SUMMARY
Hand1 regulates development of numerous tissues within the embryo, extraembryonic mesoderm and trophectoderm. Systemic loss of Hand1 results in early embryonic lethality but the cause has remained unknown. To determine if Hand1 expression in extraembryonic mesoderm is essential for embryonic survival, Hand1 was conditionally deleted using the HoxB6-Cre mouse line that expresses Cre in extraembryonic and lateral mesoderm. Deletion of Hand1 using HoxB6-Cre resulted in embryonic lethality identical to systemic knockout. To determine if lethality is due to Hand1 function in extraembryonic mesoderm or lateral mesoderm, we generated a Tlx2-Cre mouse line expressing Cre in lateral mesoderm but not extraembryonic tissues. Deletion of Hand1 using the Tlx2-Cre line results in embryonic survival with embryos exhibiting herniated gut and thin enteric smooth muscle. Our results show that Hand1 regulates development of lateral mesoderm derivatives and its loss in extraembryonic mesoderm is the primary cause of lethality in Hand1-null embryos.
Keywords: mouse, embryo, Cre recombinase, Hand1, neural crest, nervous system
The Hand1 bHLH transcription factor is expressed in a number of lineages within the developing embryo including the heart, neural crest (NC) derivatives and lateral mesoderm while extraembryonically, it is expressed in mesoderm and trophectoderm (Cserjesi et al., 1995; Morikawa and Cserjesi, 2004; Riley et al., 1998). Systemic knockout of Hand1 leads to early embryonic lethality but the cause of the lethality has not been determined (Firulli et al., 1998; Morikawa and Cserjesi, 2004; Riley et al., 1998). The role of Hand1 in extraembryonic mesoderm has been studied and shown to be essential during vasculogenesis (Morikawa and Cserjesi, 2004) while in the trophectoderm lineage, it is required during placental development (Riley et al., 1998). These studies suggest that the early lethality in Hand1 null embryos is due to its functions in one of these extraembryonic lineages. In support, conditional deletion of Hand1 in the NC lineage (Barbosa et al., 2007) or heart (McFadden et al., 2002) does not result in early lethality.
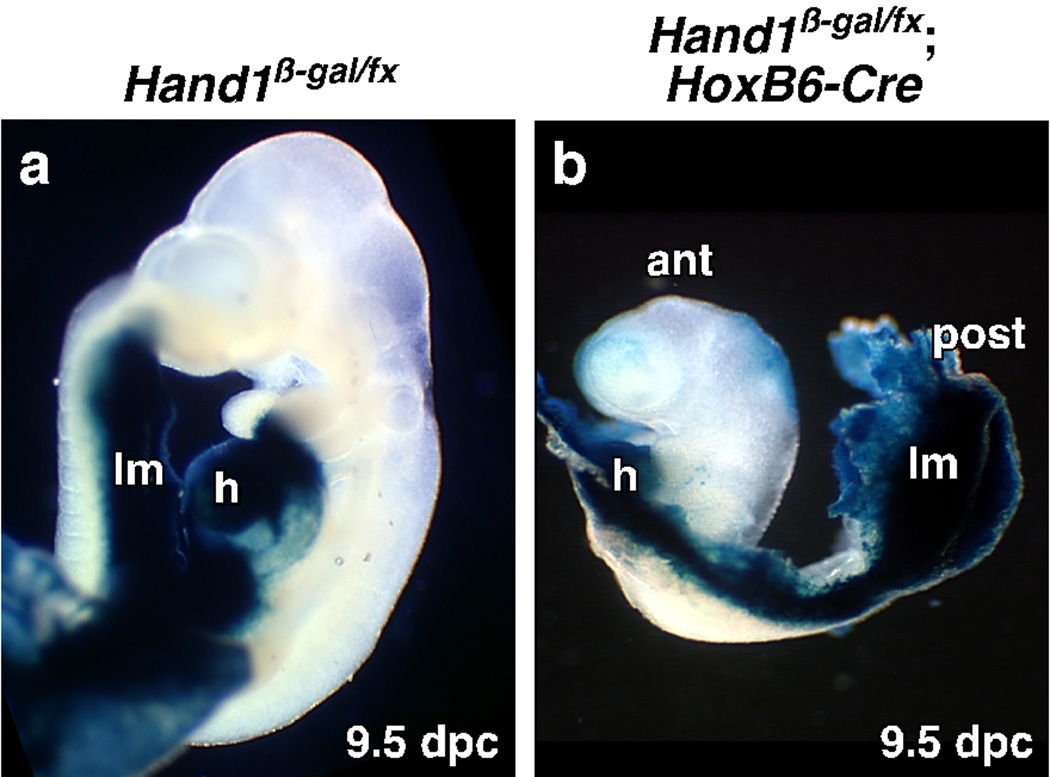
To determine if Hand1 expression in lateral and extraembryonic mesoderm is essential for embryonic survival, we deleted Hand1 in these tissues but not trophectoderm using the HoxB6-Cre deleter line (Lowe et al., 2000). Deletion of Hand1 using HoxB6-Cre (Fig. 1) recapitulates the phenotype of the Hand1 systemic knockout (Firulli et al., 1998; Morikawa and Cserjesi, 2004; Riley et al., 1998). Mutant embryos survive in the expected Mendelian ratio until 8.5 dpc. Most survive to 9.5 dpc but fail to turn with develop halting at the 7-somite stage (8.5 dpc) (Fig. 1a, b), a phenotype identical to the Hand1 systemic knockout. Previous analysis suggested that the lethality of the systemic Hand1 knockout may be due to its function in trophectoderm (Riley et al., 1998). To confirm that Cre is not expressed in trophectoderm, the HoxB6-Cre line was crossed with the R26R reporter line. No Cre activity was observed in trophectoderm derivatives (date not shown). These results show that lethality caused by systemic loss of Hand1 is due to loss of Hand1 in lateral or extraembryonic mesoderm.
Fig. 1. Deletion of Hand1 in extraembryonic and lateral mesoderm results in embryonic lethality.
Hand1 was conditionally deleted in lateral and extraembryonic mesoderm using the HoxB6-Cre deleter line and Hand1 expressing tissues were marked using a β-galactosidase gene inserted into the Hand1 gene. (a) Control embryos show the distribution of Hand1 expression at 9.5 dpc. (b) Conditional mutant embryos at 9.5 dpc are developmentally retarded failing to turn and do not develop past the 7-somite stage. ant: anterior, h; heart, lm; lateral mesoderm, post: posterior.
Although Hand1 is abundantly expressed in lateral mesoderm, and may be the cause of the early lethality, its role has not been investigated due to the lack of an appropriate Cre deleter line. To delete Hand1 in lateral but not in extraembryonic mesoderm, we generated a transgenic mouse line expressing Cre under the control of Tlx2 regulatory sequences. The Tlx2 gene (previously called Hox11L1, Enx, and Ncx) is first expressed in the primitive streak ectoderm (Tang et al., 1998) and subsequently expressed in the peripheral nervous system (PNS) (Hatano et al., 1997), neural tube (NT) (Uchiyama et al., 1999) and posterior lateral mesoderm, extraembryonic ectoderm, and ectoplacental cone by 7.0 dpc (Tang et al., 1998).
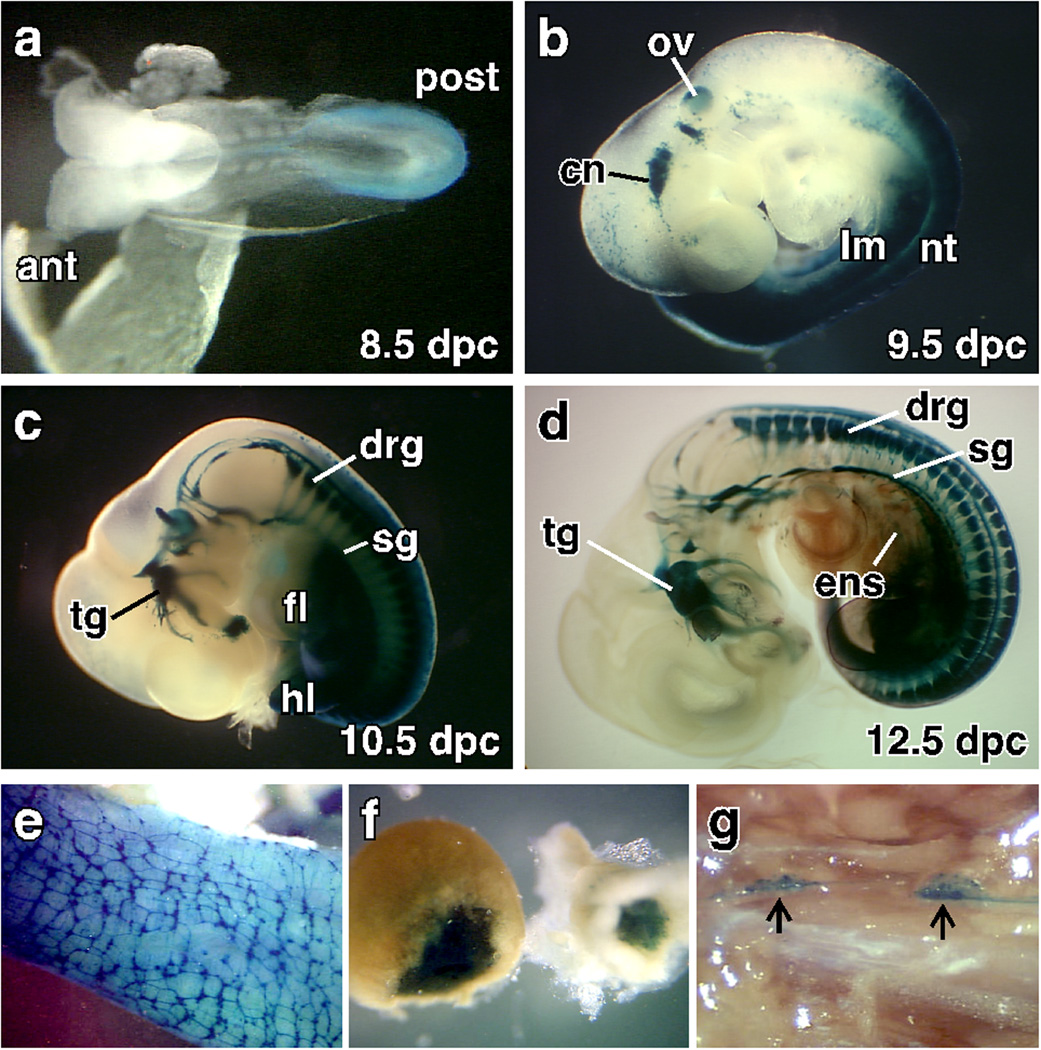
In order to find a regulatory region of the Tlx2 gene that activates expression in the embryo but not extraembryonic lineages, we analyzed the Tlx2 promoter region in vivo. We generated transgenic mouse lines with a 3 kb 5’ sequence known to preferentially drives expression in NC-derived cell lines (Iitsuka et al., 1999), linked to LacZ. The expression pattern was determined by examining embryos and adult tissues for β-galactosidase activity (Fig. 2). Unlike the expression of the endogenous Tlx2 gene, which begins to express in the primitive streak, extraembryonic ectoderm, and ectoplacental cone by 7.0 dpc (Kapur et al., 2005; Tang et al., 1998), expression of the transgene begins at 8.5 dpc and is not detected in extraembryonic tissues. At 8.5 dpc, the transgene recapitulates expression of the endogenous Tlx2 gene with expression first appearing in the neuroectoderm at the caudal half of the embryos at 8.5 dpc (Fig. 2a). By 9.5 dpc, expression is robust in the lateral mesoderm, caudal neural tube, and developing cranial nerves (CN) (Fig. 2b). In addition, ectopic expression is seen in the otic vesicle (Fig. 2b). At 10.5 dpc, expression is seen in much of the PNS (Fig. 2c). In the head, expression is seen in the glossopharyngeal ganglion (CN IX), inferior vagal ganglia (CN X), facioacoustic complex (CN VII–VIII) and trigeminal nerves (CN V). In the trunk, expression is high in the dorsal root ganglia (DRG), sympathetic ganglia (SG), enteric nervous system (ENS), with expression continuing in the lateral mesoderm (Fig. 2c). At 12.5 dpc, intense staining continues to be seen in the PNS but expression in the CNs and lateral mesoderm is decreased (Fig. 2d) resulting in weaker β-galactosidase staining by 14.5 dpc (data not shown). In the trunk, the transgene continues to be expressed in the PNS until birth (data not shown). In adults, transgene expression becomes highly restricted to the regions of endogenous gene expression (Parisi et al., 2003). In the gut, expression remains high throughout the myenteric plexus and in a subset of smooth muscle cells (Fig 2e). Expression in the adrenal gland remains high (Fig. 2f) but in the trunk SG, expression is restricted in a subset of cells (Fig. 2g). The 3 Kb regulatory region of Tlx2 closely resembles expression of endogenous gene in the embryos but not in extraembryonic tissues.
Fig. 2. A 3 kb region of the Tlx2 gene promotes endogenous gene expression within the embryos.
The Tlx2-LacZ transgenic line was analyzed for expression during development and in adults. Expression of the transgene is first observed at 8.5 dpc (a) with expression restricted to the dorsal region of the closing neural tube (NT) rostrally and neural folds caudally. At 9.5 dpc (b), Tlx2-LacZ expression is seen in the condensing cranial ganglia, otic vesicle, lateral mesoderm and NT. At 10.5 dpc (c), expression begins to be detected in the DRG and SG while expression is maintained in the cranial nerves, neural tube and lateral mesoderm. In the lateral mesoderm derivatives, expression is restricted caudal to the posterior half of forelimb. At 12.5 dpc (d), expression of the transgene remains robust in cranial ganglia, DRG, SG and express can be seen in the ENS with decreased expression in the NT and lateral mesoderm. Prior to imaging, 12.5 dpc embryos were cleared in benzyl alcohol and benzyl benzoate. Expression of Tlx2-LacZ continues in adult ENS (e), adrenal gland (f), and a subset of the cells in the SG (g). ant: anterior, cn: cranial nerves, drg: dorsal root ganglia, ens; enteric nervous system, fl: forlimb, hl: hindlimb, lm: lateral mesoderm, nt: neural tube, ov: otic vesicle, post: posterior, sg: sympathetic ganglia, tg: trigeminal ganglia,
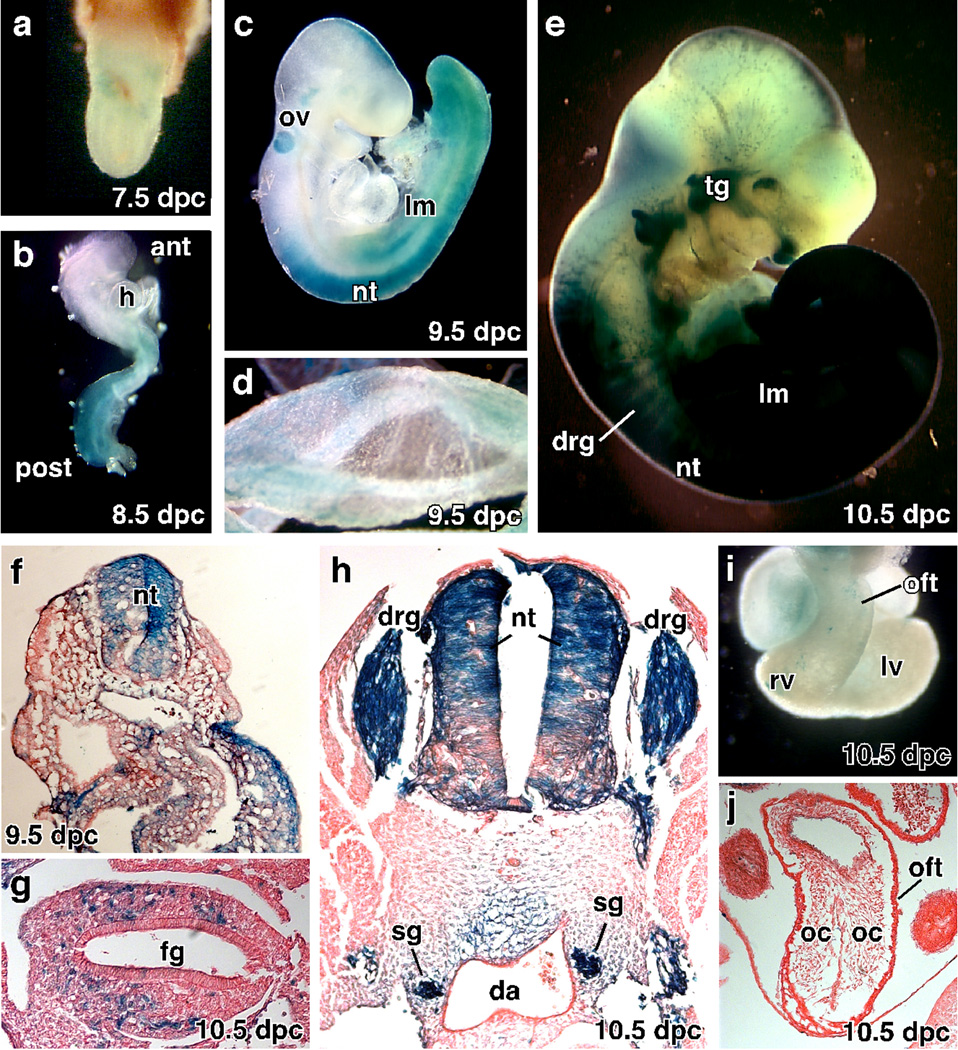
Since this regulatory region of the Tlx2 does not promote expression in extraembryonic tissues, we generated a Tlx2-Cre deleter line. The Tlx2-Cre construct was generated by replacing the LacZ region of Tlx2-LacZ with the Cre recombinase gene. We generated a stable line of Tlx2-Cre and examined the expression of Cre by crossing with the R26R reporter line. Analysis of β-galactosidase expression from 7.5 dpc to 10.5 dpc demonstrates that the Tlx2-Cre transgene recapitulates expression of the Tlx2-LacZ line (Fig. 3). Recombination of LacZ by Tlx2-Cre was not detected at 7.5 dpc (Fig 3a) but was observed at 8.5 dpc in the NT (data not shown) and lateral plate mesoderm in the caudal half of the embryo (Fig. 3b). By 9.5 dpc, β-galactosidase activity is seen in the otic vesicle and continues in the NT and lateral plate mesoderm (Fig. 3c). Unlike Tlx2-LacZ, a few cell in the extraembryonic mesoderm express β-galactosidase (Fig. 3d). At 10.5 dpc, Cre activity is found in the PNS (Fig. 3e, g, h), including CNs, DRG, SG and ENS. To visualize the distribution of Cre expressing cells in greater detail, embryos were sectioned. At 10.5 dpc, Cre activity is localized to the dorsal region of NT and lateral mesoderm and is not expressed in paraxial mesoderm (Fig. 3f).
Fig. 3. The Tlx2-Cre line expresses Cre in the PNS, otic vessel and lateral mesoderm.
The Tlx2-Cre mouse line was analyzed for β-galactosidase expression by crossing with the R26R reporter line. (a) Little or no expression of β-galactosidase was observed at 7.5 dpc. (b) Cre recombinase activity was observed at 8.5 dpc in caudal half of the embryo. (c) At 9.5, β-galactosidase expression is seen in the neural tube, lateral mesoderm, and otic vesicle. (d) Limited punctate expression of β-galactosidase was observed in yolk sac. (e) β-galactosidase expression is activated in the PNS by 10.5 dpc and remains expressed in the neural tube and lateral plate mesoderm but not in NC-derived cranial tissues. (f, g, h) Histological analysis of 9.5 dpc (f) and 10.5 dpc (g, h) embryos shows Cre activity in the dorsal neural tube, lateral mesoderm and the NC-derived sensory, sympathetic and enteric nervous systems. (i, j) β-galactosidase is not expressed in the outflow tract (oft) of the heart. ant: anterior, drg: dorsal root ganglia, ens: enteric nervous system, fg: forgut, h: heart, lm: lateral mesoderm, lv: left ventricle, nt: neural tube, oc: outflow tract cardiac cushion, oft: outflow tract, ov: otic vesicle, post: posterior, rv: right ventricle, sg: sympathetic ganglia, tg: trigeminal ganglia.
The expression pattern of the Tlx2-Cre line suggests it will be useful for conditional inactivation of genes in a number of lineages. Of particular interest will be its use in development studies of the NC-derived PNS. Early deletion of genes regulating sympathetic, sensory and enteric nervous system development have relied to a great extent on the Wnt1-Cre mouse line which expresses Cre in the premigratory NC (Danielian et al., 1998). However, Wnt1-Cre deletes genes in all NC often resulting in cardiovascular and facial defects leading to death at birth (Morikawa and Cserjesi, 2008; Morikawa et al., 2009; Stottmann et al., 2004; Xiong et al., 2009). The Tlx2-Cre line deletes genes in the NC-derived PNS (Fig. 3h, g) and in placode derived cranial ganglia (Fig. 3e) but does not express Cre in the cranial NC (Fig. 3e) or cardiac NC (Fig. 3i, j), allowing deletion of the genes regulation of PNS development without affecting the cranial or cardiac NC lineages.
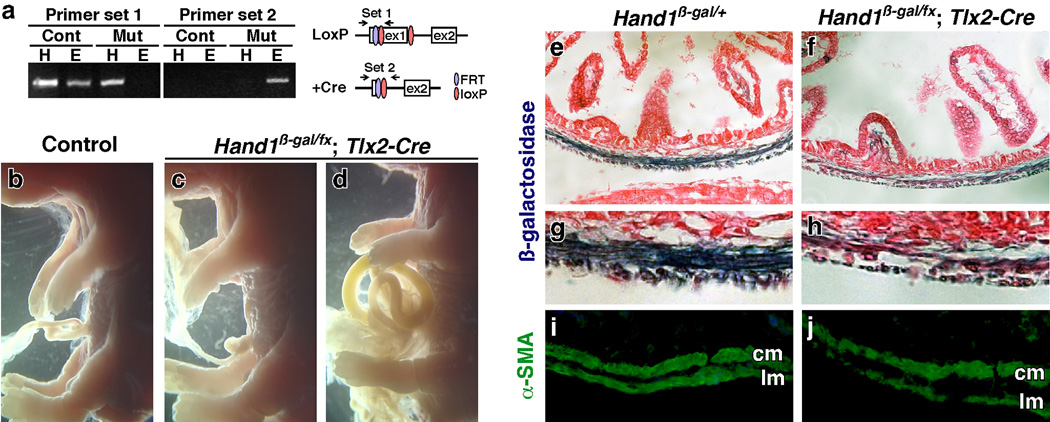
Tlx2-Cre and Hand1 are co-expressed in the SG and lateral mesoderm (Cserjesi et al., 1995; Hatano et al., 1997; Kapur et al., 2005; Morikawa and Cserjesi, 2004). To investigate the role of Hand1 in lateral mesoderm, we deleted Hand1 conditionally by crossing with the Tlx2-Cre line. The level of Hand1 recombination was determined by PCR analysis. In enteric smooth muscle (ESM), which is derived from Cre expressing lateral mesoderm, recombination occurs at a high level (Fig. 4a). Survival analysis of mutant embryos shows that all survive until 14.5 dpc with 6 out of 8 mutants dying between 14.5 dpc and birth with the remaining surviving to adulthood. Gross analysis of 18.5 dpc mutant embryos shows that the defects in conditional knockout embryos range from no apparent defects (Fig. 4b, c) to those with ventral closure defects resulting in gut hernias of variable severity (Fig. 4d). The results show that Hand1 plays an important function in lateral plate derived mesoderm.
Fig. 4. Deletion of Hand1 in lateral mesoderm results in gut herniation and enteric smooth muscle defects.
Hand1 was deleted in lateral mesoderm by crossing the Hand1β-gal/fx and Tlx2-Cre mouse lines. (a) PCR analysis of recombination efficiency of the conditional Hand1 allele. Recombination of Hand1β-gal/fx (cont) and Hand1β-gal/fx; Tlx2-Cre (mut) tissues from heart (H) and enteric smooth muscles (E) was determined by analysis of the first loxP site (primer set 1) and the recombined loxP site (primer set 2). A high level of recombination is seen in enteric smooth muscle. (b–d) Morphological analysis of control (b) and conditional Hand1 knockout (c, d) embryos at 18.5 dpc shows that loss of Hand1 can result in gut herniation. Mutant embryos present variable penetrance ranging from no gut herniation (c) to severe herniation (d). (e–j) Histological analysis of gut musculature of Hand1β-gal/+ heterozygous (e, g, i) and conditional Hand1 mutant (f, h, j) 18.5 dpc embryos. Staining for β-galactosidase expression from the Hand1β-gal allele (d, f) shows that Hand1 is expressed in a subset of cells in the smooth muscle layers. Loss of Hand1 in lateral mesoderm results in thinner and disorganized smooth muscle layers. (i, j) Enteric smooth muscle cells in control (i) and conditional Hand1 mutant (j) embryos were analyzed for α-smooth muscle actin (α-SMA) expression by immunohistochemistry. Enteric smooth muscle in conditional Hand1 knockout embryos is thin and disorganized. cm: circular muscle, lm: longitudinal muscle.
Hand1 is expressed in the ESM throughout development and in adults (Cserjesi et al., 1995; D'Autreaux et al., 2007; Morikawa and Cserjesi, 2004) and we have shown a role in vascular smooth muscle cell recruitment in extraembryonic mesoderm (Morikawa and Cserjesi, 2004). We examined if Hand1 deletion using Tlx2-Cre affects ESM development (Fig. 4e–h). Compared to control embryos (Fig. 4e, g), the ESM layers in mutant embryos were thinner and disorganized (Fig. 4f, h). To determine if the ESM cells are differentiated, the guts were analyzed for expression of α-smooth muscle actin (α-SMA) by immunofluorescent analysis (Fig. 4i, j). The cells in longitudinal and circular muscle layers of the gut express α-SMA in both control (Fig. 4i) and conditional mutant (Fig. 4j) embryos, suggesting that as in extraembryonic mesoderm, Hand1 is not required for differentiation of ESM cells but is required for its organization.
The combined deletional analysis of Hand1 using the HoxB6-Cre and Tlx2-Cre lines shows for the first time that the early lethality of systemic loss of Hand1 is due to its function in extraembryonic mesoderm. In addition, our analysis of Hand1 function in lateral mesoderm has uncovered roles in ventral wall closure and ESM development.
MATERIALS AND METHODS
Generation of Transgenic Constructs and Mouse Lines
A Tlx2 sequence containing −3003 bp to +706 was amplified from C57BL6 genomic DNA (Promega) using LA Taq polymerase (TaKaRa) and the primers 5’-AGA CAC CCT CAC CCC TAC CCC AAC CCT CAA -3’ and 5’-TCC TCC CCC AAC CAC AGA AGC CTC ATC AAG -3’. The amplified region was cloned into pCR-XL-Topo vector (Invitrogen) to generate Tlx2-pCR-XL. To generate the Tlx2 promoter/enhancer β-galactosidase fusion construct pTlx2-LacZ, the Tlx2-pCR-XL construct was digested with HindIII and NcoI to produce a fragment containing Tlx2 sequence from −3003 bp upstream to the start of translation. The fragment was cloned into the HindIII and NcoI sites of pBS-Hsp68-LacZ (Kothary et al., 1989) resulting in the simultaneous loss of the Hsp68 promoter sequence and fusion of the Tlx2 translational start site of LacZ. The pTlx2-LacZ insert was excised for proneural injection by digestion with SalI.
The pTlx2-Cre vector was generated by replacing the LacZ of pTlx2-LacZ with the gene encoding Cre recombinase. The Cre gene was excised as an NcoI-NotI fragment from Hsp68-Cre (provided by Brian Black, University of California San Francisco) and cloned into the NcoI-NotI site of pTlx2-LacZ. The Tlx2-Cre insert was released by digestion with SalI.
Generation of the transgenic lines was by pronuclear injection by the Tulane Transgenic Mouse Facility. Tlx2-LacZ transgenic mouse lines were identified by PCR analysis of tail clippings using the primers GCA ATT TAA CCG CCA GTC AGG and AGG CGG TCG GGA TAG TTT TCT T to amplify a fragment of 462 bp located in the LacZ gene. Mice carrying Tlx2-Cre were identified by PCR genotyping using primer sequences CTG GAA AAT GCT TCT GTC CGT TTG and ACG AAC CTG GTC GAA ATC AGT GCG located within the Cre gene producing a 316 bp fragment (Lowe et al., 2000).
Mouse Husbandry
Tlx2-LacZ males were mated with Black Swiss females (Taconic) and the resulting Tlx2-Cre males were mated with R26R (Gt(ROSA)26Sortm1Sho) reporter females (Mao et al., 1999). HoxB6-Cre (Lowe et al., 2000) and Tlx2-Cre lines were mated with the Hand1β-gal/+ line (Morikawa and Cserjesi, 2004) to obtain Hand1β-gal/+; HoxB6-Cre or Hand1β-gal/+; Tlx2-Cre lines. These mice were crossed with Hand1fx/fxfemale mice (provided by Dr E. Olson, UT Southwestern Medical Center)(McFadden et al., 2005) to obtain conditional knockout embryos. To obtain embryos, mice were sacrificed using CO2 in accordance with Tulane’s Institutional Animal Care and Use Committee.
LoxP site and recombined allele were genotyped by PCR analysis. Primer set 1 (GGG AGG GAC ATA GGC GGG GCG GGT TTT and GG GTG CGG CGG GTG TGA GTG GTG) amplifies across the first loxP site producing a 600 bp product. The recombined allele was genotyped using primer set 2 (CCT TCA GGC TCC CAC GAT and AGC AAA ATT CTA TGT TCA CTC AGC) that amplifies an 800 bp region.
β-galactosidase staining and histological analysis
Embryos and tissues were fixed in 4% paraformaldehyde in PBS for 15 minutes, rinsed in PBS, and stained overnight with 1 mg/ml X-gal, in 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2 in PBS. For analysis at 14.5 dpc, embryos were cleared after staining by dehydration in ethanol then clearing in BABB (2:1 benzylaclohol:benzyl benzoate). For histological analysis, tissues were embedded in paraffin and sectioned at 10 µm. Sections were counter-stained for eosin.
Immunohistochemistry
Immunohistochemical analysis on cryosections was performed as previously described (Morikawa and Cserjesi, 2004; Morikawa et al., 2007). The antibody used in this study was FITC conjugated anti-α-SM actin, (Sigma) at a 1:200 dilution.
ACKNOWLEDGEMENT
The authors thank Dr. Eric Olson (University of Texas Southwestern Medical Center) for providing the conditional Hand1 mouse line and Dr. Brian Black (University of California San Francisco) for providing the Hsp-Cre plasmid. This work was supported by grants to Y. M. from AHA (SDG) and AHA (Grant-in-Aid), NSF (IOS-0529746, MCB-0529746) to P. C and NIH (NS15547).
LITERATURE CITED
- Barbosa AC, Funato N, Chapman S, McKee MD, Richardson JA, Olson EN, Yanagisawa H. Hand transcription factors cooperatively regulate development of the distal midline mesenchyme. Dev Biol. 2007;310:154–168. doi: 10.1016/j.ydbio.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Lyons GE, Olson EN. Expression of the novel basic helix-loop-helix gene eHAND in neural crest derivatives and extraembryonic membranes during mouse development. Dev Biol. 1995;170:664–678. doi: 10.1006/dbio.1995.1245. [DOI] [PubMed] [Google Scholar]
- D'Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development. 2007;134:2237–2249. doi: 10.1242/dev.003814. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Muccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN. Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet. 1998;18:266–270. doi: 10.1038/ng0398-266. [DOI] [PubMed] [Google Scholar]
- Hatano M, Iitsuka Y, Yamamoto H, Dezawa M, Yusa S, Kohno Y, Tokuhisa T. Ncx, a Hox11 related gene, is expressed in a variety of tissues derived from neural crest cells. Anat Embryol (Berl) 1997;195:419–425. doi: 10.1007/s004290050061. [DOI] [PubMed] [Google Scholar]
- Iitsuka Y, Shimizu H, Kang MM, Sasagawa K, Sekiya S, Tokuhisa T, Hatano M. An enhancer element for expression of the Ncx (Enx, Hox11L1) gene in neural crest-derived cells. J Biol Chem. 1999;274:24401–24407. doi: 10.1074/jbc.274.34.24401. [DOI] [PubMed] [Google Scholar]
- Kapur RP, Clarke CM, Doggett B, Taylor BE, Baldessari A, Parisi MA, Howe DG. Hox11L1 expression by precursors of enteric smooth muscle: an alternative explanation for megacecum in HOX11L1-/- mice. Pediatr Dev Pathol. 2005;8:148–161. doi: 10.1007/s10024-005-1126-0. [DOI] [PubMed] [Google Scholar]
- Kothary R, Clapoff S, Darling S, Perry MD, Moran LA, Rossant J. Inducible expression of an hsp68-lacZ hybrid gene in transgenic mice. Development. 1989;105:707–714. doi: 10.1242/dev.105.4.707. [DOI] [PubMed] [Google Scholar]
- Lowe LA, Yamada S, Kuehn MR. HoxB6-Cre transgenic mice express Cre recombinase in extra-embryonic mesoderm, in lateral plate and limb mesoderm and at the midbrain/hindbrain junction. Genesis. 2000;26:118–120. doi: 10.1002/(sici)1526-968x(200002)26:2<118::aid-gene5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mao X, Fujiwara Y, Orkin SH. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci U S A. 1999;96:5037–5042. doi: 10.1073/pnas.96.9.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Extra-embryonic vasculature development is regulated by the transcription factor HAND1. Development. 2004;131:2195–2204. doi: 10.1242/dev.01091. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Cserjesi P. Cardiac neural crest expression of Hand2 regulates outflow and second heart field development. Circ Res. 2008;103:1422–1429. doi: 10.1161/CIRCRESAHA.108.180083. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, D'Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–126. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development. 2009;136:3575–3584. doi: 10.1242/dev.038133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi MA, Baldessari AE, Iida MH, Clarke CM, Doggett B, Shirasawa S, Kapur RP. Genetic background modifies intestinal pseudo-obstruction and the expression of a reporter gene in Hox11L1-/- mice. Gastroenterology. 2003;125:1428–1440. doi: 10.1016/j.gastro.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Hoodless PA, Lu Z, Breitman ML, McInnes RR, Wrana JL, Buchwald M. The Tlx-2 homeobox gene is a downstream target of BMP signalling and is required for mouse mesoderm development. Development. 1998;125:1877–1887. doi: 10.1242/dev.125.10.1877. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Otsuka R, Hanaoka K. CHox11L2, a Hox11 related gene, is expressed in the peripheral nervous system and subpopulation of the spinal cord during chick development. Neurosci Lett. 1999;273:97–100. doi: 10.1016/s0304-3940(99)00637-0. [DOI] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang Z, Lan Y, Jiang R, Cserjesi P, Chen Y. Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol. 2009;330:131–141. doi: 10.1016/j.ydbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]