Abstract
Small intestinal adenocarcinomas are uncommon neoplasms that are rarely reported in non-human primates. These neoplasms are also rare in man, although they are thought to share a similar pathogenesis with the more common colorectal carcinoma. Herein we report the clinical, histologic, immunohistochemical, and molecular characteristics of small intestinal adenocarcinoma in 10 common marmosets (Callithrix jacchus). Retrospective analysis of necropsy records revealed small intestinal carcinoma to be the most common neoplastic cause of morbidity and mortality in aged common marmosets. The average age of affected animals was 6.6 years old and there was no sex predilection. 9/10 (90%) of the tumors arose within the proximal small intestine near the interface with the duodenum. All cases were characterized by disorganization, loss of polarity, and proliferation of neoplastic epithelial cells along the crypt to mid-villous interface. 2/10 (20%) were defined as carcinoma in situ. 8/10 (80%) had some degree of invasion with lymphatic invasion and lymph node metastasis present in 6/10 (60%) of the animals. Immunohistochemically, 10/10 (100%) expressed cytokeratin, 7/9 (77%) expressed E-cadherin, and 8/9 (88%) expressed β-catenin. The expression of E-cadherin and β-catenin was decreased in the cell membrane and increased in the cytoplasm. No Helicobacter-like bacteria were observed via silver stain and callitrichine herpesvirus-3 was detected by PCR with equal frequency from neoplastic and non-neoplastic intestinal sections. The tumors described in this population illustrate comparable features to human cases of small intestine carcinoma and may serve as a potential animal model for small intestinal carcinomas.
Keywords: Common marmoset, intestinal adenocarcinoma, β-catenin
Introduction
Gastrointestinal carcinomas are relatively common in man; however the bulk of these neoplasms arise in the colon and rectum with a small percentage of cases recorded in the small intestine.4,9 Those that arise in the small intestine are more prevalent in the duodenum compared to the mid- and distal jejunum and are directly linked to advanced age; however no other causative factors have been found in human medicine.7 In non-human primates, a similar scenario exists in which gastrointestinal carcinomas are most commonly described in the large intestine whereas those arising in the small intestine are described only rarely.1,2,18 The prototypical gastrointestinal carcinomas in non-human primates are ileocecal adenocarcinomas in the rhesus macaque (Macaca mulatta) and colorectal adenocarcinoma in aged cotton-top tamarins (Saguinus oedipus). The proposed pathogenesis of colorectal adenocarcinoma in cotton-top tamarins involves an association between chronic lymphoplasmacytic colitis and the development of crypt dysplasia that progresses to carcinoma.10 Other New World primates, including the common marmoset (Callithrix jacchus) have rarely been reported to develop small intestinal carcinomas; however too few cases have been analyzed to determine any specific trends.1,2
In man, a variety of infectious, genetic, and environmental factors can contribute to gastrointestinal tumorigenesis. Bacteria, like Helicobacter sp., are implicated in the development of gastric and colonic carcinomas in man and immunodeficient mice.7,12 Helicobacter species have been recovered from the feces and gastric mucosa of common marmosets; however no known disease association exists.5,16,20 In the cotton-top tamarin, novel intestinal Helicobacter species have been isolated from animals with chronic colitis.16 However the relationship with bacterial infection, colitis, and eventual tumor formation is not known and whether a similar scenario exists in other non-human primate species is purely speculative. Epstein-Barr virus (EBV), a human gamma herpesvirus that is most commonly associated with lymphoproliferative diseases including lymphosarcoma, is also associated with gastric adenocarcinoma formation.17 An Epstein-Barr related herpesvirus (callitrichine herpesvirus-3; CHV-3) has been isolated from common marmosets with lymphosarcoma; however an association with other malignancies in the common marmoset has not been determined.3
There are broad similarities in the histologic appearance of large intestinal carcinoma from small intestinal carcinoma and a similar molecular pathogenesis is proposed for the two sites. Commonly implicated in the pathogenesis of colorectal carcinoma is derangement and dysregulation of the Wnt pathway.7,21 Wnts are signaling proteins that prevent β-catenin degradation in the cytoplasm, thereby allowing β-catenin to translocate to the nucleus and accumulate in a complex with various transcription factors that in turn regulate target gene transcription.13,14 If Wnt signaling is absent, excess β-catenin is degraded within the cytoplasm so that excess transcription factor activation does not occur.13,14 A second function of β-catenin exists outside of the Wnt pathway and involves binding to the cytoplasmic domain of type I cadherins. This complex facilitates linkage to the actin cytoskeleton and allows for normal cellular structural organization.13,14 Alterations in the conventional Wnt pathway will lead to accumulation of β-catenin and modified progression through the cell cycle. Perturbed interaction between β-catenin and type I cadherins can destabilize cell-cell interactions and promote loss of cell cohesion, an important aspect of metastatic spread. In human colorectal carcinogenesis, the primary dysregulation of the Wnt signaling pathway occurs through mutations in the adenomatous polyposis coli (APC) gene, a potent tumor suppressor.14 Normally, the APC protein complexes with β-catenin and causes phosphorylation of β-catenin rendering it marked for degradation.14 Therefore, β-catenin can exert control on both nuclear transcription and cell adhesion and is central to the pathogenesis of gastrointestinal tumors.
Herein we characterize the spontaneous development of small intestinal carcinomas in ten common marmosets. We use silver stains to assess the presence of Helicobacter-like bacteria, immunohistochemistry for CD3, CD20, cytokeratin, E-cadherin, and β-catenin as well as PCR for CHV-3 to better elucidate the pathogenesis of these tumors in the common marmoset.
Materials and Methods
Necropsy records at the New England Primate Research Center (NEPRC) were searched from 1991–2009 for intestinal malignancies in common marmosets. Excluding neonates and research necropsies, 384 common marmosets were necropsied during this time period. All cases recorded were localized to the small intestine. All animals were necropsied within 24 hours of death and representative sections of all major organs were collected, fixed in 10% neutral buffered formalin (NBF), embedded in paraffin, sectioned at 5μm, and stained using hematoxylin and eosin (HE). Additional sections of affected intestine were stained via the Warthin-Starry method.
To characterize the neoplasms using immunohistochemical methods we used standard immunoperoxidase staining for CD3, CD20, E-cadherin, cyotokeratin AE1/AE3, and β-catenin. Formalin fixed, paraffin embedded sections were deparaffinized, rehydrated and subsequently blocked with hydrogen peroxide. Pre-treatment for all antibodies involved microwaving for 20 minutes in 0.01M citrate buffer followed by 20 minutes of cooling. All steps were followed by a tris-buffered saline (TBS) wash. Prior to application of primary antibodies, all slides were treated with Dako protein block for 10 minutes. Sections were incubated with anti-human CD3 (Dako [Carpinteria, CA, USA], polyclonal, 1:600, 30′ at room temperature), anti-human CD20 (Dako, monoclonal, 1:175, overnight in refrigerator), anti-human E-cadherin (Labvision [Fremont, CA, USA], monoclonal, 1:100, overnight in refrigerator), anti-human cytokeratin AE1/AE3 (Dako, monoclonal, 1:140, overnight in refrigerator), and anti-human β-catenin (Santa Cruz Biotechnology [Santa Cruz, CA, USA], monoclonal, 1:100, overnight in refrigerator). Slides were then incubated with secondary antibody biotinylated goat anti-rabbit (Vector laboratories, [Burlingame, CA, USA],1:200, 30′ at room temperature) for CD3 and E-cadherin and biotinylated horse anti-mouse (Vector laboratories, 1:200, 30′ at room temperature) for CD20, cytokeratin AE1/AE3, and β-catenin. This was followed by 30 minute incubation at room temperature with Vectastain ABC Elite [Vector laboratories] (CD3, E-cadherin, cytokeratin AE1/AE3, and β-catenin) or Vectastain ABC standard [Vector laboratories] (CD20). All slides were developed with DAB chromagen (Dako) and counterstained with Mayer’s hematoxylin. In all cases, step sections were incubated with isotype-specific irrelevant antibodies for negative controls. Positive controls consisted of sections of lymph node (CD3 and CD20) and small intestine (E-cadherin, cytokeratin AE1/AE3, and β-catenin) from age matched common marmosets.
Isolation of DNA from affected frozen small intestine was performed on case 5 and 8 and was carried out using the DNeasy Tissue Kit (Qiagen, Valencia, CA). In order to amplify DNA, degenerate herpesvirus primers were used to screen the sections of frozen small intestine. The degenerate primers used corresponded to DFASA (5′-GTGTTCGACTTYGCNAGYYTNTAYCC-3′) and GDTD1B (5′-CGGCATGCGACAAACACGGAGTCNGTRRCNCCRTA-3′).15 Amplifications were carried out in 25 ul of 1x GoTag Green Master Mix (Promega, San Diego, CA) with 20 pmoles of each primer and 50 ng of template DNA. The cycling condition consisted of 2 minutes denaturation at 94C followed by 35 cycles of 1min denaturation at 94C, 1 min of annealing at 60C, and 1 min of extension at 72C followed by a final 10 min extension step at 72C. An aliquot (2 to 5%) of these amplification products (DFASA-GDTD1B) was then used as a template in a subsequent nested PCR with the primer pool VYGA (5′- ACGTGCAACGCGGTGTAYGGNYTNACNGG-3′) and GDTD1B. The products of the secondary nested PCR amplification were electrophoresed in a 2% agarose gel and visualized by irradiation with UV in the presence of ethidium bromide. The nested PCR product (-236 bp) were cloned into pCR 2.1-TOPO vector (Invitrogen) and sequenced (Retrogen, Inc., San Diego, CA).
In order to compare the relative levels of viral replication in neoplastic and non-neoplastic gastrointestinal tract, a 10 fold serial dilution was made with the DNA samples and a 306 bp fragment was amplified with CHV3 specific primers (CHV3F (5′-TTGACTTCGCCAGCCTCTAT-3′) and CHV3R (5′-TTGCAGGTGCACTTGATAGC-3′) derived from DNA sequences from the GenBank (AF319782.2). The cycling condition consisted of 3 minutes denaturation at 94C followed by 5 cycles of 30 sec at 94C, 30 sec at 60C, and 30 sec at 72C followed with 35 cycles of 30 sec at 94C, 30 sec at 55C, and 30 sec at 72C followed by a final 10 min extension step at 72C.
Results
Clinical and Gross findings
Age and sex data, clinical findings, and gross findings for the ten animals are presented in table 1. 9/10 of the animals were euthanized due to intractable diarrhea that was variably bloody and weight loss with the average age at time of euthanasia of 7.6 years (range 3.2 to 13.4 years). Animals with small intestinal adenocarcinoma corresponded to 3.8% of the total non-experimental and non-neonatal common marmosets necropsied during this period. Six animals were female and 4 were male. Clinical chemistry analysis revealed hypoalbuminemia in 4/10 animals. 5/10 animals had a mildly decreased hematocrit. Fecal cultures were performed on seven of the animals with Escherichia coli (5/7), Klebsiella sp (3/7), Enterococcus sp. (2/7), and Proteus mirabilis (1/7) isolated. One animal was fecal positive for Giardia by antigen capture (Triage, Biosite).
Table 1.
| Case Number | Age (yrs) | Sex | Clinical findings | Gross Findings |
|---|---|---|---|---|
| 1 | 6.9 | Male | Diarrhea, decreased weight | Narrowing and thickening of proximal jejunum (5 cm distal to pylorus) |
| 2 | 3.8 | Female | Diarrhea, decreased weight | Thickening at the duodenal-jejunal interface with mucosal ulceration |
| 3 | 6.1 | Female | Diarrhea, decreased weight | Thickening of middle jejunum |
| 4 | 3.2 | Female | Diarrhea | None |
| 5 | 7.3 | Male | Diarrhea | Stricture in proximal jejunum |
| 6 | 7.2 | Male | Diarrhea, bloating, weight loss | None |
| 7 | 8.8 | Female | Diarrhea, decreased weight | Thickened proximal jejunum |
| 8 | 8.1 | Male | Weight loss | None |
| 9 | 11.1 | Female | Weight loss | Stricture in proximal jejunum |
| 10 | 13.4 | Female | None | None |
Grossly, 5/10 of the animals were noted to have thickening of the distal duodenum and proximal jejunum with stricture observed in 2/10 animals. 1/10 was noted to have thickening in the middle jejunum. Gross lesions in the small intestine were not identified in 4/10 animals.
Histologic Findings
In 9/10 cases, there was a locally extensive region of hypercellularity along the crypt to mid-villus zone of the duodenum and proximal jejunum. In one of the cases (case 3) there was a similar region in the middle jejunum. Two of the cases (case 4 and 8) were classified as carcinoma in situ in which there was no obvious breach of the basement membrane zone, but clear disorganization in the overlying crypt region (Fig. 1). Irrespective of invasiveness, all cases were characterized by loss of cell polarity and effacement of the crypts and villi by a homogenous population of neoplastic epithelial cells that multifocally breached the basement membrane zone and infiltrated into the tunica submucosa and smooth muscle layers (Fig. 2). Neoplastic cells had moderate amounts of deeply basophilic cytoplasm and large prominent nuclei with eccentrically located, 1–2 nucleoli. Mitotic figures were variable, but averaged 0–1 per high power field. Roughly 50% of the neoplastic cells in all cases had large, solitary, intracytoplasmic, clear vacuoles that marginated the nucleus (signet ring cells) (Fig. 3). In one of the cases (case 10), the neoplastic cells were admixed with abundant mucin (Fig. 4). The neoplastic cells were embedded in a relatively scant stroma, except for one case (case 5) that was embedded in an abundant schirhous response leading to marked narrowing of the intestinal lumina (Fig. 5). In three of the affected animals (case 1, 3, and 10), the neoplasm was extensively ulcerated and covered by a thick plaque of degenerate neutrophils, sloughed epithelial cells, and hemorrhage.
Fig. 1.
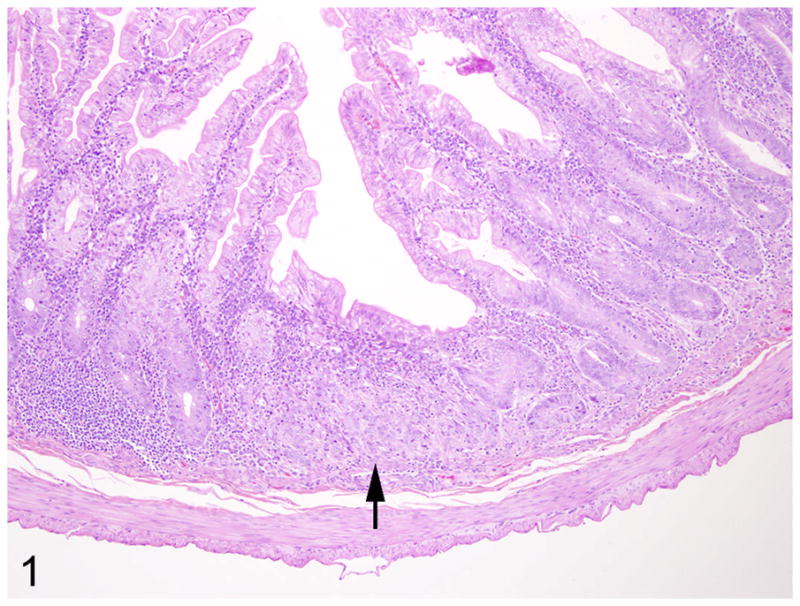
Proximal jejunum, common marmoset, case No. 4. Focally, the crypts are disorganized with loss of cell polarity (arrow). The lamina propria is filled with moderate numbers of lymphocytes and plasma cells. HE.
Fig. 2.
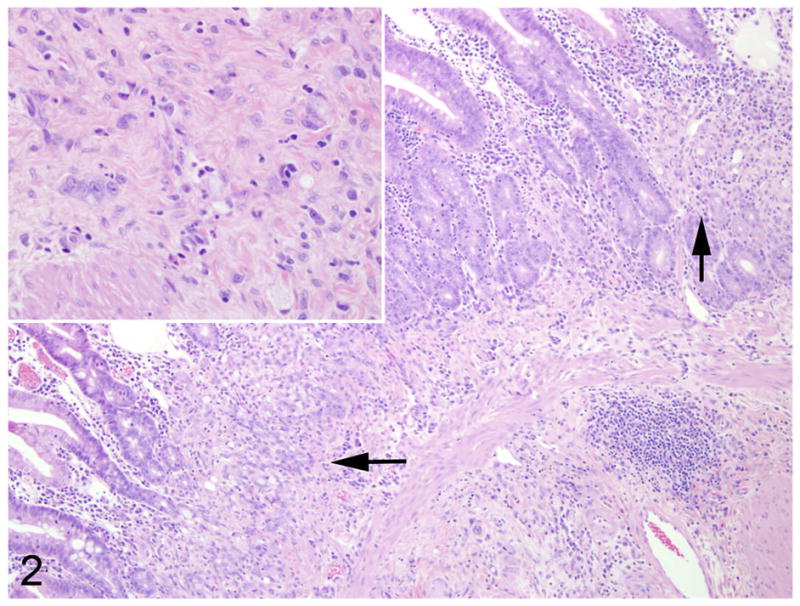
Proximal jejunum, common marmoset, case No. 7. Multifocally, crypt and mid-villous regions are replaced by neoplastic epithelial cells (arrows). Within the underlying submucosa are small aggregates of solitary neoplastic cells (inset). HE.
Fig. 3.
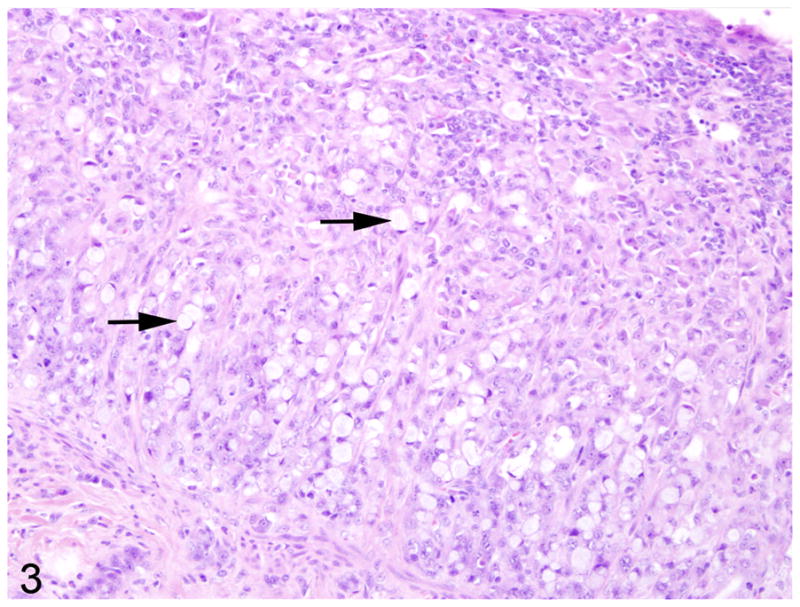
Proximal jejunum, common marmoset, case No. 1. Large numbers of neoplastic cells have intracytoplasmic vacuoles that displace the nucleus to the periphery (signet ring cells) (arrows). HE.
Fig. 4.
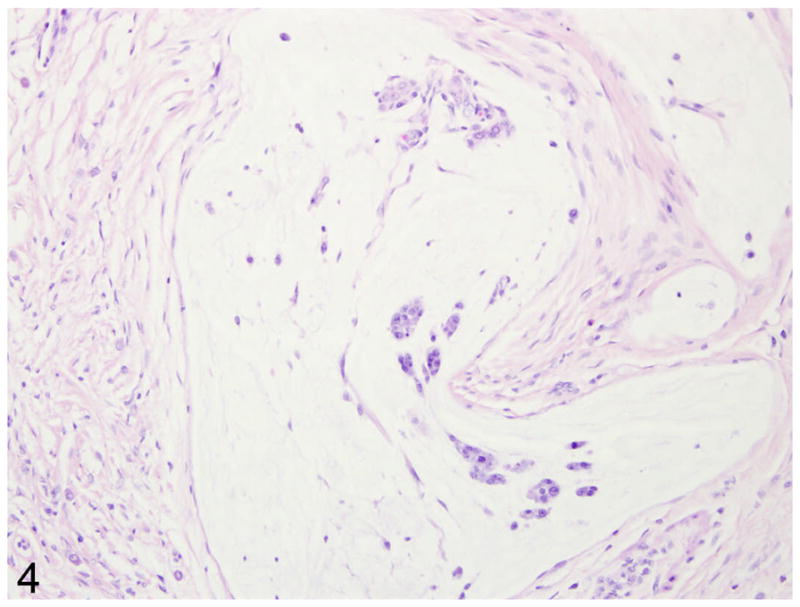
Proximal jejunum, common marmoset, case No. 9. Within the smooth muscle layers are large deposits of mucin (asterisk) that contain rare rafts of neoplastic cells (arrow). HE.
Fig 5.
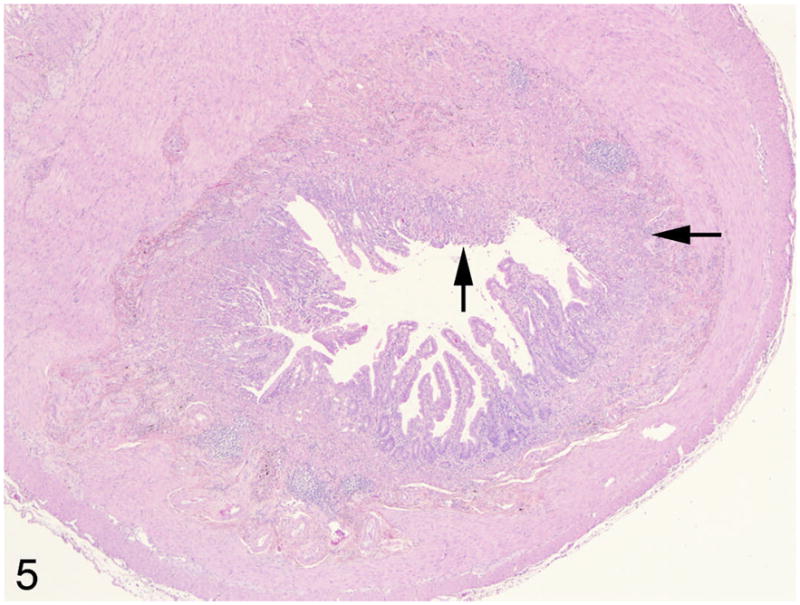
Middle jejunum, common marmoset, case No. 3. The lumen of the small intestine is markedly narrowed due to a marked schirhous response accompanying the neoplastic epithelial cells (arrows). HE.
In 6/10 cases (cases 1, 3, 5, 6, 9, 10) there were nests and acini of neoplastic cells within the submucosal lymphatics and multifocally expanding the subcapsular sinuses of the draining mesenteric lymph nodes (Fig. 6). Cases 4, 6, and 9 (3/10) were accompanied by a moderate enteritis that was characterized by large numbers of small lymphocytes and fewer plasma cells that expanded the lamina propria in both the affected and unaffected portions of the small intestine. Additional co-morbid histologic findings included: cholecystitis (1/10), mesangioproliferative glomerulonephritis (2/10), atherosclerosis (1/10), pancreaticodochitis (1/10), and typhlitis with attaching and effacing Escherichia coli (1/10).
Fig. 6.
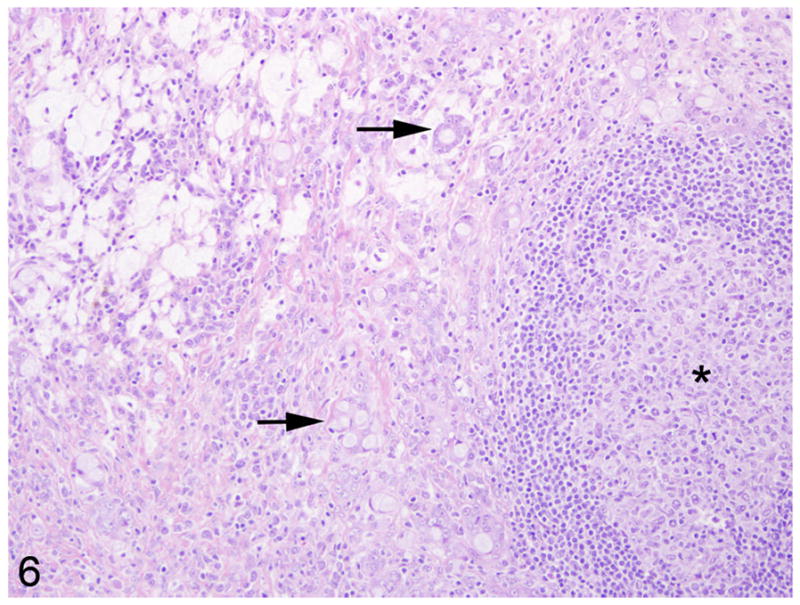
Mesenteric lymph node, common marmoset, case No. 6. The subcapsular sinus and paracortex is replaced by sheets and rafts of neoplastic cells that occasionally form pseudo-acinar structures (arrows). The remnant follicles are hyperplastic with prominent germinal centers (asterisk).
Histochemical and Immunohistochemical Findings
Warthin-Starry staining of all cases revealed no spiral bacteria noted within the affected and unaffected sections of small intestine. Paraffin embedded, neoplastic small intestine was not available from case 2 and therefore was not included in the immunohistochemical studies. Nearly three-quarters (75%) of the inflammatory cells in the affected small intestine had strong, membranous CD3 immunoreactivity (Fig. 7). Approximately 15% of the inflammatory cells in the affected small intestine had strong, membranous CD20 immunoreactivity (Fig. 8) whereas roughly 10% of the inflammatory cells did not stain for either CD3 or CD20. There was no difference in the percentage of CD3 and CD20 positive cells in the affected and unaffected sections of small intestine in the 9 cases. Cytokeratin immunoreactivity was strong and diffusely cytoplasmic in the neoplastic cells and highlighted the invasiveness and lymph node metastasis of the neoplastic epithelial cells (Fig. 9). E-cadherin immunoreactivity was detected in 7/9 cases examined. There was no E-cadherin immunoreactivity in both neoplastic and non-neoplastic tissue from cases 3 and 4. E-cadherin immunoreactivity was predominately and strongly associated with the cell membrane and to a lesser degree the cytoplasm in the normal sections of small intestine. In the affected sections as well as affected lymph nodes, neoplastic cells had scant to absent membrane staining and increased intracytoplasmic, often punctuate to granular staining (Fig. 10). Aggregates of neoplastic cells (i.e. pseudo-acini) often retained strong membrane staining and only scattered intracytoplasmic immunoreactivity. β-catenin immunoreactivity was detected in 8/9 cases examined. No immunoreactivity to β-catenin was recorded in case 4. Normal small intestine had uniform, strong cell membrane staining with faint cytoplasmic staining. In the neoplastic cells, there was decreased cell membrane staining with increased cytoplasmic immunoreactivity and minimal nuclear reactivity (Fig. 11). Neoplastic cells that formed pseudo-acini often retained strong cytoplasmic membrane staining (Fig. 12).
Fig. 7.
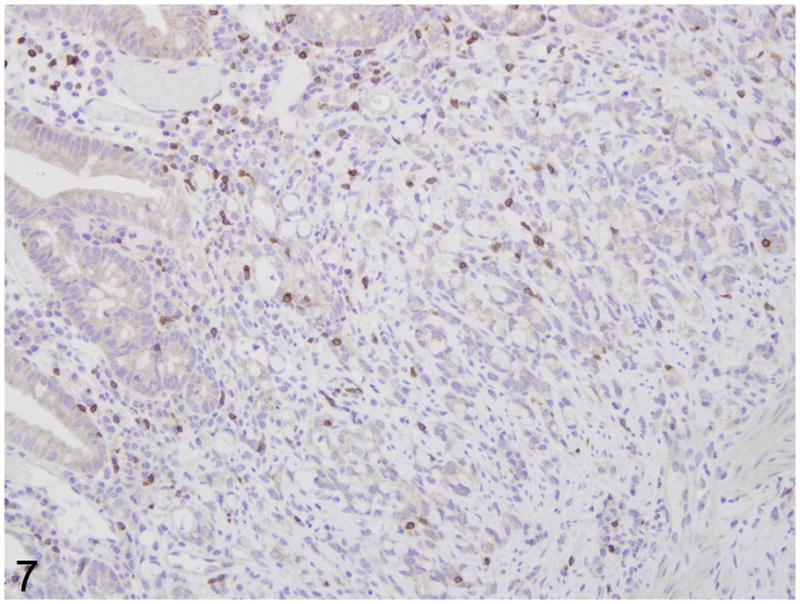
Proximal jejunum, common marmoset, case No. 7. Moderate numbers of CD3 positive T lymphocytes in the lamina propria. Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Fig. 8.
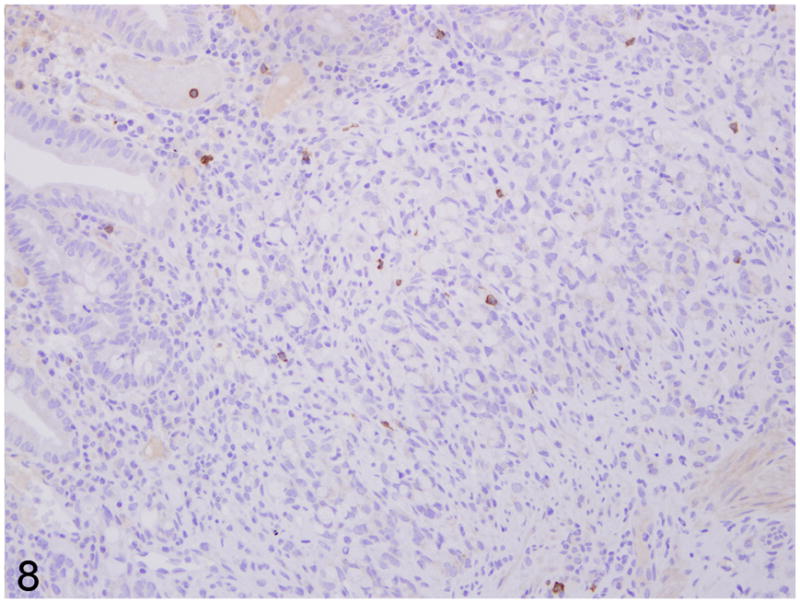
Proximal jejunum, common marmoset, case No. 7. Rare CD20 positive B lymphocytes in the lamina propria. Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Fig. 9.
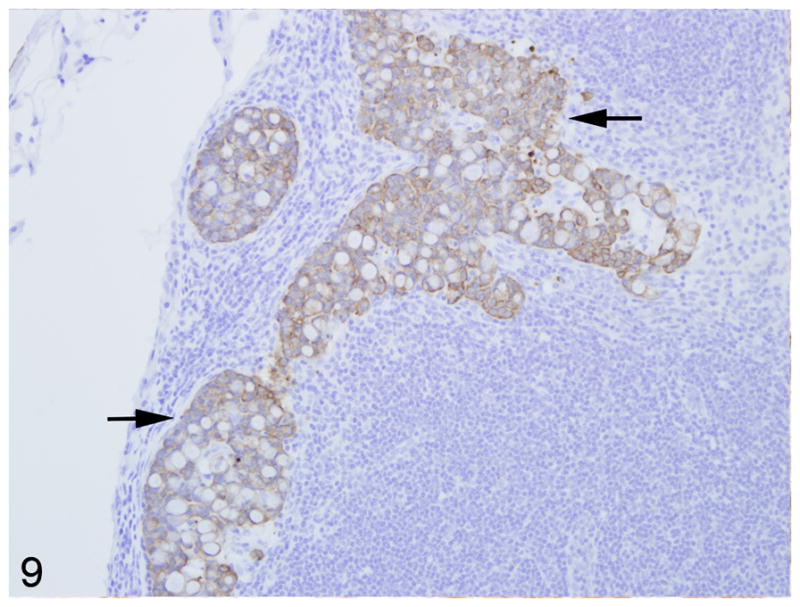
Mesenteric lymph node, common marmoset, case No. 10. Large aggregates of neoplastic epithelial cells that strongly express cytokeratin fill the subcapsular sinus and compress the adjacent cortex. Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Fig. 10.
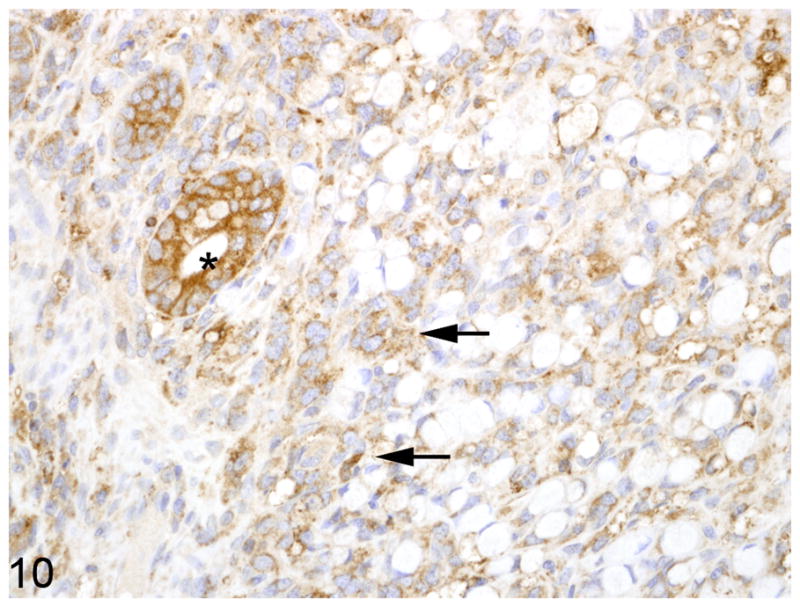
Proximal jejunum, common marmoset, case No. 1. Neoplastic epithelial cells exhibit reduced membranous and increased cytoplasmic, punctuate to granular immunoreactivity for E-cadherin (arrows). Normal crypt epithelial has strong membranous and cytoplasmic immunoreactivity (asterisk). Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Fig. 11.
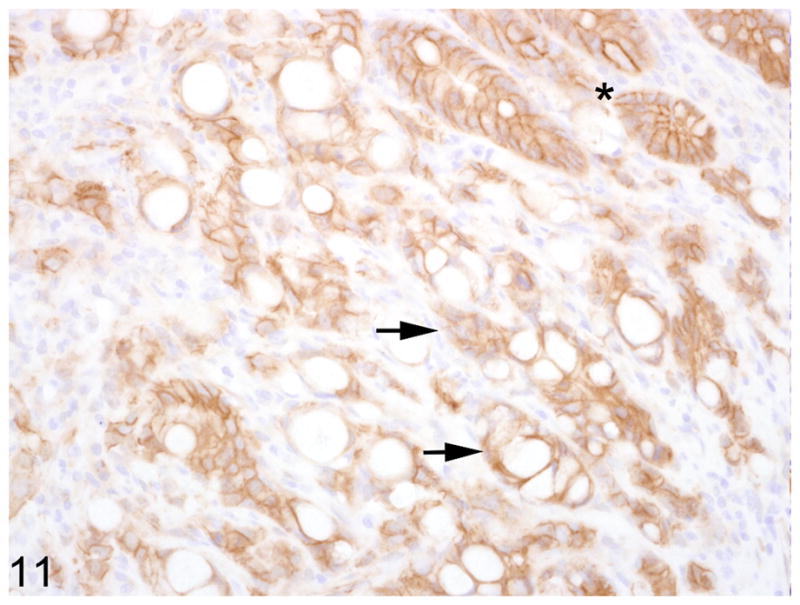
Proximal jejunum, common marmoset, case No. 10. Neoplastic epithelial cells exhibit reduced membranous immunoreactivity for β-catenin (arrows). Normal crypt epithelial cells (asterisk) have strong membranous immunoreactivity (asterisk). Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Fig. 12.
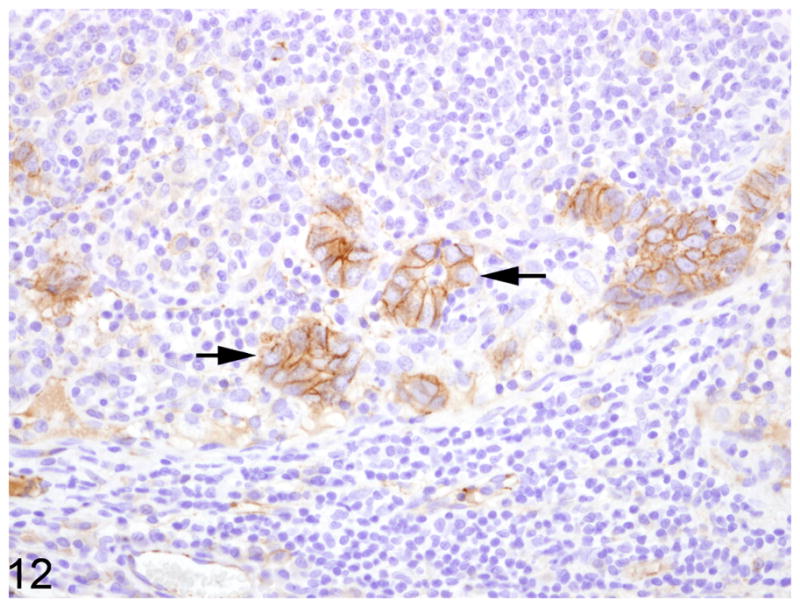
Mesenteric lymph node, common marmoset, case No. 6. Neoplastic acinar structures within a the subcapsular sinus retain strong membranous immunoreactivty for β-catenin. Immunoperoxidase staining, DAB chromagen, Mayer’s hematoxylin counterstain.
Sequencing analysis
DNA isolated from paraffin embedded and fresh frozen, neoplastic small intestine and unaffected large intestine from cases 5 and 8 were screened for the presence of CHV-3 DNA using nested PCR. Positive results were obtained from both the neoplastic small intestine and unaffected large intestine in both cases. Sequence analysis of amplicons generated with the nested broad based herpesvirus primers indicated alignment with CHV-3 and revealed 94% identity with CHV-3 sequences previously reported. The PCR product was distinct from EBV and other old world primate lymphocryptoviruses. Following serial dilution of DNA isolated from the two regions of interest, there was no difference in the ability to detect CHV-3 between neoplastic small intestine and large intestine from both animals tested indicating that viral copy number was equivalent in neoplastic and non-neoplastic tissue.
Discussion
In primate species, carcinomas confined to the small intestine are exceedingly rare and have been reported in two rhesus macaques (Macaca mulatta) and four common marmosets.1,2,6,11 In those previously reported, the location of tumor development varied and included the proximal small intestine (2), the mid-small intestine (1), and the ileum (1).1,2 Herein we report the clinical, histologic, immunohistochemical, and molecular characteristics of 10 cases of small intestinal adenocarcinoma in common marmosets (Callithrix jacchus). Interestingly, 9/10 of the animals reported herein developed small intestine adenocarcinoma in the proximal jejunum, immediately distal to the duodenum, certainly suggesting an increased propensity for tumors to develop at this site in the common marmoset. There were no consistent bloodwork findings in animals with tumors and fecal cultures, when performed, revealed a mixed population of common intestinal bacteria. Six of the cases had gross lesions of thickening and structure at the site of the neoplasm. Four were considered incidental findings on histologic examination; however of these four only two were categorized as carcinoma in situ whereas the other two had spread extensively into the muscle layers. Many of the cases had only moderate invasion into the underlying submucosa and muscle layers and carcinomatosis was not present in any of the studied animals; however most cases had marked lymphatic invasion and metastasis to mesenteric lymph nodes. This indicates that although these tumors are small and confined to a specific region, metastatic spread is a common and likely an early event. Signet ring cell differentiation was typical in all cases; however only one case had large areas of mucin deposition. Signet ring cells are more common in cases of gastric and colonic carcinoma; however in one large human study 37% of the total cases of small intestinal adenocarcinoma were classified as signet ring carcinoma.4 Currently in human medicine there is no prognostic significance to the presence of signet ring cells in cases of small intestinal carcinoma and where a similar scenario exists in the common marmoset is not known.4 Mucin deposition has been described previously in cases of small intestinal carcinoma; however it is more commonly observed in large intestinal carcinomas.1
Although the small intestine has a similar epithelial turnover and proliferative rate compared to the large intestine, epithelial tumors of the small intestine are very rare compared to their colonic counterpart. The reason for this is not known; however various hypotheses have been put forth that include: (a) digesta in the small intestine has a faster transit time when compared to the large intestine, thereby decreasing exposure to preformed and metabolized toxins; (b) digesta is more liquid than that in the large intestine leading to less mucosal irritation; (c) the small intestine has a lower total bacterial count than the colon; (d) there are higher levels of IgA in the small intestine in addition to more abundant mucosal-associated lymphoid tissue which provides a cytoprotective effect through immune surveillance; and (e) stem cells that line the crypts are further away from the lumen that their counterpart in the colon and are therefore exposed to fewer luminal carcinogens.7,8 In man, while many of these proposed hypotheses are thought to play a role, the true pathogenesis of spontaneous small intestinal adenocarcinoma remains poorly defined; although chronic inflammation, bacterial and viral infection, and geneticmutations all likely play a role in promoting tumor development.
Chronic inflammation progressing to intestinal carcinoma is well described in man and cotton-top tamarins (Saguinus oedipus). In the cotton-top tamarin, chronic lymphoplasmacytic colitis is a common cause of wasting in middle to advanced age. A large proportion of these animals, with chronic colitis, progress through dysplasia and eventual carcinoma.10 This pathogenesis mimics human ulcerative colitis/carcinoma progression. In 3/10 cases presented herein the tumor was accompanied by a moderate lymphoplasmacytic enteritis. The majority of lymphocytes were T cells with only a small percentage of B cells. There was no difference in the percentage of inflammatory cells in the neoplastic sections of small intestine and the non-neoplastic regions. It is unlikely that the chronic inflammation induced the tumor formation in these three animals; however it is likely that the inflammation contributed to an altered local environment that could have potentiated tumor growth.
In man, Epstein-Barr virus (EBV) has been associated with the genesis of gastric carcinoma however there has been no association made between EBV infection and small intestine adenocarcinoma.17,19 We analyzed tissue from three of the cases reported herein for the presence of callitrichine herpesvirus-3 (CHV-3), a lymphocryptovirus related to EBV, that has previously been recovered from common marmosets with lymphosarcoma.3 All three cases analyzed had CHV-3 DNA in both the neoplastic small intestine and the normal colon. Even after titrated PCR, the levels of virus remained the same in both locations indicating that although these animals had been exposed to and infected with CHV-3, it likely plays no role in the pathogenesis of small intestinal carcinomas in this species. Helicobacter sp. have been associated with chronic enteritis, intestinal metaplasia, and gastric carcinoma in a variety of species including man and immunodeficient mice.7,12 Helicobacter species have previously been recovered from the feces and gastric mucosa of marmosets; although no disease association has been made.5,20 No spiral bacteria were detected in the cases presented herein and they are unlikely to play a role in the pathogenesis.
The molecular basis of small intestinal adenocarcinomas in man is not as well characterized as the more prevalent colorectal adenocarcinoma; however many similarities exist between carcinomas at the two sites. In the colorectal region, progression of adenomas to carcinomas involves the deregulation of both oncogenes and tumor suppressor genes. These include APC genes, P53, K-ras, and a variety of DNA mismatch repair genes (including hMSH2 and PMS2).8,9,14 Also of vital importance in the pathogenesis of colorectal carcinomas is dysregulation of Wnt signaling. The most commonly observed aberrant Wnt signaling is via altered β-catenin function. β-catenin serves a dual functioning protein that acts both as a transcription factor activator as well as a link between membranous proteins, like cadherins, and the cytoskeleton.13 Altered levels of β-catenin can lead to a shift in balance either toward increased nuclear gene expression or altered cell adhesion. In the current study, only several cases had no immunoreactivity to E-cadherin and β-catenin which was interpreted as secondary to altered fixation times rather than true loss of expression. In those cases that displayed E-cadherin and β-catenin expression there was decreased cell membrane staining and increased cytoplasmic staining in the tumor cells compared to the normal small intestinal epithelium. This cell membrane staining was preserved in cohesive populations of cells. Although this does not prove that derangement in either protein is what triggers the initiation of small intestinal adenocarcinoma development in common marmosets, the decreased expression suggests that loss of cell-cell adhesion is an early change in the pathogenesis of these tumors.
In conclusion, we report 10 cases of small intestinal adenocarcinoma in common marmosets (Callithrix jacchus). These tumors were almost entirely located within the proximal small intestine, immediately distal to the duodenum. Signet ring cell differentiation, lymphatic invasion, and metastatic spread to regional lymph nodes were present in the majority of cases. There was no association between expression of Callitrichine-herpesvirus-3 and tumor development. Similarly there was no association between the presence of inflammation or Helicobacter in regards to tumor development. Immunohistochemistry for E-cadherin and β-catenin indicates decreased cell membrane immunoreactivity for both of these proteins with a concomitant increase in cytoplasmic staining suggesting an important role for these proteins in tumorigenesis.
Acknowledgments
The authors wish to thank Kristen Toohey for assistance with photography and Liz Curran for tissue procurement. This research was funded, in part, by NIH grants RR00168 and RR07000.
References
- 1.Brack M. Gastrointestinal tumors observed in nonhuman primates at the German primate center. J Med Primatol. 1998;27:319–324. doi: 10.1111/j.1600-0684.1998.tb00082.x. [DOI] [PubMed] [Google Scholar]
- 2.Chalifoux LV. Adenocarcinoma of the small intestine in a common marmoset (Callithrix jacchus) Lab Anim Sci. 1990;40:210–212. [PubMed] [Google Scholar]
- 3.Cho Y, Ramer J, Rivailler P, Quink C, Garber RL, Beier DR, Wang F. An Epstein-Barr-related herpesvirus from marmoset lymphomas. Proc Natl Acad Sci U S A. 2001;98:1224–1229. doi: 10.1073/pnas.98.3.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabaja BS, Suki D, Pro B, Bonnen M, Ajani J. Adenocarcinoma of the small bowel: presentation, prognostic factors, and outcome of 217 patients. Cancer. 2004;101:518–526. doi: 10.1002/cncr.20404. [DOI] [PubMed] [Google Scholar]
- 5.de Mello MF, Monteiro AB, Fonseca EC, Pissinatti A, Ferreira AM. Identification of Helicobacter sp. in gastric mucosa from captive marmosets (Callithrix sp.; callitrichidae, primates) Am J Primatol. 2005;66:111–118. doi: 10.1002/ajp.20131. [DOI] [PubMed] [Google Scholar]
- 6.Fanton JW, Hubbard GB, Witt WM, Wood DH. Adenocarcinoma of the small intestine in two rhesus monkeys. J Am Vet Med Assoc. 1984;185:1377–1378. [PubMed] [Google Scholar]
- 7.Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Latz PE, Isaacson PG. Gastrointestinal Pathology: An Atlas and Text. 3. Lippincott Williams & Wilkins; Philadelphia, USA: 2008. pp. 471–489. [Google Scholar]
- 8.Howe JR, Karnell LH, Menck HR, Scott-Conner C. Adenocarcinoma of the small bowel: review of the National Cancer Data Base, 1985–1995. Cancer. 1999;86:2693–2706. doi: 10.1002/(sici)1097-0142(19991215)86:12<2693::aid-cncr14>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 9.Hutchins RR, Bani Hani A, Kojodjojo P, Ho R, Snooks SJ. Adenocarcinoma of the small bowel. ANZ J Surg. 2001;71:428–437. doi: 10.1046/j.1440-1622.2001.02149.x. [DOI] [PubMed] [Google Scholar]
- 10.Johnson LD, Ausman LM, Sehgal PK, King NW., Jr A prospective study of the epidemiology of colitis and colon cancer in cotton-top tamarins (Saguinus oedipus) Gastroenterology. 1996;110:102–115. doi: 10.1053/gast.1996.v110.pm8536845. [DOI] [PubMed] [Google Scholar]
- 11.Lingeman CH, Garner FM. Comparative study of intestinal adenocarcinomas of animals and man. JNatl Cancer Inst. 1972;48:325–346. [PubMed] [Google Scholar]
- 12.Nagamine CM, Sohn JJ, Rickman BH, Rogers AB, Fox JG, Schauer DB. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2(−/−) Apc(Min/+) mouse. Infect Immun. 2008;76:2758–2766. doi: 10.1128/IAI.01604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phelps RA, Broadbent TJ, Stafforini DM, Jones DA. New perspectives on APC control of cell fate and proliferation in colorectal cancer. Cell Cycle. 2009;8:2549–2556. doi: 10.4161/cc.8.16.9278. [DOI] [PubMed] [Google Scholar]
- 15.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr, Thouless ME, Tsai CC, Bosch ML. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders KE, Shen Z, Dewhirst FE, Paster BJ, Dangler CA, Fox JG. Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J Clin Microbiol. 1999;37:146–151. doi: 10.1128/jcm.37.1.146-151.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truong CD, Feng W, Li W, Khoury T, Li Q, Alrawi S, Yu Y, Xie K, Yao J, Tan D. Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S.A. J Exp Clin Cancer Res. 2009;28:14. doi: 10.1186/1756-9966-28-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valverde CR, Tarara RP, Griffey SM, Roberts JA. Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.) Comp Med. 2000;50:540–544. [PubMed] [Google Scholar]
- 19.von Rahden BH, Langner C, Brucher BL, Stein HJ, Sarbia M. No association of primary adenocarcinomas of the small bowel with Epstein-Barr virus infection. Mol Carcinog. 2006;45:349–352. doi: 10.1002/mc.20163. [DOI] [PubMed] [Google Scholar]
- 20.Won YS, Vandamme P, Yoon JH, Park YH, Hyun BH, Kim HC, Itoh T, Tanioka Y, Choi YK. Helicobacter callitrichis sp. nov., a novel Helicobacter species isolated fromthe feces of the common marmoset (Callithrix jacchus) FEMS Microbiol Lett. 2007;271:239–244. doi: 10.1111/j.1574-6968.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang MQ, Chen ZM, Wang HL. Immunohistochemical investigation of tumorigenic pathways in small intestinal adenocarcinoma: a comparison with colorectal adenocarcinoma. Mod Pathol. 2006;19:573–580. doi: 10.1038/modpathol.3800566. [DOI] [PubMed] [Google Scholar]


