Abstract
We investigate the system size-dependent rheological response of branched type I collagen gels. When subjected to a shear strain, the highly interconnected mesh dynamically reorients, resulting in overall stiffening of the network. When a continuous shear strain is applied to a collagen network, we observe that the local apparent modulus, in the strain-stiffening regime, is strongly dependent on the gel thickness. In addition, we demonstrate that the overall network failure is determined by the ratio of the gel thickness to the mesh size. These findings have broad implications for cell-matrix interactions, the interpretation of rheological tissue data, and the engineering of biomimetic scaffolds.
The increasing variety and availability of purified extra- and intracellular matrix proteins provides an unprecedented opportunity to quantify the mechanical properties of the cellular environment. Reconstituted biopolymer fiber networks can exhibit nonlinear rheology that is dramatically different than synthetic polymer gels (1–5). Tying the microscopic physical behavior of the network constituents to the meso- and macroscale rheology is critical for determining the mechanosensory mechanisms that underlie such diverse processes as cell motility, cancer metastasis, and tumor proliferation. In vivo, cells sense, and respond to forces generated within or transmitted through the extracellular matrix (ECM). In turn, the regulation of sensory cues can be highly susceptible to changes in the ECM stiffness on length scales comparable to the cell size. However, despite the increasing number of experimental and theoretical investigations of the nonlinear rheological behavior of semiflexible and stiff polymer networks (6–9), the effects of system size on strain-stiffening remains essentially unexplored. Most theoretical models focus on the global response of biopolymer networks using three distinct microscopic mechanisms: nonaffine deformations of crosslinked rigid rods (9); entropic penalties of fiber stretching in entangled networks (3); or changes to the network geometry (10). There is little experimental data with which to verify these microscopic mechanisms directly, in part because of the difficulty in extracting the relevant quantities experimentally (11,12). In addition, the nonlinear rheology of stiff networks can only be investigated with bulk techniques, so the behavior at biologically relevant microscopic and mesoscopic scales is largely unknown.
Collagen is the most abundant ECM protein, and a common component of in vitro cell cultures and bioengineered scaffolds. Under appropriate conditions, type I collagen self-assembles in vitro to form percolated networks (gels) (13) with mesh sizes ξ that depend on the concentration and polymerization conditions (Fig. 1, A–C) (14,12). These branched networks substantially strain-stiffen over a broad range of concentrations (15–17); one interpretation of the biomechanical function associated with nonlinear stiffening is a passive protection for soft tissues when shear deformations become large. Collagen networks are not only excellent ECM model systems, but provide a platform for investigating cellular motility within three-dimensional microenvironments (18), and provide biocompatible scaffolds for tissue growth and organ regeneration (19–22).
Figure 1.
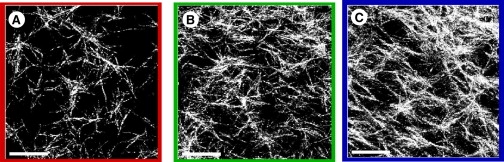
Confocal reflectance images of branched type I rat tail collagen fiber networks corresponding to the concentrations: (A) 1 mg/mL, (B) 2 mg/mL, and (C) 3 mg/mL. Each image is a 10-μm-thick maximum-projection; scale bar = 25 μm.
In this work, we investigate strain-stiffening of collagen networks under steady shear and observe that the nonlinear rheological response is strongly dependent on the material thickness. Moreover, the apparent moduli near yield decreases dramatically in thin samples in a process that is controlled by the ratio of the sample size to the network mesh size. The system size dependence is not accounted for in current models of nonlinear strain-stiffening in biopolymer networks.
We apply continuous shear strains to collagen networks using a model No. MCR-301 bulk rheometer (Anton Paar, Graz, Austria) with a 25-mm-diameter parallel-plate geometry within a temperature- and humidity-controlled environment. All continuous shear strain experiments are performed at a strain rate of s–1. Type I rat tail collagen (BD Bioscience, San Mateo, CA; 3.32 mg/mL) is polymerized at 23°C for 45 min in 10× phosphate-buffered saline at pH 7.0 with ionic strengths I = {0.044, 0.087, 0.13} for the concentrations c = {1.0, 2.0, 3.0} mg/mL, respectively.
The plate tool provides precise control of the sample thickness through a change of the rheometer gap h. At low strains, the measured stress σ is proportional to the applied strain γ, for all values of h. Intermediate strains reveal that the network undergoes a substantial nonlinear increase of σ, indicative of strain-stiffening (3). Specifically, for h > 150 μm, σ(γ) is approximately h-independent. However, for smaller gaps, we observe that the maximum of σ(γ) moves to larger values of γ (Fig. 2). The magnitude of σ at the peak remains relatively constant. The yield strain in thin samples is more than twice that of thick samples, and the apparent modulus of thin samples can be up to three times smaller than the bulk value. To ensure that the changes in h are not introducing variability in the rheological measurements, particularly at small h, we measure the linear viscoelastic modulus G0 using oscillatory rheology at a frequency of ω = 2π rads s–1 at a strain of γ = 1.0%. For each concentration, we observe that G0 is constant over the entire range of h (Fig. 3 A). Higher strains lead to irreversible deformations and yielding, as evidenced by a reduction in the overall modulus.
Figure 2.
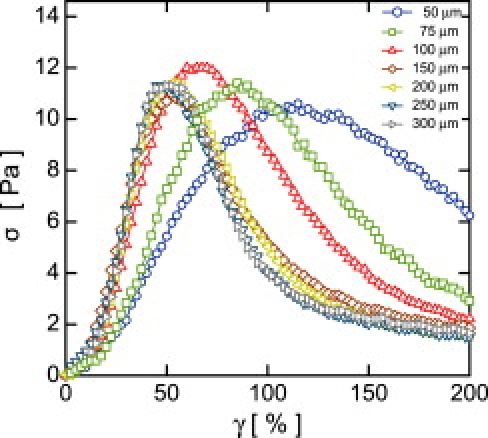
Stress-strain rheology curves for 1 mg/mL collagen gels at different gaps h (strain rate = 1% s–1); gel networks are polymerized at 23°C and pH 7.0. All lines connecting points are guides for the eye.
Figure 3.
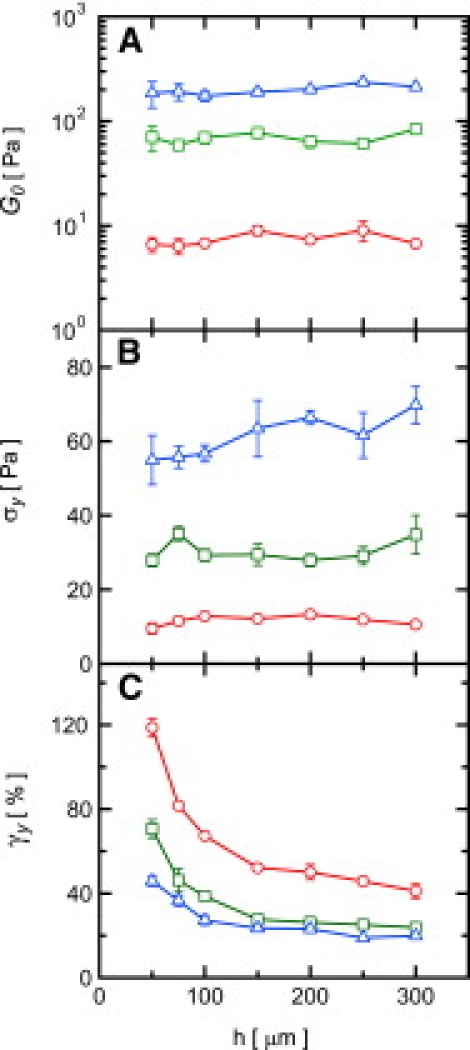
For all graphs, (○) 1 mg/mL, (□) 2 mg/mL, and (▵) 3 mg/mL. (A) Linear elastic modulus G0 from oscillatory rheology versus gap height h. (B) Yield stress extracted from the stress-strain curves σy versus h shows strong ξ-dependence and limited h-dependence for all concentrations. (C) Yield strain γy versus h for each collagen concentration extracted from the peaks in the stress-strain curves (Fig. 2). Error bars represent standard error from four trials; lines are guides to the eye.
To quantify the h-dependence of the nonlinear rheology, we perform a least-squares polynomial fit to the peaks of the rheology curves in Fig. 2 A. We use the peak positions to define the yield values for the stress σy and strain γy. We determine γy as a function of h for three different collagen concentrations. For each concentration, γy exhibits a clear increase for small h and approaches a constant value for large h (Fig. 3 C). Interestingly, for the entire range of h and concentration, the values of σy show weak or no h-dependence (Fig. 3 B). We speculate that σy is determined by the strength of fiber-fiber or fiber-boundary junctions, and depends only on the total applied stress, independent of h.
To elucidate the role of the microscopic length scale ξ in the observed size-dependent rheology, we replot the data in Fig. 3 C with h rescaled by ξ. To quantify the mesh size ξ, we analyze the spacing between fibers extracted along horizontal and vertical lines within z-resolved planar confocal images (14). We fit the distribution of the fiber spacing to an exponential decay for each concentration, c = {1.0, 2.0, 3.0} mg/mL, to obtain the characteristic mesh sizes ξ = {14.7, 10.4, 9.4} μm (Fig. 4 A). To account for the concentration-dependent modulus, we also rescale γy by the ξ-dependent plateau values γp, determined by fitting γy(h) to an exponential decay with an offset. The data from the three concentrations collapse onto a single curve (Fig. 4 B).
Figure 4.
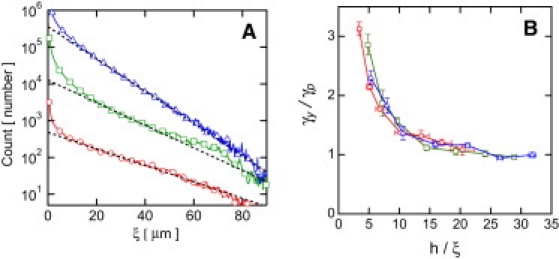
(A) Mesh size distributions for (○) 1 mg/mL, (□) 2 mg/mL, and (▵) 3 mg/mL. Exponential fits (given by the dashed lines) provide characteristic mesh sizes: (○) 14.7 μm, (□) 10.4 μm, and (▵) 9.4 μm. These curves have been shifted along the ordinate for clarity. (B) Rescaling γy by the extrapolated plateau yield strain γp and h by ξ reduces Fig. 3C to a universal yielding curve for all concentrations; (○) 1 mg/mL, (□) 2 mg/mL, and (▵) 3 mg/mL.
We conclude that the nonlinear rheology of collagen is determined by the interplay between the macroscopic material thickness and the mesh size. We do not claim that the mesh size determines the network rheology; this is most likely determined by some combination of the network structure and topology. In addition, we note that the range of gaps investigated directly corresponds to the radial gap separation in many cone-plate tool geometries, and is equivalent to length scales of many important biological processes.
The observed size dependence of the nonlinear rheology indicates that the material response is nonuniform on scales significantly larger than the mesh size, and is therefore inconsistent with models that attribute strain stiffening to the affine deformation of networks of semiflexible filaments. Collagen fibers are relatively stiff, and therefore more likely described by athermal models that attribute strain stiffening to nonaffine filament stretching (23). The nonaffine length scale can be significantly larger than the mesh size (24), but to our knowledge the implications for system size dependence has not been investigated. Alternatively, heterogeneous localization of strain within the network that is either uniformly distributed through the gel or localized near the boundaries could produce size-dependent effects. Reflectance images do not show any measurable variation in collagen structure near the boundary, but more-subtle boundary effects may also play a role. Finally, the long persistence length of the collagen fibers may directly contribute to a finite size effect. Whatever the physical mechanism, the size-dependent rheology extends to important length scales for a range of fundamental studies and applications.
Acknowledgments
We thank P. Janmey, F. MacKintosh, and Andreas Bausch for helpful discussions, and W. Rosoff and D. Koch for the collagen preparation. We also thank P. Kumar for assistance with the image analysis.
This work was funded by the National Science Foundation through grant No. DMR-0804782 and from the Air Force Office of Scientific Research through grant No. FA9550-07-1-0130.
References and Footnotes
- 1.Djabourov M., Lechaire J.-P., Gaill F. Structure and rheology of gelatin and collagen gels. Biorheology. 1993;30:191–205. doi: 10.3233/bir-1993-303-405. [DOI] [PubMed] [Google Scholar]
- 2.Gardel M.L., Shin J.H., Weitz D.A. Elastic behavior of cross-linked and bundled actin networks. Science. 2004;304:1301–1305. doi: 10.1126/science.1095087. [DOI] [PubMed] [Google Scholar]
- 3.Storm C., Pastore J.J., Janmey P.A. Nonlinear elasticity in biological gels. Nature. 2005;435:191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 4.Liu J., Koenderink G.H., Weitz D.A. Visualizing the strain field in semiflexible polymer networks: strain fluctuations and nonlinear rheology of F-actin gels. Phys. Rev. Lett. 2007;98:198304. doi: 10.1103/PhysRevLett.98.198304. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri O., Parekh S.H., Fletcher D.A. Reversible stress softening of actin networks. Nature. 2007;445:295–298. doi: 10.1038/nature05459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacKintosh F.C., Käs J., Janmey P.A. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995;75:4425–4428. doi: 10.1103/PhysRevLett.75.4425. [DOI] [PubMed] [Google Scholar]
- 7.Wilhelm J., Frey E. Elasticity of stiff polymer networks. Phys. Rev. Lett. 2003;91:108103. doi: 10.1103/PhysRevLett.91.108103. [DOI] [PubMed] [Google Scholar]
- 8.Head D.A., Levine A.J., MacKintosh F.C. Distinct regimes of elastic response and deformation modes of cross-linked cytoskeletal and semiflexible polymer networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2003;68:061907. doi: 10.1103/PhysRevE.68.061907. [DOI] [PubMed] [Google Scholar]
- 9.Heussinger C., Schaefer B., Frey E. Nonaffine rubber elasticity for stiff polymer networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007;76:031906. doi: 10.1103/PhysRevE.76.031906. [DOI] [PubMed] [Google Scholar]
- 10.Onck P.R., Koeman T., van der Giessen E. Alternative explanation of stiffening in cross-linked semiflexible networks. Phys. Rev. Lett. 2005;95:178102. doi: 10.1103/PhysRevLett.95.178102. [DOI] [PubMed] [Google Scholar]
- 11.Stein A.M., Vader D.A., Sander L.M. An algorithm for extracting the network geometry of three-dimensional collagen gels. J. Microsc. 2008;232:463–475. doi: 10.1111/j.1365-2818.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y.-L., Leone L.M., Kaufman L.J. Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy. Biophys. J. 2009;97:2051–2060. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forgacs G., Newman S.A., Sackmann E. Assembly of collagen matrices as a phase transition revealed by structural and rheologic studies. Biophys. J. 2003;84:1272–1280. doi: 10.1016/S0006-3495(03)74942-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman L.J., Brangwynne C.P., Weitz D.A. Glioma expansion in collagen I matrices: analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005;89:635–650. doi: 10.1529/biophysj.105.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janmey P.A., McCormick M.E., MacKintosh F.C. Negative normal stress in semiflexible biopolymer gels. Nat. Mater. 2007;6:48–51. doi: 10.1038/nmat1810. [DOI] [PubMed] [Google Scholar]
- 16.Kang H., Wen Q., MacKintosh F.C. Nonlinear elasticity of stiff filament networks: strain stiffening, negative normal stress, and filament alignment in fibrin gels. J. Phys. Chem. B. 2009;113:3799–3805. doi: 10.1021/jp807749f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vader D., Kabla A., Mahadevan L. Strain-induced alignment in collagen gels. PLoS ONE. 2009;4:e5902. doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mierke C.T., Rösel D., Brábek J. Contractile forces in tumor cell migration. Eur. J. Cell Biol. 2008;87:669–676. doi: 10.1016/j.ejcb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott H.C., Matthiesen T.S., Taylor D.A. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 20.Sabass B., Gardel M.L., Schwarz U.S. High resolution traction force microscopy based on experimental and computational advances. Biophys. J. 2008;94:207–220. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beningo K.A., Lo C.-M., Wang Y.-L. Flexible polyacrylamide substrata for the analysis of mechanical interactions at cell-substratum adhesions. Methods Cell Biol. 2002;69:325–339. doi: 10.1016/s0091-679x(02)69021-1. [DOI] [PubMed] [Google Scholar]
- 22.Gaudet C., Marganski W.A., Wong J.Y. Influence of type I collagen surface density on fibroblast spreading, motility, and contractility. Biophys. J. 2003;85:3329–3335. doi: 10.1016/S0006-3495(03)74752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heussinger C., Frey E. Role of architecture in the elastic response of semiflexible polymer and fiber networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2007;75:011917. doi: 10.1103/PhysRevE.75.011917. [DOI] [PubMed] [Google Scholar]
- 24.Lieleg O., Claessens M.M.A.E., Bausch A.R. Mechanics of bundled semiflexible polymer networks. Phys. Rev. Lett. 2007;99:088102. doi: 10.1103/PhysRevLett.99.088102. [DOI] [PubMed] [Google Scholar]


