Abstract
Background
Although tacrolimus-based immunosuppression has made intestinal transplantation feasible, the risk of the requisite chronic high-dose treatment has inhibited the widespread use of these procedures. We have examined our 1990–1997 experience to determine whether immunomodulatory strategies to improve outlook could be added to drug treatment.
Study Design
Ninety-eight consecutive patients (59 children, 39 adults) with a panoply of indications received 104 allografts under tacrolimus-based immunosuppression: intestine only (n = 37); liver and intestine (n = 50); or multivisceral (n = 17). Of the last 42 patients, 20 received unmodified adjunct donor bone marrow cells; the other 22 were contemporaneous control patients.
Results
With a mean followup of 32 ± 26 months (range, 1–86 months), 12 recipients (3 intestine only, 9 composite grafts) are alive with good nutrition beyond the 5-year milestone. Forty-seven (48%) of the total group survive bearing grafts that provide full (91%) or partial (9%) nutrition. Actuarial patient survival at 1 and 5 years (72% and 48%, respectively) was similar with isolated intestinal and composite graft recipients, but the loss rate of grafts from rejection was highest with intestine alone. The best results were in patients between 2 and 18 years of age (68% at 5 years). Adjunct bone marrow did not significantly affect the incidence of graft rejection, B-cell lymphoma, or the rate or severity of graft-versus-host disease.
Conclusions
These results demonstrate that longterm rehabilitation similar to that with the other kinds of organ allografts is achievable with all three kinds of intestinal transplant procedures, that the morbidity and mortality is still too high for their widespread application, and that the liver is significantly but marginally protective of concomitantly engrafted intestine. Although none of the endpoints were markedly altered by donor leukocyte augmentation (and chimerism) with bone marrow, establishment of the safety of this adjunct procedure opens the way to further immune modulation strategies that can be added to the augmentation protocol.
Even with modern immunosuppressive therapies, clinical intestinal transplantation has not become a standard therapy for patients with irreversible intestinal failure.1,2 The management difficulties and unsatisfactory longterm outcome have stemmed largely from the inability to completely control rejection without resorting to chronic heavy immunosuppression. All too often, the consequence of efforts to prevent graft loss has been lethal infection, which has been the leading cause of death.1,2 In previous reports we identified three major immunologic risk factors: high blood tacrolimus levels, high-dose steroid requirements, and the need for adjunct OKT3 (antilymphoid) therapy.1 In order to lower the immunosuppressant requirements, we declared a 1-year moratorium in 1994, pending the results of extensive clarifying investigations by Murase and associates3 in rats.
When the program reopened, two changes in management strategy were instituted. One was an attempt to avoid, when possible, the transplantation of organs from cytomegalovirus (CMV-positive) donors to CMV-negative recipients. The other change was to give perioperative adjunct donor bone marrow when this was available, in order ro take advantage of the more tolerogenic profile of bone marrow cells compared to that of the intestinal passenger leukocytes.3-6
We report here our overall longterm results with 104 consecutive intestinal transplantations in 98 patients. The small bowel was engrafted alone, or as part of a multivisceral complex, before and after the moratorium. Of the 42 recipients in the later period, 20 were infused with bone marrow, the distinction from the other 22 being the willingness of the donor family to permit the extra procurement procedure.
METHODS
Case material
Recipient
Case accrual was between May 2, 1990 and August 11, 1997, during which 98 patients received 104 intestinal grafts: 35 alone, 48 with a liver, and 15 as part of a multivisceral graft. Of the 6 retransplantations, 2 were isolated intestine and 4 were liver-intestine or multivisceral. Children (0.5–16.8 years) outnumbered adults (18–58 years), and gender distribution was nearly equal. Other demographic and clinical features are summarized in Table 1. The causes of intestinal failure are listed in Table 2. Short gut syndrome was the most common (n = 78), with a variety of causes: predominantly thrombotic disorders, Crohn's disease, and trauma in adults and mostly volvulus, gastroschisis, necrotizing enterocolitis, and intestinal atresia in children.
Table 1.
Clinical Features of Intestinal Allograft Recipients
| Isolated intestine | Composite graft | Total | |
|---|---|---|---|
| Total no. of patients | 35 | 63 | 98 |
| Children/adults | 16/19 | 43/20 | 59/39 |
| Age (y) | |||
| Children* | 8 ± 5 | 4±4 | 5 ± 5 |
| Adults | 36 ± 11 | 32 ± 9 | 34 ± 10 |
| Male/female | 16/19 | 34/29 | 50/48 |
| Duration of TPN (mo) | 53 ± 47 | 39 ± 41 | 44 ± 43 |
| Total bilirubin (mg/dL)* | 1.2 ± 0.7 | 17.4 ± 14 | 12 ± 14 |
| No. of abdominal operations* | 5.1 ± 4.3 | 2.7 ± 2.2 | 3.6 ± 3.3 |
| Operative time (h)* | 12 ± 4 | 15 ±4 | 14 ±4 |
| No. of allografts | 37 | 67 | 104 |
| Cold ischemia time (h)* | 7 ± 2 | 9 ± 3 | 8±3 |
| HLA (-A, -B, -DR) mismatch | 4.5 ± 1.2 | 4.7 ± 1.1 | 4.6 ± 1.1 |
| Positive cross-match | 3 (8%) | 10 (15%) | 13 (13%) |
| Bone marrow augmentation | 8 (22%) | 12 (18%) | 20 (19%) |
| Followup (mo) | 23 ± 21 | 24 ± 26 | 24 ± 24 |
p < 0.05.
HLA, human leukocyte antigen; TPN, total parenteral nutrition.
Table 2.
Causes of Intestinal Failure and Indications for Transplantation
| Isolated intestine |
Composite graft |
Total |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Short gut syndrome | 28 | 80 | 50 | 79 | 78 | 80 |
| Dysmotility syndrome | 4 | 11 | 7 | 11 | 11 | 11 |
| Intestinal neoplasm* | 2 | 6 | 4 | 6 | 6 | 6 |
| Enterocyte dysfunction | 1 | 3 | 2 | 3 | 3 | 3 |
Desmoid, polyposis, and gastrinoma.
Interestingly, three of the pediatric patients had undergone liver replacement 4–10 years before intestinal transplantation. One of these is a 17-year-old boy who developed short gut syndrome because of midgut volvulus at age 4 and was placed on total parenteral nutrition (TPN). At age 13, he received a liver allograft at another transplant center because of TPN-induced cholestatic liver failure. The hepatic allograft was subsequently destroyed by TPN and the patient was referred for combined liver-intestine transplantation. The second patient had pseudo-obstruction after birth that was not diagnosed at the time of his liver transplantation for biliary atresia. The third patient developed short gut syndrome 10 years after his liver transplantation after extensive resection of the small bowel volvulus.
All 35 recipients of 37 intestine-only grafts were suffering from frequent central line sepsis, vanishing central venous access, and variable but usually minor biochemical evidence of nonicteric hepatic dysfunction. These patients had undergone an average of 5.1 previous operations. Forty-six (73%) of the 63 recipients of 67 composite allografts (multivisceral or liver-intestine) were United Network of Organ Sharing starus I (intensive care unit [ICU] bound), or II (permanently hospital bound).
Of the 15 multivisceral grafts, 13 included the liver; the liver was removed from the other 2 grafts and given to other patients. All 48 liver-intestine recipients and the 13 whose multivisceral grafts included liver had advanced hepatic disease (mean bilirubin > 17 mg/100 mL) that was TPN induced in most cases. Ischemia of the upper abdominal organs (adults) and diffuse irreversible gastrointestinal disease involving the foregut (mostly children) were the usual reasons for choosing multivisceral in preference to liver-intestine replacement.
The mean followup to September 8, 1997 is 24 ± 24 months (range, 1.0–86 months) for the total group, and 32 ± 26 months (range, 1–86 months) for 47 current survivors with grafts in place. Twelve of these recipients (three isolated intestine, nine composite) have had functional grafts for more than 5 years.
Donor
The grafts were all obtained from ABO blood type-identical cadaveric donors. Human leukocyte antigen (HLA) matching was random and uniformly poor (Table 1), with no examples of zero -A, -B, -DR mismatches. The lymphocytotoxic cross-match was positive after dithiothreitol treatment in 13 patients (Table 1). Because of the reported adverse effect of positive donor CMV serology on outcome,1,7 attempts were made in the recent cases to avoid using CMV-positive intestinal donors for CMV-negative recipients, particularly those waiting for intestine-only transplantation.
Operative procedure
Donor
Management policies and retrieval operations have been described previously.8-10 No attempts were made to modulate the graft immunologic tissue by donor or bowel irradiation, antilymphoid antibody treatment, or other modalities during intact donor circulation or subsequently. The University of Wisconsin solution was used for graft preservation in all but the first case, and the cold ischemia time ranged from 2.8 to 17 hours (Table 1). In two donors, both the pancreas and intestine were harvested en bloc and separated on the back table for two different recipients. The enteric and celiac ganglia have been preserved for the past 16 grafts in an attempt to reduce postoperative graft dysmotility.11
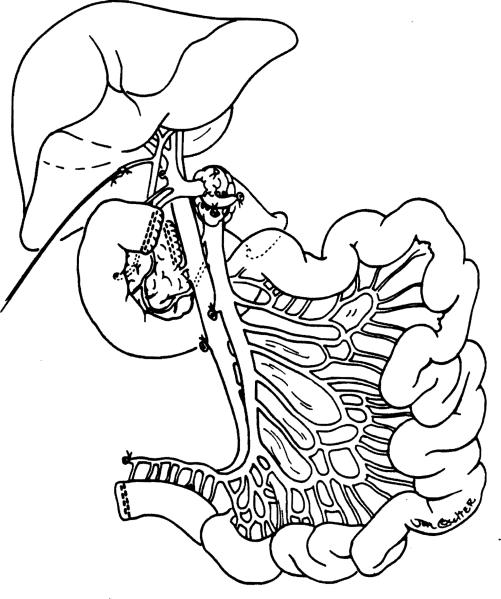
In the four most recent combined liver-intestinal allografts, the standard technique has been modified, with the harvest of the graft duodenum in continuity with the jejunum and allograft biliary system (Fig. 1). In one of these grafts, the left lateral hepatic segment was successfully used after an in situ split was performed to overcome a donor/recipient size mismatch in an ICU-bound 3-year-old child with hepatointestinal failure. In this case, the vascular and biliary structures of the left lateral liver segment were maintained in continuity with the intestine.
Figure 1.
Composite liver and intestinal graft with preservation of the duodenum in continuity with the graft jejunum and hepatic biliary system. Note transection of the pancreas to the right of the portal vein with preservation of the pancreatoduodenal arterial and venous arcade. The technique (used in four cases) reduces the time of the recipient operation and avoids the potential risks of biliary reconstruction.
Recipient
The principles and various modifications of the three generic intestinal transplantation procedures (intestine, liver-intestine, and multivisceral) have been reported elsewhere.8,10,12-17 The venous outflow from 28 (76%) of the 37 isolated intestinal grafts was drained into the recipient portal system; and in the other 9, it was directed into the inferior vena cava. When combined liver-intestinal transplantation was performed (n = 48), preliminary portocaval shunt of the native vessels was performed in order to prevent portal hypertension and bleeding during dissection and removal of the host organs. The shunt was left permanently in 33 (69%) of the operations, but in the remaining 15 it was disconnected after graft revascularization and the host portal vein was anastomosed to the side of the allograft portal vein.
A segment of large intestine was included with 32 (31%) of the allografts: 12 of the 37 (32%) isolated intestines, and 20 of the 67 (30%) composite grafts. Inclusion of the colon was abandoned after 1994 because it appeared to increase mortality.1
Management and monitoring
Nutrition
The conversion from parenteral to enteral alimentation was highly variable, necessitating flexible and complex metabolic and surgical management, which differed among the three cohorts of intestinal allograft recipients. This has been comprehensively described elsewhere.12,15,18,19 Resumption of oral diet was later in the composite graft recipients than in those receiving intestine-only.
Immunosuppression
Therapy was based on tacrolimus and prednisone. Adjunct prostaglandin E1 was infused intravenously during the early postoperative period to all but the first eight recipients. In the last 23 patients (10 isolated intestine, 13 composite), cyclophosphamide (cytoxan) was given at a dose of 2–3 mg/kg/day for 4 weeks and then switched to mycophenolate mofetil (15–30 mg/kg/day) or azathioprine. In a few cases, azathioprine was given as a third drug from the outset. Episodes of rejection were treated with adjustments of tacrolimus dose or supplemental prednisone (or both), and, if necessary, OKT3. Upward dose adjustments of mycophenolate mofetil, azathioprine, or steroids were frequently needed to compensate for tacrolimus dose reductions mandated by tacrolimus-related adverse effects.12,15,18
Bone marrow augmentation
With informed consent from both donor and recipient families, bone marrow (BM) cells were recovered from the intestinal donor, prepared, and infused intravenously into the recipient perioperatively. Intestinal recipients, for whom permission for BM harvest could not be obtained, were considered to be prospective contemporaneous control patients. The preparation of the “unpurged” cell infusate from donor thoracolumbar vertebrae has been described elsewhere.20-22 A single infusion of 3–5 × 108 cells/kg body weight was given for 20 minutes, 2–12 hours after revascularization of the intestinal graft (n = 20). All of the bowel transplantations were primary except in one patient of the BM group. Except for a disparity in gender distribution, the two cohorts were similar (Table 3).
Table 3.
Clinical Features of Bone Marrow-Augmented Intestinal Recipients and Control Patients
| BM-augmented group (n = 20) | Control group (n = 22) | |
|---|---|---|
| Children/adult | 13/7 | 16/6 |
| Male/female | 9/11 | 16/6 |
| Allograft | ||
| Intestine | 8 (40%) | 7 (32%) |
| Composite | 12 (60%) | 15 (68%) |
| Duration of TPN (mo) | 59 ± 53 | 49 ± 49 |
| Total bilirubin (mg/dL) | 17 ± 15 | 21 ± 16 |
| No. of abdominal operations | 5 ± 4 | 4 ± 3 |
| Operative time (h) | 14 ± 6 | 14 ± 4 |
| Cold ischemia time (h) | 9.7 ± 2.8 | 9.2 ± 3.0 |
| HLA (-A, -B, -DR) mismatch | 4.4 ± 1.1 | 4.4 ± 1.1 |
| Positive cytotoxic cross-match | 2 (10%) | 6 (27%) |
| Followup (mo) | 13 ± 12 | 13 ± 11 |
BM, bone marrow; TPN, total parenteral nutrition.
Chimerism
The presence of donor cells in the recipient's peripheral blood mononuclear cells were evaluated serially after transplantation by either flow cytometry or polymerase chain reaction (PCR). Monoclonal antibodies specific for donor HLA class I molecules were used for single color immunofluorescence analysis, which was performed using EPICS Elite flow cytometry (Coulter Corp., Hialeah, FL). The procedure used for cell staining has been detailed elsewhere.20 For PCR analysis, primers specific for donor HLA class II allele or else the SRY region of the Y chromosome (in male donors to female recipients) were used.
Rejection surveillance
Acute rejection was diagnosed by histopathologic studies of random and endoscopically guided multiple mucosal biopsies, usually of the ileum. A new rejection episode was defined by documentation of new histologic changes. Chronic rejection was diagnosed by full-thickness histologic examination of the enterectomy specimens. The criteria adopted for diagnosis of acute and chronic rejection have been described elsewhere.23 A total of 4,472 gastrointestinal allograft biopsies (4,093 small bowel, 272 colon, and 107 stomach), and 258 liver allograft biopsies were obtained during the study period and were examined by a single transplant pathologist. All of the liver rejection episodes tabulated here were biochemically suspected, histo-logically documented, and medically treated.
Graft-versus-host disease surveillance
Suspicious skin or gastrointestinal lesions were biopsied and studied by conventional histopathologic methods. Immunohistologic staining for donor-specific HLA antigens, and in situ hybridization technique using the Y chromosome-specific probe permitted skin chimerism to be ruled in or our.24-26 Other target organs of graft-versus-host disease (GVHD) (lungs, bone marrow, and liver, when relevant) were evaluated repetitively.
Infection
Protocols for prophylactic and active treatment of viral, bacterial, and fungal infections15,27 were adopted from those developed for liver transplantation. During the last half of the series, the newly developed technique of semiquantitative PCR assay of Epstein-Barr virus (EBV) in the peripheral blood was routinely used for early detection, and monitoring of EBV viremia, which forewarns of the development of B-cell lymphoma. CMV-specific hyperimmune globulin (Cytogam) was added to the early postoperative antiviral prophylaxis and used as an adjunct to ganciclovir for active EBV and CMV diseases.27
Statistical analysis
Data were collected for the total group, pooled at first, and then stratified according to the type of transplanted graft. Data analyses in the patients treated with adjunct BM focused on comparison of results with those of contemporaneous control patients. Continuous variables were presented as mean ± standard deviation (SD), and categorical data as proportions. Differences in group means were tested using the standard two-tailed sample t-test and differences in proportions by Fisher's exact test. The two modified multivisceral grafts (without a liver) were excluded from any comparative analysis between isolated intestine and composite grafts.
Patient and graft survival curves were generated using the Kaplan-Meier method,28 and group comparisons were done using the log-rank test.29,30 The isolated intestinal recipients in whom the allograft was removed, and immunosuppression was stopped, were censored at the time of their hospital discharge. The cumulative risk of rejection and graft loss from rejection were estimated using the Kaplan-Meier method.
Risk factors for mortality, graft loss, and morbidity were analyzed using Cox's proportional hazards model.31 Factors independently associated with Outcomes were identified using a stepwise (backward elimination method) procedure.
The mean values for the tacrolimus and steroid doses and rhe measured 12-hour tacrolimus whole blood trough levels were collected for each patient at 7 days, 30 days, 90 days, 6 months, 12 months, and every year after. The trough plasma levels that were obtained early in the study were converted to whole blood values by multiplying each plasma level by a factor of 10.32 The values were pooled for the BM-augmented and control cohorts and the mean value ± standard error calculated.
The dependence between recurrent intestinal rejection and graft failure (ie, death or retransplantation) and mortality was examined by incorporating recurrent events as time-dependent covariates, in Cox's proportional hazards model.33 The effect of recurrent rejection was adjusted for the various relevant factors.
All analyses were performed using SPSS (SPSS, Inc., Chicago, IL) and S-Plus (Stat Sci Division, Seattle, WA) for Windows software.
RESULTS
Patient survival
Overall
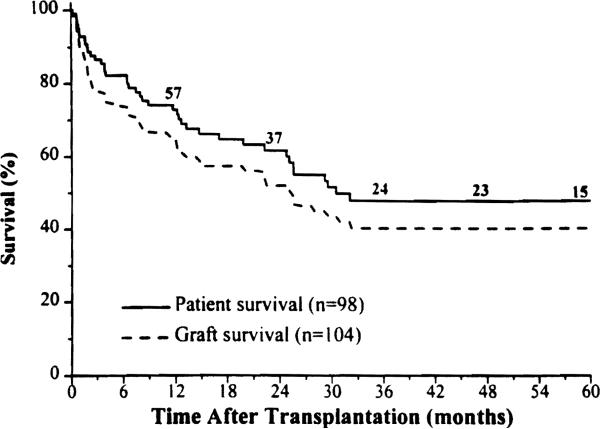
Current survivors who are still bearing their grafts (n = 47) have been followed up for 32 ± 26 months (range, 1–86 months); 2 are successful retransplant recipients (one isolated intestine, and one combined liver-intestine). Fifteen recipients survived more than 5 years. Kaplan-Meier survival rate is 72% at 1 year and 48% at 5 years (Fig. 2). Most of the deaths occurred during the first 30 postoperative months.
Figure 2.
Kaplan-Meier patient and graft survival rates for the total patient group.
The survival rate was similar among the three different types of transplantation (p = 0.6). Of the seven isolated intestinal recipients who were censored posthospitalization following graft enterectomy, five died later of TPN-related complications. The other two are alive and at home on TPN.
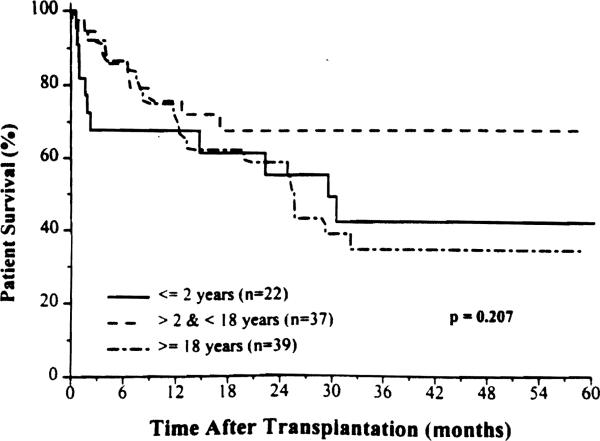
The effect of age on survival is shown in Figure 3. Patients 2 years and younger had a high early post- operative mortality, with technical failure being the leading cause of death (50%). The best results were in patients between 2 and 17 years of age, in whom the 5-year cumulative survival rate was 68%.
Figure 3.
Patient survival according to recipient age.
BM augmentation study
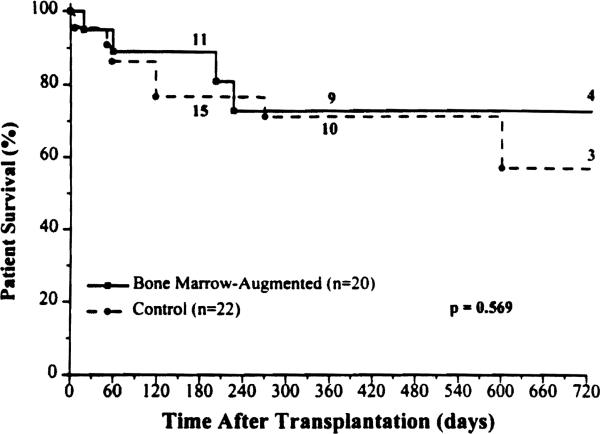
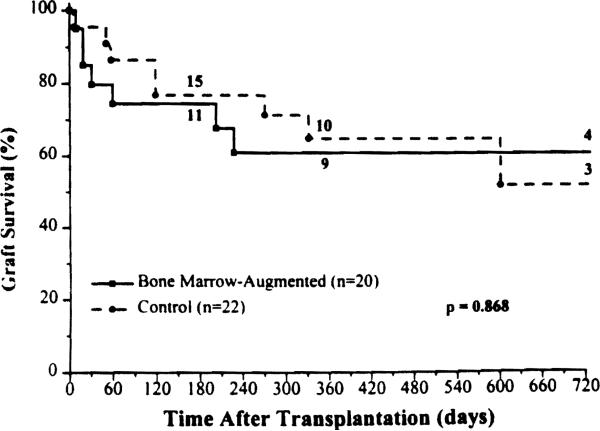
The 6-month and 2-year patient survival rate in the study group was 89% and 72%, respectively (Fig. 4), compared with 77% and 57%, respectively, in the control group. Importantly, no mortality occurred beyond the first postoperative year in the BM group. Fourteen patients in each cohort are currently alive bearing functioning grafts, all with nutritional autonomy.
Figure 4.
Patient survival rates for the bone marrow-augmented and control groups.
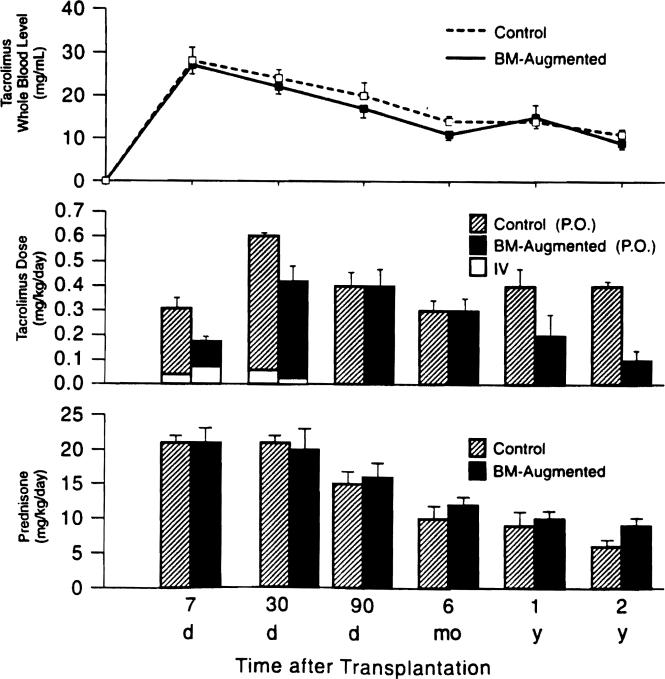
The time and cause of death in both groups are listed in Table 4. Of interest, only one of the four deaths in the experimental group was from a classic complication of immunosuppression, versus four of the seven deaths in the control group plus a fifth from intractable rejection. This did not correlate with a greater dependence on high dose prednisone or tacrolimus during the dangerous early posttransplant period, compared with the requirements in the BM-augmented cohort (Fig. 5).
Table 4.
Time and Cause of Death Among Bone Marrow-Augmented Intestinal Recipients and Control Patients
| Pt. No. | Graft | Time (days) | Cause |
|---|---|---|---|
| BM-augmented (n = 20) | |||
| 1 | L/I | 19 | Hepatic artery thrombosis |
| 2 | L/I | 60 | Pancreatitis and gastric bleeding |
| 3 | MV | 203 | Aspergillosis |
| 4 | L/I | 228 | Acute dissection of ascending thoracic aorta |
| Control (n = 22) | |||
| 1 | L/I | 6 | Arterial thrombosis |
| 2 | L/I | 51 | Cerebral infarction* |
| 3 | MV | 58 | Acute rejection |
| 4 | I | 119 | Aspergillosis |
| 5 | L/I | 119 | Aspergillosis |
| 6 | L/I | 27 | CMV/B-lymphoma |
| 7 | L/I | 602 | Aspergillosis |
Intraoperative cardiac arrest.
Pt. No., patient number; BM, bone marrow; L/I, liver-intestine; MV, multivisceral; I, intestine; CMV, cytomegalovirus.
Figure 5.
Tacrolimus whole blood trough levels and tacrolimus and prednisone doses in the bone marrow (BM)-augmented and control groups. IV, intravenous; PO, oral.
The beginning dosages of the multiple agents were similar in both groups, except for an arbitrarily higher rate of cyclophosphamide use for induction in the control patients (61 % versus 41 %). It was noteworthy, however, that the oral tacrolimus doses required to maintain equivalent blood level were lower in the BM cohort (Fig. 5). In addition, OKT3 was thought necessary to control rejection in only four (20%) of the BM group versus nine (41%) of the control cohort.
Graft survival
Overall survival
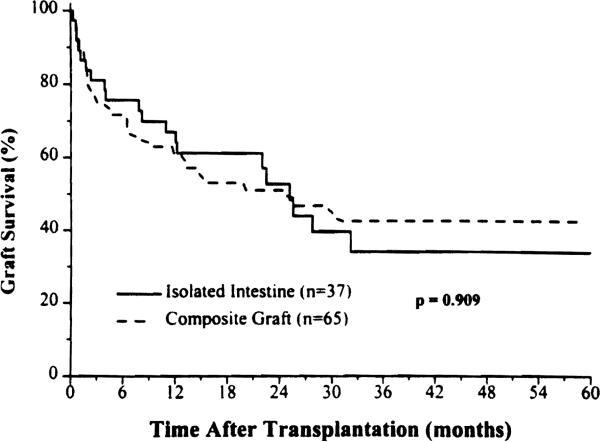
The estimated 1- and 5-year survival (n = 104) was 64% and 40% (Fig. 2), with no difference between the intestine-alone and composite grafts (p = 0.97). Examination of the survival curves, however, showed a crossing of lines between 2 and 3 years whereby the superior earlier survival of the isolated intestinal grafts was lost (Fig. 6). This correlated with a higher cumulative risk of graft loss from rejection (see later).
Figure 6.
Allograft survival of the intestine-only or as part of a composite graft that contained liver.
BM augmentation study
Survival of the BM-augmented grafts was 75% at 6 months, and 61% at 2 years compared with 77% and 52% in the control group (Fig. 7). It should be noted, however, that two of the graft losses in the experimental group were unequivocally caused by management or technical errors (one example each). One of these patients is alive after retransplantation; the other died of liver failure more than 2 years after resuming TPN.
Figure 7.
Graft survival rates for the bone marrow-augmented and control groups.
One (5%) of the BM-augmented, and 2 (9%) of the control grafts were lost because of acute rejection.
Graftectomy and retransplantation
Intestine alone
Thirteen graft enterectomies were performed in 12 recipients (8 adults, 4 children), 9–840 days from the time of transplantation (median, 285 days) because of refractory acute (n = 6) or chronic rejection (n = 2), B-cell lymphoma (n = 2), neuronal demyelinization (n = 1), CMV retinitis/enteritis (n = 1), or pseudomonas pneumonia (n = 1).
Although retransplantation was done in three isolated intestine recipients, using intestine-only (n = 2) or a multivisceral graft (n = 1) 16, 61, and 340 days after primary enterectomy, two of the recipients died 20 days and 5 months later, respectively; the third is well 32 months after the second intestine-only retransplantation. Of the other nine patients whose intestine-only grafts were excised, two died of sepsis after their enterectomies, five died later of TPN-related complications, and two are alive 28 and 40 months after enterectomy.
Composite grafts
Two of the 48 liver-intestine recipients had their failing primary grafts removed and replaced at 30 and 59 days. A third underwent multivisceral transplantation 455 days after the primary liver-intestine procedure, and a fourth had attempted replacement of an infarcted liver but not the intestine, which still functioned. The only survivor, who is well 3 months after retransplantation, was the one with intervention at 30 days. The two others whose entire grafts were removed died after 1.5 and 2 months from intractable acute rejection and B-cell lymphoma, respectively.
No multivisceral retransplantations were performed.
Causes of Death
In addition to the foregoing 13 deaths, 36 patients died with their primary graft in place. The primary causes of these 36 mortalities were infection (n = 15), technical/management errors (n = 8), B-cell lymphoma (n = 6), rejection (n = 5), and other (n = 2). All of the technical-, lymphoma-, and rejection-related deaths were among the composite allograft recipients. At the time of their death, 18 (50%) of these 36 recipients had already achieved and maintained full nutritional autonomy, and 6 (17%) had partially functioning intestinal allografts.
Risk Factors
We previously reported six risk factors: high tacrolimus blood level, steroid bolus therapy, use of OKT3, length of operation, CMV disease, and inclusion of a segment of colon with the graft.1 With univariate and multivariate analyses, other important ones have emerged. Both patient and graft survival were significantly influenced by number of intestinal rejection episodes, development of B-cell lymphoma, cold ischemia time, number of previous abdominal operations, and male donor to male recipient.
Rejection
Overall
Acute rejection severe enough to be treated was diagnosed by histologic findings in 97 (93%) of the 104 intestinal allografts; most were graded mild to moderate. The degree of HLA antigen mismatch did not affect the incidence (p = 0.9) nor the frequency of rejection (p = 0.6). More than 50% of the episodes occurred within the first 90 days after transplantation. Only 14% were diagnosed and treated beyond the second year after transplantation.
The incidence of intestinal rejection was similar for both positive and negative lymphocyrotoxic cross-match grafts (p = 0.3). The mean number of episodes, however, was significantly higher in positive cross-match grafts (5.4 ± 2.6) compared with those who had negative cross-match grafts (3.3 ± 3) (p = 0.03).
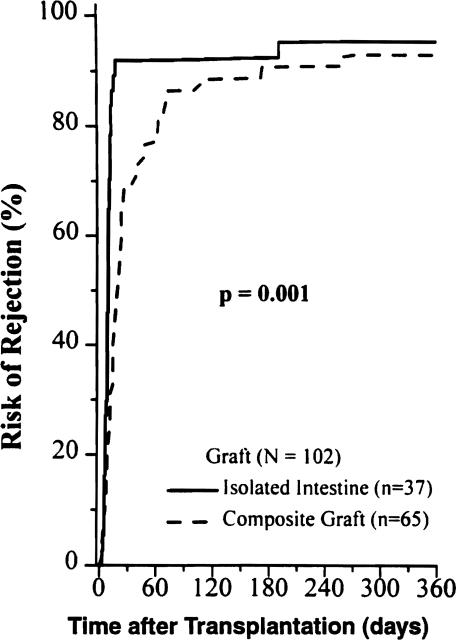
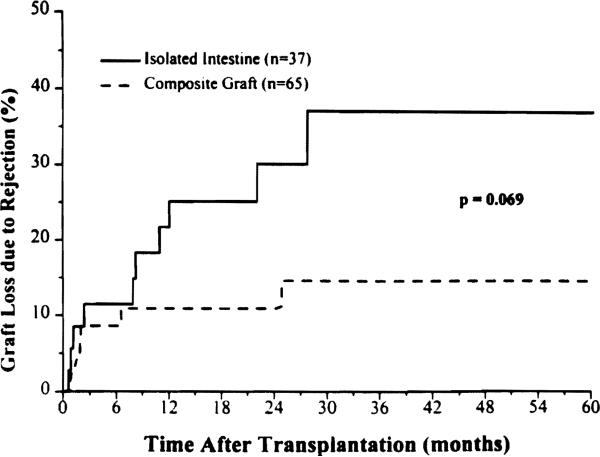
The isolated intestine had a significantly higher incidence of rejection (92%) compared with intestine contained in a composite graft (66%) during the first 30 postoperative days (p = 0.004), a gap that narrowed during the rest of the first postoperative year but remained significant (p = 0.001) (Fig. 8). Although the cumulative rejection rate of intestine contained in a composite graft approached that of isolated intestine (Fig. 8), the rate of graft loss from rejection was less than half (Fig. 9).
Figure 8.
Cumulative risk of intestinal rejection for the isolated intestine and composite grafts that contained liver.
Figure 9.
Cumulative risk of graft loss from rejection in the intestine-only and composite visceral grafts that contained liver.
The median postoperative time to the first episode of intestinal rejection was 9 days for the isolated intestine, and 19 days for the composite grafts (p = 0.0001). OKT3 was required to treat steroid-resistant rejection in 20 (54%) of the isolated intestine, and 15 (23%) of the composite grafts (p = 0.002). Systemic venous drainage of the allografted intestine did not increase the risk of rejection or graft loss (p = 0.7).
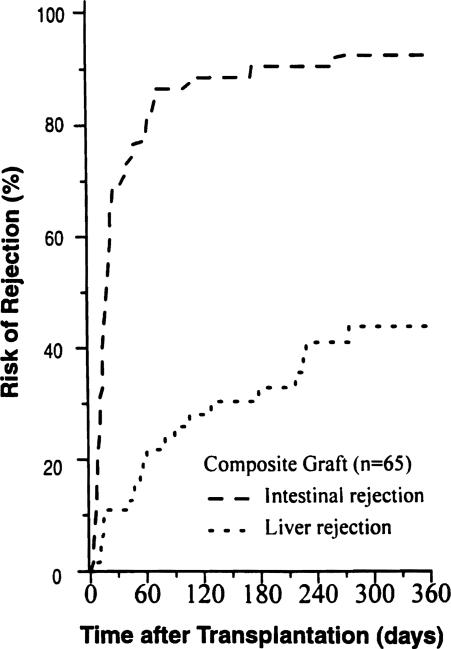
In the recipients of composite grafts, the incidence of liver rejection was less than half of that in the intestine (Fig. 10). The median time to diagnosis of the first episode was 58 days, and the mean number of episodes in all cases was 0.78 ± 1.5 days.
Figure 10.
Cumulative risk of both intestinal and hepatic rejection in the composite visceral allografts.
Eleven (34%) of the 32 intestinal allografts, which included colon, showed histologic evidence of colonic rejection at some time. Two (12%) of the multivisceral grafts had histologically proved gastric rejection, and another two (12%) had pancreatic rejection.
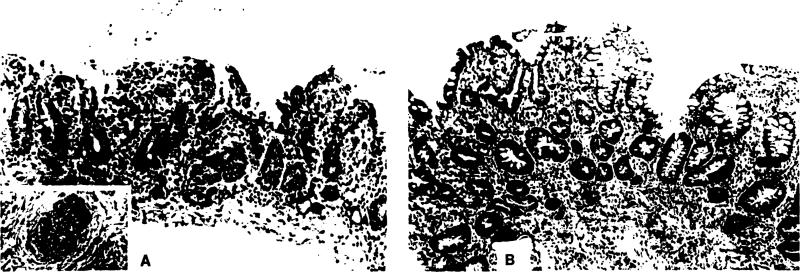
Acute vascular rejection was histologically documented in three of the isolated intestinal allografts, two of which were from strongly positive lymphocytotoxic cross-matched donors. One positive and one negative cross-match graft eventually were lost to rejection; the third fully recovered (Fig. 11).
Figure 11.
Acute vascular rejection of positive cross-match isolated intestinal graft. (A) This biopsy specimen obtained 4 hours after transplantation shows typical changes of acute vascular rejection, with pronounced hemorrhage and congestion obscuring the lamina propria. The villi, although intact, are shortened, and the surface epithelium is focally lost. Inflammatory infiltration is minimal. Small arterioles demonstrated endothelial hypertrophy, mural thickening, platelet thrombi, and focally sparse neutrophil infiltration (insert). (B) Fifteen days after transplantation and after treatment with steroids and OKT3, the mucosa displays prominent evidence of mucosal regeneration. The crypts are irregular, branched, and lined by hyperplastic epithelium with crowded, pseudostratified cells. Toward the luminal surface, early villus formation can be appreciated. The lamina propria retains a mild component of inflammatory cells, primarily small lymphocytes and scattered plasma cells.
Chronic rejection was diagnosed in three enterectomy specimens for an incidence with isolated intestinal transplantation of 8%. The only example of chronic rejection of a composite graft was in a patient whose donor was strongly cross-match positive; both the intestine and liver were affected, and the recipient died of combined hepatic and intestinal failure. Because the diagnosis of chronic rejection requires a transmural specimen that is not deliberately obtained in biopsies, the incidence of chronic rejection undoubtedly was grossly underestimated.
BM augmentation study
Although the incidence of acute rejection during the first postoperative month was higher (p = 0.2) in the BM cohort (85%) compared with the control patients (64%), the overall incidence, frequency, and median time of onset of the first episode were similar in the two groups. OKT3 was used to control rejection in four (20%) of the BM recipients, and nine (41%) of the control patients (p = 0.2).
Viral infections
CMV disease
Thirty-five (36%) of the 98 recipients had at least one episode of clinically significant new or reactivated CMV infection, with a median onset of 65 days after transplantation (range, 10–276 days). The incidence according to the donor and recipient CMV serologic status was 68% for positive to negative; 54% for positive to positive; 36% for negative to positive; and 8% for negative to negative. The difference in risk of CMV disease in adults (44%) and children (31%) was not significant (p = 0.2). There was no difference in the incidence and onset of CMV disease between isolated and composite graft recipients, or during the BM augmentation trial between patients given the adjunct treatment (35%) and control patients (44%) (p = 0.8).
EBV-associated b-cell lymphoma
Twenty patients (20%) developed posttransplant EBV-related B-cell lymphoma at a median time of 8.7 months after transplantation (range, 1.1–61.8 months), involving both native and transplanted organs. The abnormality was found in the autopsy specimens of four of these recipients. Children (27%) were at a significantly higher risk than adults (11 %) (p = 0.02). The disease was lethal in 9 (45%) of the 20 patients (7 children, 2 adults).
The multivisceral patients (33%) were more prone than the recipients of liver-intestine (21%), and intestine-only (11%) grafts (p = 0.2). In the BM augmentation trial period, the incidence was 2 (10%) of 20 in the experimental group and 4 (18%) of 22 in the control patients (p = 0.60).
Using univariate and multivariate analyses, young age, type of intestinal graft (multivisceral), and recipient splenectomy were the three significant risk factors for the development of B-cell lymphoma.
Graft-versus-host disease
Skin changes suspicious for GVHD were observed in 11 patients, but histologically verified in only 5 (5%). Two of the 5 had received intestine-only and the other 3 composite grafts. The GVHD was lethal in only one patient, a previously reported child34 with preexisting IgA deficiency who received a liver-intestine graft. One patient, an adult recipient of a multivisceral graft, developed chronic GVHD, which was diagnosed by biopsy of the buccal mucosa. This patient eventually died of disseminated B-cell lymphoma, with the chronic GVHD still active.
The disease was self-limited in the other three patients, one of whom had received adjunct BM. The latter patient developed a mild skin rash 188 days after liver-intestine transplantation. The disease was easily controlled in this and another intestine-only recipient by an increase in the daily tacrolimus and steroid doses. The third patient experienced transient GVHD 6 days after graft enterectomy and withdrawal of immunosuppression because of the development of B-cell lymphoma.
Chimerism
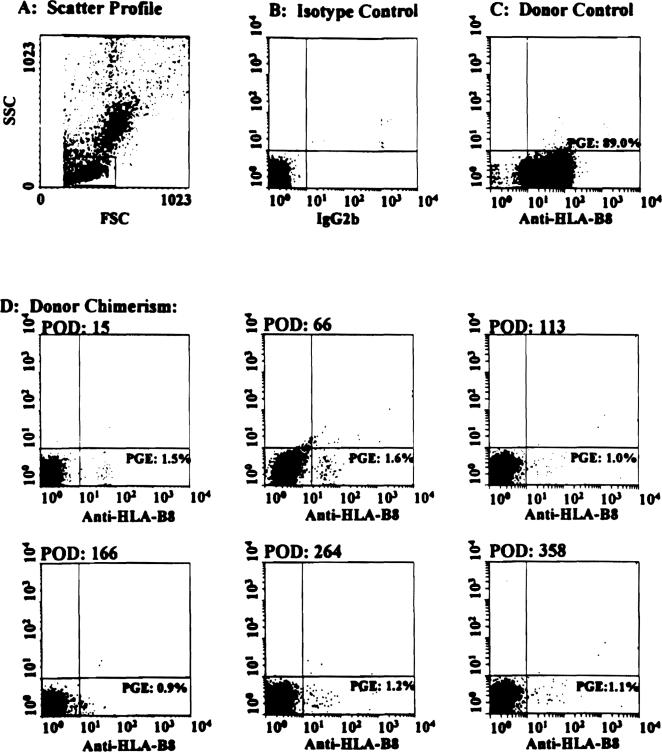
The nonavailability of either an appropriate monoclonal antibody or the donor-specific primer precluded the analysis of chimerism in four BM-augmented and seven control patients. Using these techniques, however, at their most recent followup, 16 (100%) of 16 study and 12 (80%) of 15 control patients had evidence for the presence of donor cell chimerism. Additionally, when followed up serially after transplantation, BM-augmented recipients had much higher levels of donor cell chimerism than control patients (Fig. 12); an observation similar to that published previously for other BM-augmented organ transplant recipients.35
Figure 12.
(A–D) Serial determination of donor cell chimerism in the peripheral blood of a bone marrow-augmented small bowel recipient for up to 358 days after transplantation. (D) For the determination of chimerism, FITC-conjugated monoclonal antibody specific for donor HLA class I (anti-B8) was used. (B) Cells stained with fluorochromeconjugated, irrelevant, isotype-matched (IgG2b monoclonal antibody and (C) those obtained from the cadaveric donor were used as negative and positive controls, respectively. (A) Orthogonal and side scatter profiles were used to establish gates for lymphocyte subpopulation, analysis of which was done using an EPICS Elite flow cytometer (Coulter, Hialeah, FL). As evident in Figure D, 0.90%–1.6% of donor cell chimerism was present in the peripheral blood of this recipient and has remained relatively stable for up to 1 year after transplantation. SSC, side scatter channel; FSC, forward scatter channel; HLA, human leukocyte antigen; PGE, percentage gated events; POD, postoperative day.
Longterm rehabilitation
Forty-three (91%) of the 47 current survivors are home and completely off TPN, with full nutritional autonomy. Two of the remaining four recipients require home parenteral nutrition because of chronic graft dysmotility. The other two patients are receiving partial intravenous nutritional support while recovering from a recent episode of severe intestinal rejection. No attempts have been made to date to electively reduce or wean any of the intestinal recipients, including the BM-augmented recipients, off immunosuppression.
DISCUSSION
Empirical progress in organ transplantation depended for 30 years on a search for more potent baseline immunosuppressants, without understanding the basis for the prototypic immunologic confrontation (rejection) and involution (“graft acceptance”) that was first observed in kidney recipients treated with azathioprine and prednisone.36 The advent of cyclosporine elevated the expectations with transplantation of most organs to the level of patient service, but the resulting revolution largely bypassed the intestine. Nevertheless, prolonged alimentary nutrition (ie, > 6 months) from human intestine, transplanted as a component of composite grafts37-40 or alone41,42 was achieved for the first time using cyclosporine-based immunosuppression between November 1987 and the end of 1989. These were rare cases, however, and only the pediatric patient of Goulet and associates,42 who received an isolated neonatal intestine, remains alive.
With the clinical introduction of tacrolimus in 198943 and the demonstration of superior clinical results with a variety of organ allografts, compared with results previously with cyclosporine,44 interest was intensified in intestinal transplantation. After preclinical studies showed the superiority of the drug in animal models of intestine-alone and multivisceral transplantation,45-48 large-scale trials of intestinal and composite visceral transplantation were undertaken in 1990.1,12-15 The present report accounts for all cases accrued since then at the University of Pittsburgh, divided into two cohorts, three-fifths before and two-fifths after the 1-year moratorium of 1994.
With 12 patients from the first period in good nutritional condition beyond the 5-year posttransplant milestone, there is little residual doubt about the feasibility, if not the reliability, of all three bowel transplant procedures. It has also become evident from data reported previously,1,15 and now, that the management strategy must be modified further before these operations find a respected place in the surgical armamentarium. The observations described here, combined with major advances in transplantation immunology (see later), provide insight into how this may be done safely.
During the same 1990-1997 period of the intestinal transplant case accrual, an explanation applicable to all organs evolved for the previously enigmatic ability to transplant HLA-incompatible allografts. This began with the discovery that the hematolymphopoietic cells of BM origin (passenger leukocytes), which are normal constituents of all organs, migrate and engraft peripherally after successful transplantation.24-26,49 Until this time, clinical trials of transplantation of the leukocyte-rich intestine had been inhibited by the fear that the lymphoid cells would cause GVHD, such as occurs after conventional BM transplantation from HLA mismatched donors to cytoablated recipients.
As a result of this anxiety, many experimental studies of intestinal transplantation until the late 1980s were designed for the pretransplant destruction of the lymphoid-rich passenger leukocytes.50-52 It became clear that a mutual nullification of the host-versus-graft (rejection) and graft-versus-host reactions could be expected in the immunologically intact recipient under conditions of immunosuppression that equally affect both donor and recipient cell populations.53 The bidirectional induction of specific nonreactivity occurs primarily by a mechanism of clonal exhaustion/deletion that takes place in organized lymphoid centers,24,49 in the same way as tolerance to noncytopathic intracellular parasites (viral, bacterial, and protozoa) leads to a “carrier state.”54 Because the exhaustion/deletion that takes place in organized lymphoid collections is neither absolute nor irreversible, its maintenance is dependent on the persistence of donor leukocyte chimerism that may be present in these locations at very low levels (microchimerism).
In a second mechanistic analogy to infectious immunity,54 the migration of passenger leukocytes from the graft and their replacement by those in a reverse traffic from the recipient suggests a second less quantifiable mechanistic contribution of immune indifference. Because a large number of the cells departing the graft find their way to the skin and other nonlymphoid centers, immune indifference could be operational at the graft level or peripherally, or at both sites.54 In experimental models, pure examples of immune indifference can be produced by pretransplant depletion of the passenger leukocytes, but the chimerism-dependent donor-specific tolerance is not thereby induced.55-57
These two tolerance mechanisms are generic with the successful engraftment of all organs.24,49,53,54 It has been shown in rat experiments, however, that the lineage profile of passenger leukocyte in intestine has inferior tolerogenic qualities (ie, fewer undifferentiated stem, precursor, and myeloid cells and more mature T and B lymphocytes than in BM and solid organs, especially the liver).3,58 In the clinical context of our intestinal transplant series, the consequent protective effect on the intestine of the contemporaneously transplanted liver was obvious, and explained by the similarity of the hepatic passenger leukocyte lineage profile to that of BM. The cumulative risk of intestinal loss to rejection in a composite graft was only one third that incurred by intestine that was transplanted alone (Fig. 9).
With the information already available in 1994 from many of the foregoing clinical and experimental investigations, it was possible to proceed with the trial of donor leukocyte augmentation1 without the historically rooted fear of an increased risk of GVHD that has been exemplified by the warnings of Gruessner and coauthors.59 The absence of added risk was evident in the results. There were no examples of lethal GVHD in the 20 patients treated with adjunct BM, and the incidence of less significant GVHD was no greater than in the contemporaneous control group. Other possible complications of BM augmentation (eg, a greater risk of rejection or of B-cell lymphomas) were not seen.
Although the trial has established the safety of BM augmentation, definitive evidence of efficacy is expected to take 5 to 10 years to be manifest, as has been the case with adjunct BM infusion with other organs.20,60 Because the time of greatest need for improvement is the morbidity- and mortality-ridden first posttransplantation months, however, we consider the augmentation trial to be only the first step in developing an immune modulation strategy involving cytoablation of the graft passenger leukocytes that will ameliorate the early posttransplantation risk. In our pretacrolimus cases of multivisceral transplantation,8,37,61 this was done by treating the cadaveric donor with large doses of OKT3 before organ extirpation, followed by low-dose donor intestinal irradiation before or after graft revascularization.
In these early cases, and in virtually all others elsewhere in which similar graft modification was attempted, the recipients developed lethal operative B-cell lymphomas in the allografts and systemically.8 Consequently, this approach was abandoned despite evidence that intestinal cytoablation in experimental models caused a reduction in GVHD incidence and improved survival.50-52 In retrospect, the lymphoma complication seen in patients may have been from the imbalance of the T-cell to B-cell population in the grafts caused by administration to the donors of the T-cell directed antilymphoid agents.
Graft cytoreduction with relatively low doses of ex vivo irradiation (500–100 R), as reported by Williams and associates52 in a canine model is thought to proportionately reduce mature T and B cells without killing of stem and precursor cells and without seriously damaging either the vascular or epithelial components of the intestinal graft (Murase and coworkers, unpublished observations). In experiments using the same GVHD-prone rat intestinal transplant models used originally to demonstrate the dynamics of passenger leukocyte traffic,3,58 a dramatic benefit has been demonstrated of ex vivo irradiation combined with systemic donor BM cell infusion (Murase and coworkers, unpublished observations). Consequently, we plan to bring the combined strategy of graft irradiation plus adjunct donor BM infusion to clinical trial.
Acknowledgment
We would like to thank Dolly Martin, Lynn Ostrowski, Anita Krajack, Mauricio Geraldo, MD, and Scott Miller for their great help in data collection.
This study was supported in part by Project Grant No. DK 29661 from the Nalional Institutes of Health, Belhesda, MD.
Footnotes
Presented at the American College of Surgeons 83rd Annual Clinical Congress, Chicago, October 1997.
References
- 1.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;3:270–282. doi: 10.1097/00000658-199509000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant D. Current results of intestinal transplantation. The International Intestinal Transplant Registry. Lancet. 1996;347:1801–1803. doi: 10.1016/s0140-6736(96)91619-0. [DOI] [PubMed] [Google Scholar]
- 3.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murase N, Demetris AJ, Woo J, et al. Lymphocyte traffic and graft-versus-host disease after fully allogeneic small bowel transplantation. Transplant Proc. 1991;23:3246–3247. [PMC free article] [PubMed] [Google Scholar]
- 5.Murase N, Demetris AJ, Woo J, et al. Graft versus host disease (GVHD) after BN to LEW compared to LEW to BN rat intestinal transplantation under FK 506. Transplantation. 1993;55:1–7. doi: 10.1097/00007890-199301000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanabe M, Murase N, Demetris AJ, et al. The influence of donor and recipients strains in isolated small bowel transplantation in rats. Transplant Proc. 1994;26:4325–4332. [PMC free article] [PubMed] [Google Scholar]
- 7.Bueno J, Green M, Kocoshis S, et al. Cytomegalovirus infection after intestinal transplantation in children. Clin Infect Dis. 1997;25:1078–1083. doi: 10.1086/516113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Todo S, Tzakis A, et al. The many faces of multivisceral transplantation. Surg Gynecol Obstet. 1991;172:335–344. [PMC free article] [PubMed] [Google Scholar]
- 9.Casavilla A, Selby R, Abu-Elmagd K, et al. Logistics and technique for combined hepatic-intestinal retrieval. Ann Surg. 1992;216:605–609. doi: 10.1097/00000658-199211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furukawa F, Abu-Elmagd K, Reyes J, et al. Technical aspects of intestinal transplantation. In: Braverman MH, Tawes RL, editors. Surgical Technology International. Vol. 2. Universal Medical Press; San Francisco: 1994. pp. 165–170. [PubMed] [Google Scholar]
- 11.Hirose R, Taguchi T, Hirata Y, et al. Immunohistochemical demonstration of enteric nervous distribution after syngeneic small bowel transplantation in rats. Surgery. 1995;117:560–569. doi: 10.1016/s0039-6060(05)80256-9. [DOI] [PubMed] [Google Scholar]
- 12.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Todo S, Tzakis A, Reyes J, et al. Small intestinal transplantation in humans with or without colon. Transplantation. 1994;57:840–848. doi: 10.1097/00007890-199403270-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Todo S, Tzakis A, Abu-Elmagd K, et al. Abdominal multivisceral transplantation. Transplantation. 1995;59:234–240. doi: 10.1097/00007890-199501270-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu-Elmagd K, Todo S, Tzakis A, et al. Three years clinical experience with intestinal transplantation. J Am Coll Surg. 1994;179:385–400. [PMC free article] [PubMed] [Google Scholar]
- 16.Tzakis AG, Todo S, Reyes J, et al. Piggyback orthotopic intestinal transplantation. Surg Gynecol Obstet. 1993;176:297–298. [PMC free article] [PubMed] [Google Scholar]
- 17.Tzakis AG, Nour B, Reyes J, et al. Endorectal pull through of transplanted colon as part of intestinal transplantation. Surgery. 1995;117:451–453. doi: 10.1016/s0039-6060(05)80066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Elmagd K, Fung JJ, Reyes J, et al. Management of intestinal transplantation in humans. Transplant Proc. 1992;24:1243–1244. [PMC free article] [PubMed] [Google Scholar]
- 19.Reyes J, Tzakis AG, Todo S, et al. Nutritional management of intestinal transplant recipients. Transplant Proc. 1993;25:1200–1201. [PMC free article] [PubMed] [Google Scholar]
- 20.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Demetris AJ, Rao AS, et al. Spontaneous and iatrogenically augmented leukocyte chimerism in organ transplant recipients. Transplant Proc. 1994;26:3071–3076. [PMC free article] [PubMed] [Google Scholar]
- 22.Rao AS, Fontes P, Zeevi A, et al. Augmentation of chimerism in whole organ recipients by simultaneous infusion of donor bone marrow cells. Transplant Proc. 1995;27:210–212. [PMC free article] [PubMed] [Google Scholar]
- 23.Lee RG, Nakamura K, Tsamandas AC, et al. Pathology of human intestinal transplantation. Gastroenterology. 1996;110:1820–1834. doi: 10.1053/gast.1996.v110.pm8964408. [DOI] [PubMed] [Google Scholar]
- 24.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 25.Starzl TE, Demetris AJ, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–877. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism after liver transplantation for type IV glycogen storage disease and type I Gaucher's disease. N Engl J Med. 1993;328:745–749. doi: 10.1056/NEJM199303183281101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reyes J, Bueno J, Kocoshis S, et al. Current status of intestinal transplantation in children. J Ped Surg. 1998;33:243–254. doi: 10.1016/s0022-3468(98)90440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Matthews DE, Farewell VT. In: Using and understanding medical statistics. Matthews DE, Farewell VT, editors. Basel. Karger; New York: 1985. pp. 67–87. [Google Scholar]
- 30.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Reports. 1966;50:163–170. [PubMed] [Google Scholar]
- 31.Cox DR. Regession models and life tables [with discussion]. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 32.Warty V, Zuckerman S, Venkataramanan R, et al. Tacrolimus analysis: a comparison of different methods and matrices. Ther Drug Monit. 1995;17:159–167. doi: 10.1097/00007691-199504000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andersen PK, Gill RD. Cox's regession model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- 34.Reyes J, Todo S, Green M, et al. Graft-versus-host disease after liver and small bowel transplantation in a child. Clin Transplant. 1997;11:345–348. [PMC free article] [PubMed] [Google Scholar]
- 35.Rudert WA, Kocova M, Rao AS, Trucco M. Fine quantitation by competitive PCR of circulating donor cells in posttransplant chimeric recipients. Transplantation. 1994;58:964–965. doi: 10.1097/00007890-199410270-00022. [DOI] [PubMed] [Google Scholar]
- 36.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 37.Starzl TE, Rowe MI, Todo S, et al. Transplantation of multiple abdominal viscera. JAMA. 1989;261:1449–1457. [PMC free article] [PubMed] [Google Scholar]
- 38.Grant D, Wall W, Mimeault R, et al. Successful small bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 39.McAlister V, Wall W, Ghent C, et al. Successful small intestine transplantation. Transplant Proe. 1992;24:1236–1237. [PubMed] [Google Scholar]
- 40.Margreiter R, Konigsrainer A, Schmid T, et al. Successful multivisceral transplantation. Transplant Proc. 1992;24:1226–1227. [PubMed] [Google Scholar]
- 41.Deltz E, Schroeder P, Gundlach M, et al. Successful clinical small-bowel transplantation. Transplant Proc. 1990;22:2501. [PubMed] [Google Scholar]
- 42.Goulet O, Revillon Y, Brousse N, et al. Successful small bowel transplantation in an infant. Transplantation. 1992;53:940–943. doi: 10.1097/00007890-199204000-00046. [DOI] [PubMed] [Google Scholar]
- 43.Starzl TE, Todo S, Fung J, et al. FK 506 for human liver, kidney and pancreas transplantation. Lancet. 1989;2:1000–1004. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295–305. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murase N, Kim D, Todo S, et al. Induction of liver, heart and multivisceral graft acceptance with a short course of FK 506. Transplant Proc. 1990;22:74–75. [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffman AL, Makowka L, Banner B, et al. The use of FK 506 for small intestine allotransplantation: inhibition of acute rejection and prevention of fatal graft-versus-host disease. Transplantation. 1990;49:483–490. doi: 10.1097/00007890-199003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K, Stangl MJ, Todo S, et al. Successful orthotopic small bowel transplantation with short term FK 506 immunosuppressive therapy. Transplant Proc. 1990;22:78–79. [PMC free article] [PubMed] [Google Scholar]
- 48.Murase N, Demetris AJ, Matsuzaki T, et al. Long Survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 49.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaffer D, Maki T, DeMichele SJ, et al. Studies in small bowel transplantation: prevention of graft-versus-host disease with preservation of allograft function by donor pretreatment with antilymphocyte serum. Transplantation. 1988;45:262–269. [PubMed] [Google Scholar]
- 51.Shaffer D, Ubhl CS, Simpson MA, et al. Prevention of graft vs. host disease following small bowel transplantation with polyclonal and monoclonal antilymphocyte serum: effect on timing and route of administration. Transplantation. 1991;52:948–952. doi: 10.1097/00007890-199112000-00002. [DOI] [PubMed] [Google Scholar]
- 52.Williams JW, McClellan T, Peters TG, et al. Effect of pretransplant graft irradiation on canine intestinal transplantation. Surg Gynecol Obstet. 1988;167:197–204. [PubMed] [Google Scholar]
- 53.Starzl TE, Demetris AJ, Murase N, et al. The lost chord: microchimerism. Immunol Today. 1996;17:577–584. doi: 10.1016/s0167-5699(96)10070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Starzl TE, Zinkernagel R. The regulation of immune reactivity by antigen migration and localization: a comparison of “tolerance” to infectious agents and allografts. N Engl J Med. In Press. [Google Scholar]
- 55.Talmage DW, Dart G, Radovich J, Lafferty KJ. Activation of transplant immunity: effect of donor leukocytes on thyroid allograft rejection. Science. 1976;191:385–387. doi: 10.1126/science.1082167. [DOI] [PubMed] [Google Scholar]
- 56.Lafferty KJ, Prowse SJ, Simeonovic CJ. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Ann Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- 57.Faustman D, Hauptefeld V, Lacy P, Davie J. Prolongation of murine islet allograft survival by pretreatment of islets with antibody directed to Ia determinants. Proc Natl Acad Sci USA. 1981;78:5156–5159. doi: 10.1073/pnas.78.8.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murase N, Starzl TE, Demetris AJ, et al. Hamster-to-rat heart and liver xenotransplantation with FK506 plus antiproliferative drugs. Transplantation. 1993;55:701–708. doi: 10.1097/00007890-199304000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gruessner RWG, Uckun FM, Pirenne J, et al. Recipient preconditioning and donor-specific bone marrow infusion in a pig model of total bowel transplantation. Transplantation. 1997;63:12–20. doi: 10.1097/00007890-199701150-00004. [DOI] [PubMed] [Google Scholar]
- 60.Rao AS, Fontes P, Dodson F, et al. Augmentation of natural chimerism with donor bone marrow in orthotopic liver recipients. Transplant Proc. 1996;28:2959–2965. [PMC free article] [PubMed] [Google Scholar]
- 61.Jaffe R, Trager JDK, Zeevi A, et al. Multivisceral intestinal transplantation: surgical pathology. Pediatr Pathol. 1989;9:633–654. doi: 10.3109/15513818909022372. [DOI] [PMC free article] [PubMed] [Google Scholar]