Abstract
OBJECTIVE
Risk assessment based on plaque vulnerability would be valuable in the management of asymptomatic carotid stenosis. The purpose of this study was to compare plaque morphology in symptomatic and asymptomatic patients with significant extracranial carotid artery stenosis using MDCT angiography.
MATERIALS AND METHODS
We identified 31 patients with greater than 60% carotid artery stenosis on MDCT angiography using the criteria of the North American Symptomatic Carotid Endarterectomy Trial Collaborators. We analyzed plaque density by blinded review in Hounsfield units in the atherosclerotic plaques of 15 symptomatic and 21 asymptomatic stenotic vessels for classification as soft, intermediate, or calcified. Data were analyzed using multiple logistic regression.
RESULTS
Even with age, traditional cardiovascular risk factors, and treatment taken into account, we found that calcified plaques were 21 times less likely to be symptomatic than noncalcified plaques (95% confidence interval for odds ratio, 0.003, 0.749; p = 0.030). No significant predictive value was found between soft (p = 0.23) or intermediate (p = 0.18) plaque morphology for the occurrence of symptoms.
CONCLUSION
MDCT angiography may help risk-stratify patients with asymptomatic carotid artery stenosis. Extracranial carotid artery calcified plaques causing stenosis are significantly less likely to be symptomatic and thus may be more stable than noncalcified plaques. This finding may have implications for the interpretation of calcification of atherosclerotic plaque in other vascular beds.
Although extracranial carotid artery stenosis is accepted as a significant risk factor for cerebrovascular events, certain patients with atherosclerotic disease may be at higher risk depending on the morphology of the plaque. The vulnerable atherosclerotic plaque is characterized by an increased tendency to rupture, resulting in embolization or thrombosis and, subsequently, disruption of blood flow [1]. Conversely, stable plaques are less prone to disruption and consequently less likely to result in ischemic events. Risk stratification based on this model of plaque composition would be valuable in the management of asymptomatic carotid stenosis because low-risk patients could potentially be treated medically and those at high risk could be treated with invasive procedures.
Given its excellent spatial and temporal resolution, MDCT angiography may be effective in noninvasively discriminating plaque contents on the basis of density measurements. Although prior studies with single-detector CT and thicker axial sections had mixed results [2, 3], to our knowledge, few studies have used MDCT to evaluate plaque composition. The purpose of this study was to compare plaque morphology in symptomatic and asymptomatic patients with significant extracranial carotid artery stenosis using MDCT angiography.
Materials and Methods
After approval of the research protocol by the institutional review board of the facility, we retrospectively identified all patients (n = 43), scanned from September 2001 to December 2003, who were found to have stenosis equal to or greater than 60% (as defined by the criteria of the North American Symptomatic Carotid Endarterectomy Trial Collaborators [4]) of the internal carotid artery on MDCT angiography. The degree of luminal stenosis was measured by an experienced neuroradiologist on the basis of analysis of axial slices, multiplanar reconstructions, maximum intensity projections, and 3D volume-rendered reconstruction for optimal assessment [5]. Patients’ medical records were reviewed for ischemic neurologic symptoms, either retinal or hemispheric, that were relevant to the carotid stenosis and also for relevant medical history and imaging. Transient ischemic attack (TIA) was defined as a brief episode of neurologic dysfunction, such as hemiparesis, hemiparesthesia, dysarthria, dysphasia, or monocular blindness that was caused by ischemia without evidence of acute infarction and stroke; these conditions are persistent clinical signs or imaging characteristics that are consistent with infarction and appropriate to carotid disease [6]. Eight patients were excluded for restenosis because they had undergone carotid endarterectomy or had nonatherosclerotic causes of significant stenosis. Four patients were excluded because of concomitant conflicting lateralizing ischemic symptoms, such as those due to known cardiac thrombus or small vessel disease indicated by lacunar infarcts seen on CT and MRI.
Of the 31 patients examined, 18 had symptomatic carotid stenosis (11 men and seven women; median age, 73 years; age range, 45–93 years). All patients underwent MDCT angiography within 1 week of the ischemic event. Eleven patients had a history of TIA, and seven had symptoms of stroke attributable to the stenosis, resulting in 18 symptomatic vessels. In the asymptomatic group of 13 patients (seven men and six women), the median age was 70 years (age range, 61–86 years). Five patients with symptomatic stenosis had concomitant contralateral asymptomatic high-grade stenosis and four asymptomatic patients had bilateral stenosis, leading to a total of 22 asymptomatic vessels. Of the 40 vessels with relevant atherosclerotic plaques, four plaques (three symptomatic and one asymptomatic) were excluded from analysis because of lack of visualization of plaque burden due to small vessel caliber or tortuosity. We could analyze 36 total plaques with respect to lesion configuration.
In both groups of patients, referral for MDCT angiography was for confirmation of stenosis after B-mode sonography, as an alternative to digital subtraction angiography for surgical planning, or for nondiagnostic carotid MR angiography or sonography. All patients classified as asymptomatic had no history of ischemic neurologic symptoms, either remote or current. Patient characteristics are summarized in Table 1. The only factor significantly different in the two groups was the presence of hypercholesterolemia, which was greater in the asymptomatic group.
TABLE 1.
Clinical Characteristics of the Study Population
| Characteristic | Patients without Symptoms (%) |
Patients with Symptoms (%) |
p |
|---|---|---|---|
| Hypertension | 11 (85) | 12 (67) | 0.41 |
| Diabetes mellitus | 2 (15) | 3 (17) | 1.00 |
| Tobacco use (former and current) | 7 (54) | 10 (56) | 0.79 |
| Elevated cholesterol | 12 (92) | 9 (50) | 0.02a |
| Coronary artery disease | 7 (54) | 8 (44) | 0.88 |
| Aspirin use | 12 (92) | 11 (61) | 0.10 |
| Statin use | 9 (69) | 8 (44) | 0.32 |
p < 0.05.
MDCT was performed in all patients on either an 8-MDCT scanner (LightSpeed Ultra, GE Healthcare) or a 16-MDCT scanner (LightSpeed 16, GE Healthcare). After a delay determined by an automated bolus-timing program for the injection of 120 mL of nonionic contrast medium at a rate of 4 mL/sec, CT was performed from the aortic arch to the supraventricular white matter. Scan parameters were identical for both scanners: 1.25-mm nominal section thickness, table speed of 6.25 mm per rotation, and 0.8-sec gantry-rotation period. The image data were transferred to a computer workstation (version 4.1, Advantage Workstation, GE Healthcare) for postprocessing.
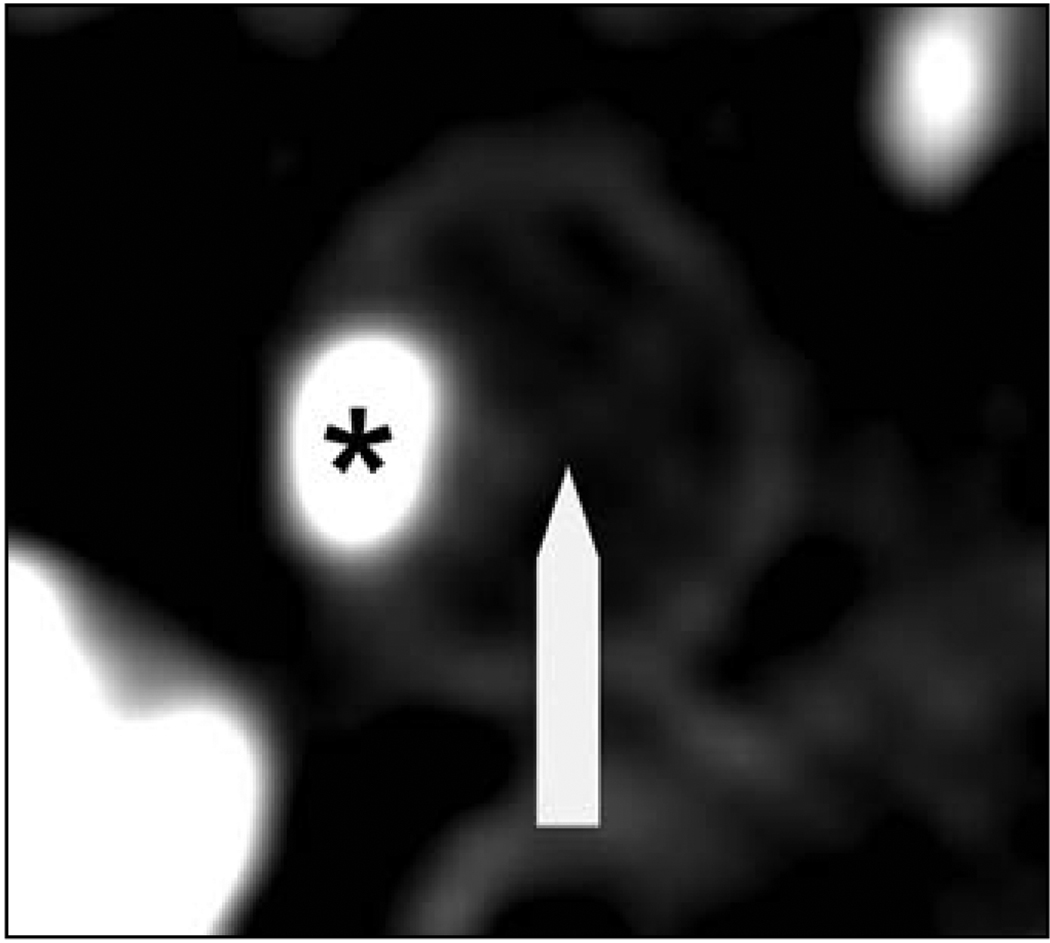
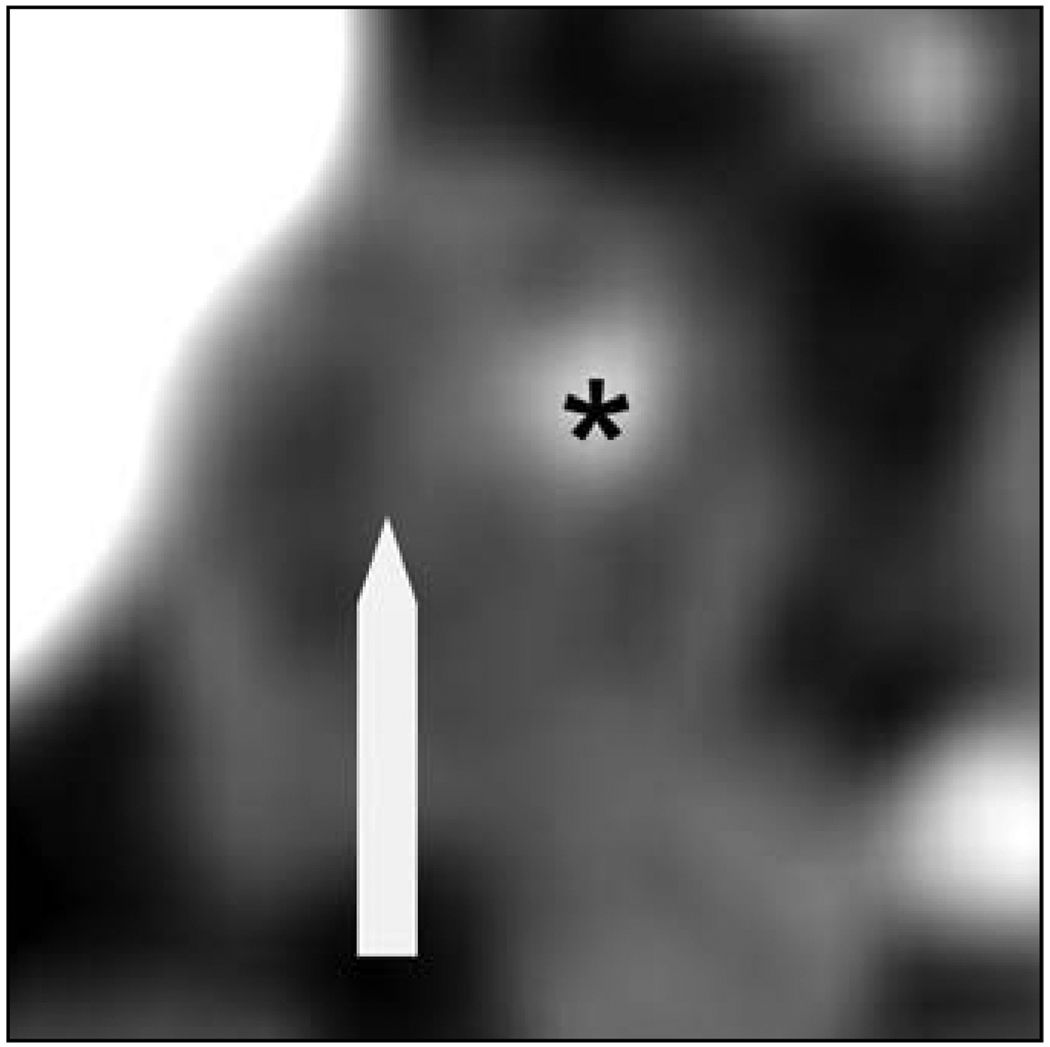
Axial CT images of the atherosclerotic plaque resulting in significant stenosis were analyzed using the consensus of two observers blinded to the clinical history. Axial sections containing the particular lesions were magnified, and density measurements in Hounsfield units (H) were made in 10 distinct 1-mm2 regions of interest on multiple axial slices within the plaque, similar to the method validated by Schroeder et al. [7], to differentiate plaque configuration in the coronary arteries. Areas showing contamination by contrast material or calcification not contributing to the stenosis were carefully avoided. Plaque type was classified according to density measurements on the basis of previously reported criteria [7]. Soft plaques associated with lipid-rich cores; were defined as those with a median density of less than or equal to 50 H (Fig. 1). Intermediate plaques, associated with large amounts of fibrous tissue, were categorized as those with a median density of 51–130 H (Fig. 2). Calcified plaques consisted of lesions having a median density of greater than 130 H (Fig. 3).
Fig. 1.
64-year-old man with stroke. Axial CT scan of internal carotid artery with soft atherosclerotic plaque (arrow and asterisk) shows contrast-enhanced lumen. Note attenuation of plaque relative to lumen and surrounding soft tissues.
Fig. 2.
79-year-old man with transient ischemic attack. Axial CT scan of internal carotid artery with intermediate plaque (arrow and asterisk) shows contrast-enhanced lumen.
Fig. 3.
86-year-old asymptomatic man. Axial CT scan of internal carotid artery with calcified plaque (arrow and asterisk) shows contrast-enhanced lumen.
Multiple logistic regression was performed to investigate the odds ratios (ORs) of plaque type (with analysis performed for calcified vs noncalcified, intermediate vs soft and calcified, soft vs intermediate and calcified, and by median numeric Hounsfield units); age; and evidence of hypertension, diabetes, hypercholesterolemia, and tobacco, aspirin, or statin drug use for the dependent variable of clinical symptomatology. Each plaque was treated as an independent experimental unit. Similar simultaneous multifactorial analysis was performed to assess factors predictive of plaque type. Continuous variables were described by their median and 25th–75th percentile. The nonparametric Kruskal-Wallis test was used to compare the medians of the density measurements of the three groups of plaques, and Wilcoxon’s rank sum test was used for pair-wise comparisons. A p value of less than 0.05 was statistically significant. All analyses were performed with SigmaStat software (version 2.03, Access Softek).
Results
On MDCT, in the symptomatic group (n = 15), 53% (n = 8) of plaques were classified as soft (Fig. 1), 40% (n = 6) as intermediate, and 7% (n = 1) as calcified. In the asymptomatic group (n = 21), 28% (n = 6) of plaques were classified as soft, 28% (n = 6) as intermediate, and 43% (n = 9) as calcified. Even with age, traditional cardiovascular risk factors, and treatment taken into account, we found that calcified plaques were 21 times less likely to be symptomatic than noncalcified plaques (95% confidence interval for OR, 0.003, 0.749; p = 0.030). No other factor was concomitantly predictive for symptoms, with all other independent variables having a p value of greater than 0.10. Calcified plaques causing stenosis were also 11 times less likely to be symptomatic than noncalcified plaques on unifactorial analysis (95% confidence interval for OR, 0.011, 0.864; p = 0.037). Moreover, there was a trend for incremental increase in the numeric density of plaques in Hounsfield units to have decreased odds for symptoms (OR, 0.997; 95% confidence interval for OR, 0.995, 1.00; p = 0.054).
No significant predictive value was found between soft (p = 0.23) or intermediate (p = 0.18) plaque type for symptomatology. After adjusted analysis, the only factor significantly predictive for plaque morphology was smoking; a history of former or current tobacco use was predictive of noncalcified plaque configuration (OR, 11.7; 95% confidence interval for OR, 1.08, 126; p = 0.043).
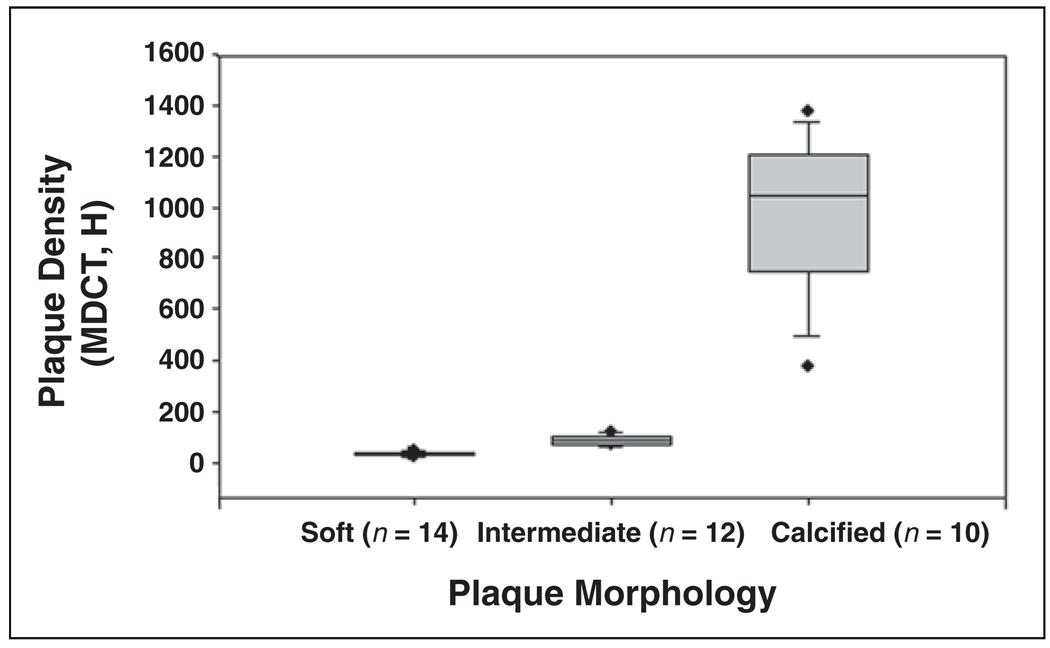
Soft plaques (n = 14) showed a median (25th–75th percentile) density of 34.2 H (29.3–39.9 H); intermediate plaques (n = 12), 83.3 H (69.2–101.1 H); and calcified plagues (n = 10) 1041.7 H (742.8–1207.7 H) with a statistically significant difference among the median plaque densities of the three groups (p < 0.0001) and a significant difference (p < 0.05) among each of the individual plaque types (Fig. 4).
Fig. 4.
Box-and-whiskers plot compares plaque morphology and plaque density in Hounsfield units on MDCT. Results of Kruskal-Wallis test are significant (p < 0.05).
Discussion
With the recent recognition of the importance of plaque vulnerability in the development of ischemic symptoms in both the carotid and coronary artery systems, considerable attention has been devoted to noninvasively determining plaque morphology. The relationship between plaque characteristics, especially calcification, and clinical behavior in the carotid circulation has been incompletely studied. Our data indicate that calcified carotid artery atherosclerotic plaques causing stenosis are less likely to be symptomatic than are noncalcified plaques, suggesting that calcification of plaques confers stability.
The role of calcification in atherosclerotic disease with regard to clinical symptoms has been studied in pathologic and sonographic studies. Calcium is postulated to confer stability by stiffening the plaque, resulting in protection against biomechanical stress and subsequent disruption [8]. In a clinicopathologic study of endarterectomy specimens from asymptomatic and symptomatic patients, Hunt et al. [9] found that patients with calcified carotid plaques had fewer cerebrovascular events than those with noncalcified plaques. On B-mode sonography, on which echo-rich carotid plaques are typically associated with calcium and fibrous tissue and echolucent plaques are associated with high lipid content, the Tromso study [10] found that echo-rich plaques carry a lower risk of neurologic symptoms. However, calcium is notably difficult to evaluate on sonography because of its variable appearance and production of acoustic shadowing, which can obscure total visualization of plaque contents.
The clinical application of plaque stability is particularly important in patients with asymptomatic high-grade carotid stenosis. Whereas the most recent consensus statement from the American Heart Association recommends endarterectomy for patients with asymptomatic carotid artery stenosis greater than 60%, provided that the morbidity and mortality associated with the procedure are within acceptable limits [11], international practice varies considerably. Classification of risk for ischemic neurologic events based on carotid plaque morphology could possibly not only aid in the identification of patients with high-risk plaques who would benefit from a particular aggressive treatment (endovascular intervention vs endarterectomy) but also monitor the effects of medical therapy in patients with high surgical risk and low-risk plaques. Statin drug therapy is believed to stabilize plaques and has been shown to decrease the lipid content of plaques [12] as well as decrease the vessel wall thickness and area [13] in human carotid artery atherosclerotic disease. In accord with our findings of calcified plaque stability, Zhao et al. [14] found that lipid-lowering therapy was not only significantly associated with lower lipid content of plaque but also with a tendency for increased plaque calcium in treated human carotid arteries compared with those of an untreated control group, although these plaques were not studied before therapy. If consistent data are found in prospective trials, an asymptomatic subgroup of patients with calcified plaques causing stenosis could potentially be followed up with medical treatment and could avoid the risks of surgery.
MDCT offers significant benefits in the evaluation of carotid atherosclerotic disease, especially as a complementary examination to sonography. This technique is robust in the assessment of calcium, has high diagnostic accuracy in the detection of stenosis, and may be an alternative to catheter angiography in diagnosing total versus near occlusion of the internal carotid artery [15]. Our results indicate that CT may also have a role in noninvasively differentiating plaque types on the basis of density measurements. Similar data in the carotid arteries using single-detector helical CT with histologic correlation [2] and in the coronary circulation using MDCT with intravascular ultrasound analysis [7] have recently been published. However, Walker et al. [3] found that CT could not effectively differentiate carotid plaque constituents on the basis of density, but spatial resolution was limited by the use of single-detector CT and thicker axial sections than were used in our study. Further trials using in vivo MDCT with histopathologic correlation of carotid atherosclerotic disease are necessary to confirm our findings.
Limitations of the current study include the retrospective identification of culprit plaques and extension of this information to future plaque vulnerability. Prospective larger outcome studies comparing the sans therapy and posttherapy histories of the three plaque types are necessary before acceptance into clinical practice. Another factor to consider is the controversy regarding the definitions of the end points in our study (i.e., stroke and TIA), because most systems are based on temporal features and clinical symptomatology, which can lead to uncertainty about the diagnosis. However, all symptomatic patients in our study would be classified as having a definite acute ischemic cerebrovascular syndrome under recently published criteria, given the nature of the symptoms and confirmed vascular disease relevant to the clinical syndrome [16]. Moreover, patients with other potential causes of ischemic symptoms, particularly cardiac thrombus and intracranial stenosis as evidenced by lacunar infarcts, were excluded from the study. Notably, the asymptomatic patients in our study did not undergo cerebral imaging and, therefore, silent ischemia could not be ruled out in these patients. Because unenhanced examinations were not performed, another issue involves high-density luminal contrast, possibly complicating the assessment of calcium. However, MDCT angiography has been shown to be effective in detecting and evaluating atherosclerotic calcium despite the presence of a contrast agent [17], and calcified plaques had mean densities considerably higher than the usual density of contrast media (350 H).
In conclusion, our results indicate that calcified carotid plaques may be more stable than noncalcified plaques; these findings may have implications not only in the risk stratification of patients with asymptomatic carotid stenosis but also potentially in the risk stratification of patients with atherosclerotic disease of other vascular beds.
References
- 1.Naghavi M, Libby P, Falk E, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies. Part I. Circulation. 2003;108:1664–1672. doi: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 2.Estes JM, Quist WC, Lo Gerfo FW, Costello P. Noninvasive characterization of plaque morphology using helical computed tomography. J Cardiovasc Surg (Torino) 1998;39:527–534. [PubMed] [Google Scholar]
- 3.Walker LJ, Ismail A, McMeekin W, Lambert D, Mendelow AD, Birchall D. Computed tomography angiography for the evaluation of carotid atherosclerotic plaque: correlation with histopathology of endarterectomy specimens. Stroke. 2002;33:977–981. doi: 10.1161/01.str.0000013562.73522.82. [DOI] [PubMed] [Google Scholar]
- 4.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 5.Dix JE, Evans AJ, Kallmes DF, Sobel AH, Phillips CD. Accuracy and precision of CT angiography in a model of carotid artery bifurcation stenosis. AJNR. 1997;18:409–415. [PMC free article] [PubMed] [Google Scholar]
- 6.Albers GW, Caplan LR, Easton JD, et al. Transient ischemic attack: proposal for a new definition. N Engl J Med. 2002;347:1713–1716. doi: 10.1056/NEJMsb020987. [DOI] [PubMed] [Google Scholar]
- 7.Schroeder S, Kopp AF, Baumbach A, et al. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37:1430–1435. doi: 10.1016/s0735-1097(01)01115-9. [DOI] [PubMed] [Google Scholar]
- 8.Wexler L, Brundage B, Crouse J, et al. Coronary artery calcification: pathophysiology, epidemiology, imaging methods, and clinical implications—a statement for health professionals from the American Heart Association. Writing Group. Circulation. 1996;94:1175–1192. doi: 10.1161/01.cir.94.5.1175. [DOI] [PubMed] [Google Scholar]
- 9.Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke. 2002;33:1214–1219. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 10.Mathiesen EB, Bonaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the Tromso study. Circulation. 2001;103:2171–2175. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 11.Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: a statement for healthcare professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1998;97:501–509. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 12.Crisby M, Nordin-Fredriksson G, Shah PK, Yano J, Zhu J, Nilsson J. Pravastatin treatment increases collagen content and decreases lipid content, inflammation, metalloproteinases, and cell death in human carotid plaques: implications for plaque stabilization. Circulation. 2001;103:926–933. doi: 10.1161/01.cir.103.7.926. [DOI] [PubMed] [Google Scholar]
- 13.Corti R, Fayad ZA, Fuster V, et al. Effects of lipid-lowering by simvastatin on human atherosclerotic lesions: a longitudinal study by high-resolution, noninvasive magnetic resonance imaging. Circulation. 2001;104:249–252. doi: 10.1161/01.cir.104.3.249. [DOI] [PubMed] [Google Scholar]
- 14.Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol. 2001;21:1623–1629. doi: 10.1161/hq1001.098463. [DOI] [PubMed] [Google Scholar]
- 15.Chen CJ, Lee TH, Hsu HL, et al. Multi-slice CT angiography in diagnosing total versus near occlusions of the internal carotid artery: comparison with catheter angiography. Stroke. 2004;35:83–85. doi: 10.1161/01.STR.0000106139.38566.B2. [DOI] [PubMed] [Google Scholar]
- 16.Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome diagnostic criteria. Stroke. 2003;34:2995–2998. doi: 10.1161/01.STR.0000098902.69855.A9. [DOI] [PubMed] [Google Scholar]
- 17.Hong C, Becker CR, Schoepf UJ, Ohnesorge B, Bruening R, Reiser MF. Coronary artery calcium: absolute quantification in nonenhanced and contrast-enhanced multi-detector row CT studies. Radiology. 2002;223:474–480. doi: 10.1148/radiol.2232010919. [DOI] [PubMed] [Google Scholar]