.pm0008675172. [DOI] [PubMed] [Google Scholar]
- 54.Raso V, Basala M. A highly cytotoxic human transferrin-ricin A chain conjugate used to select receptor-modified cells. J. Biol. Chem. 1984;259:1143–1149. [PubMed] [Google Scholar]
- 55.McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J. Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chan RY, Ponka P, Schulman HM. Transferrin-receptor-independent but iron-dependent proliferation of variant Chinese hamster ovary cells. Exp. Cell Res. 1992;202:326–336. doi: 10.1016/0014-4827(92)90082-j. [DOI] [PubMed] [Google Scholar]
- 57.Garrick MD, Dolan KG, Horbinski C, Ghio AJ, Higgins D, Porubcin M, Moore EG, Hainsworth LN, Umbreit JN, Conrad ME, Feng L, Lis A, Roth JA, Singleton S, Garrick LM. DMT1: a mammalian transporter for multiple metals. Biometals. 2003;16:41–54. doi: 10.1023/a:1020702213099. [DOI] [PubMed] [Google Scholar]
- 58.Wu KJ, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–679. doi: 10.1126/science.283.5402.676. [DOI] [PubMed] [Google Scholar]
- 59.Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic. Biol. Med. 2002;33:457–463. doi: 10.1016/s0891-5849(02)00842-0. [DOI] [PubMed] [Google Scholar]
- 60.Turi JL, Yang F, Garrick MD, Piantadosi CA, Ghio AJ. The iron cycle and oxidative stress in the lung. Free Radic. Biol. Med. 2004;36:850–857. doi: 10.1016/j.freeradbiomed.2003.12.008. [DOI] [PubMed] [Google Scholar]