Abstract
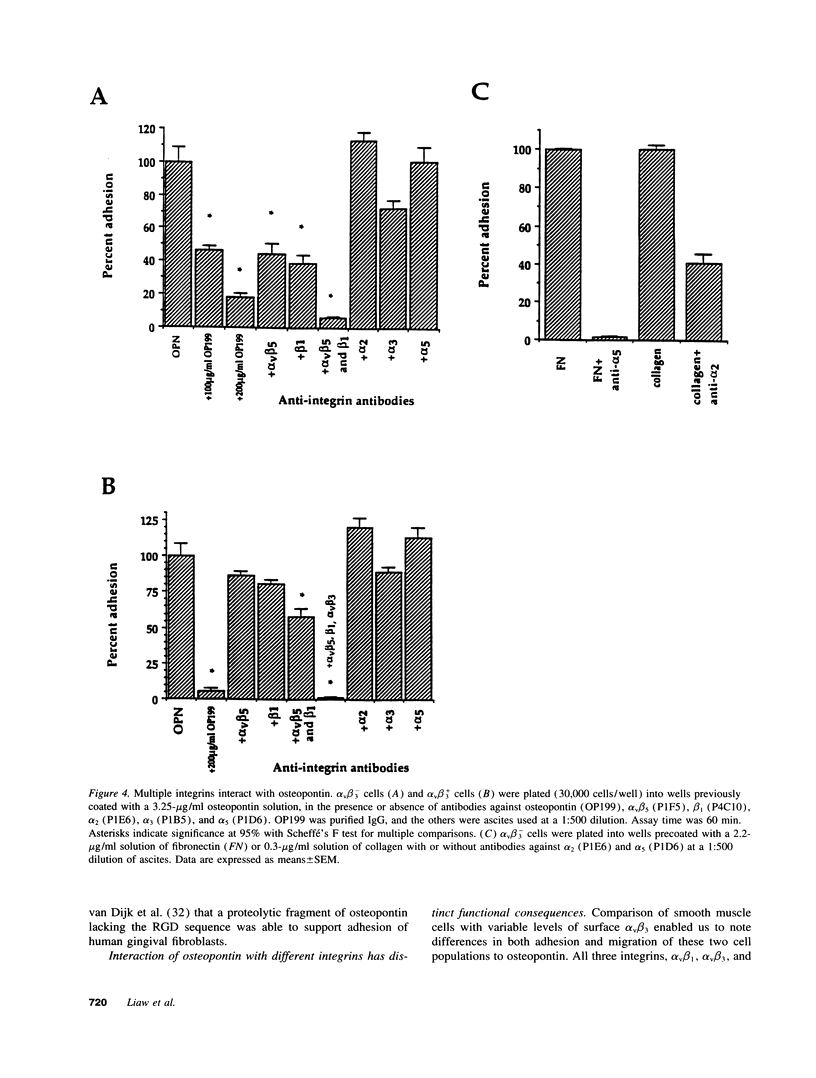
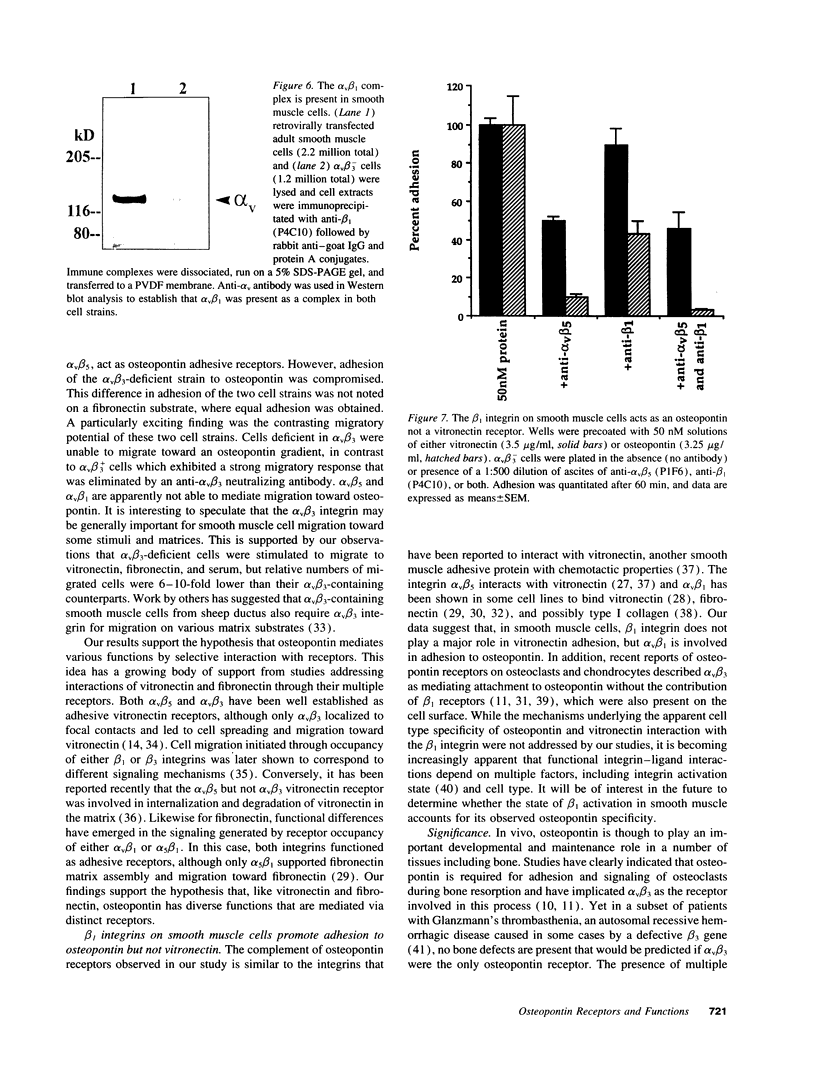
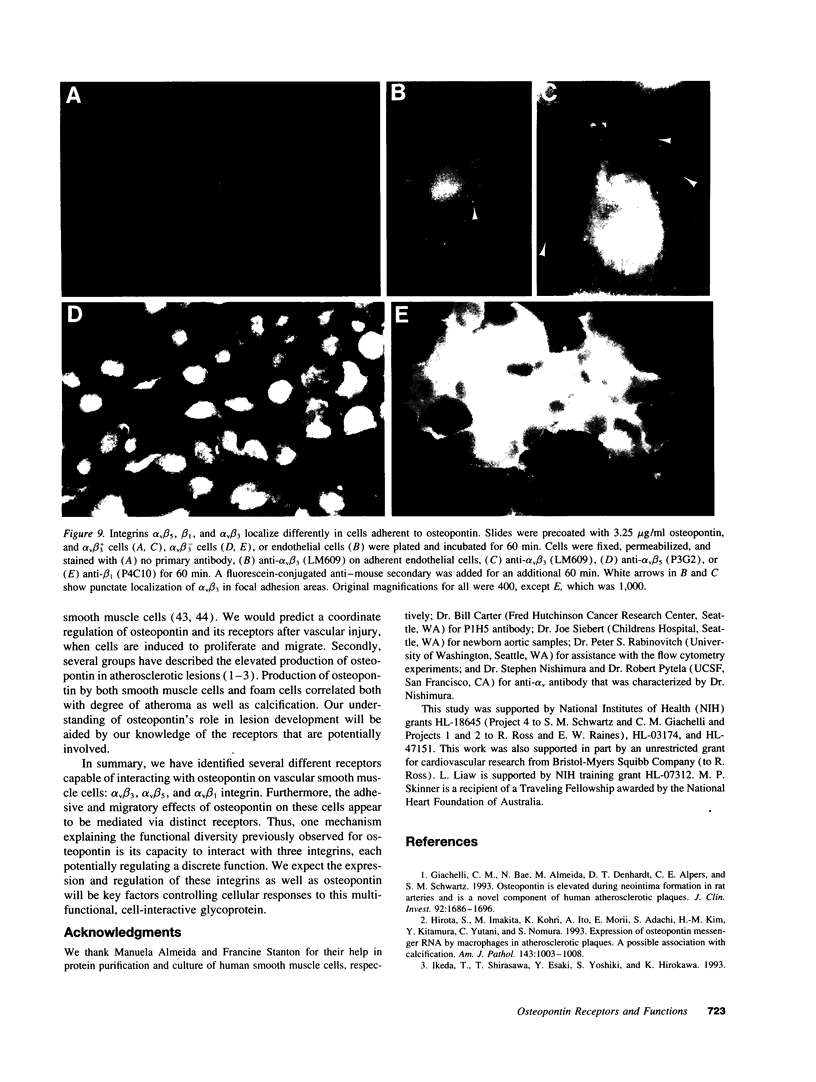
Osteopontin is an arginine-glycine-aspartate containing acidic glycoprotein postulated to mediate adhesion, migration, and biomineralization in diverse tissues. The mechanisms explaining this multifunctionality are not well understood, although it is known that one osteopontin receptor is the alpha v beta 3 integrin. In this work, we studied human smooth muscle cells varying in alpha v beta 3 levels to identify additional osteopontin receptors. We report that, in addition to alpha v beta 3, both alpha v beta 5 and alpha v beta 1 are osteopontin receptors. Moreover, the presence or absence of alpha v beta 3 on the cell surface altered the adhesive and migratory responses of smooth muscle cells to osteopontin. Adhesion of alpha v beta 3-deficient cell populations to osteopontin was only half that of cells containing alpha v beta 3, and migration toward an osteopontin gradient in the Boyden chamber was dependent on cell surface alpha v beta 3. Although alpha v beta 3-deficient smooth muscle cells were unable to migrate to osteopontin, they did migrate significantly in response to vitronectin and fibronectin. These findings represent the first description of alpha v beta 5 and alpha v beta 1 as osteopontin receptors and suggest that, while adhesion to osteopontin is supported by integrins containing beta 1, beta 3, and beta 5, migration in response to osteopontin appears to depend on alpha v beta 3. Thus, interaction with distinct receptors is one mechanism by which osteopontin may initiate multiple functions.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basson C. T., Kocher O., Basson M. D., Asis A., Madri J. A. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J Cell Physiol. 1992 Oct;153(1):118–128. doi: 10.1002/jcp.1041530116. [DOI] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Brown L. F., Berse B., Van de Water L., Papadopoulos-Sergiou A., Perruzzi C. A., Manseau E. J., Dvorak H. F., Senger D. R. Expression and distribution of osteopontin in human tissues: widespread association with luminal epithelial surfaces. Mol Biol Cell. 1992 Oct;3(10):1169–1180. doi: 10.1091/mbc.3.10.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A. F., Hota C., Prince C. W. Adhesion of metastatic, ras-transformed NIH 3T3 cells to osteopontin, fibronectin, and laminin. Cancer Res. 1993 Feb 1;53(3):701–706. [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Choi E. T., Engel L., Callow A. D., Sun S., Trachtenberg J., Santoro S., Ryan U. S. Inhibition of neointimal hyperplasia by blocking alpha V beta 3 integrin with a small peptide antagonist GpenGRGDSPCA. J Vasc Surg. 1994 Jan;19(1):125–134. doi: 10.1016/s0741-5214(94)70127-x. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Kramer R. H. Beta 1 and beta 3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992 Jun;200(2):272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Gray V. Isolation of a novel integrin receptor mediating Arg-Gly-Asp-directed cell adhesion to fibronectin and type I collagen from human neuroblastoma cells. Association of a novel beta 1-related subunit with alpha v. J Cell Biol. 1990 Jun;110(6):2185–2193. doi: 10.1083/jcb.110.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T., Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993 Dec;7(15):1475–1482. [PubMed] [Google Scholar]
- Flores M. E., Norgård M., Heinegård D., Reinholt F. P., Andersson G. RGD-directed attachment of isolated rat osteoclasts to osteopontin, bone sialoprotein, and fibronectin. Exp Cell Res. 1992 Aug;201(2):526–530. doi: 10.1016/0014-4827(92)90305-r. [DOI] [PubMed] [Google Scholar]
- Freed E., Gailit J., van der Geer P., Ruoslahti E., Hunter T. A novel integrin beta subunit is associated with the vitronectin receptor alpha subunit (alpha v) in a human osteosarcoma cell line and is a substrate for protein kinase C. EMBO J. 1989 Oct;8(10):2955–2965. doi: 10.1002/j.1460-2075.1989.tb08445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. N., Caen J. P., Nurden A. T. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990 Apr 1;75(7):1383–1395. [PubMed] [Google Scholar]
- Giachelli C. M., Bae N., Almeida M., Denhardt D. T., Alpers C. E., Schwartz S. M. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993 Oct;92(4):1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D., Mohai L. G., Schwartz S. M. Induction of polyploidy in cultures of neonatal rat aortic smooth muscle cells. Circ Res. 1986 Dec;59(6):633–644. doi: 10.1161/01.res.59.6.633. [DOI] [PubMed] [Google Scholar]
- Helfrich M. H., Nesbitt S. A., Dorey E. L., Horton M. A. Rat osteoclasts adhere to a wide range of RGD (Arg-Gly-Asp) peptide-containing proteins, including the bone sialoproteins and fibronectin, via a beta 3 integrin. J Bone Miner Res. 1992 Mar;7(3):335–343. doi: 10.1002/jbmr.5650070314. [DOI] [PubMed] [Google Scholar]
- Hirota S., Imakita M., Kohri K., Ito A., Morii E., Adachi S., Kim H. M., Kitamura Y., Yutani C., Nomura S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am J Pathol. 1993 Oct;143(4):1003–1008. [PMC free article] [PubMed] [Google Scholar]
- Horton M. A., Dorey E. L., Nesbitt S. A., Samanen J., Ali F. E., Stadel J. M., Nichols A., Greig R., Helfrich M. H. Modulation of vitronectin receptor-mediated osteoclast adhesion by Arg-Gly-Asp peptide analogs: a structure-function analysis. J Bone Miner Res. 1993 Feb;8(2):239–247. doi: 10.1002/jbmr.5650080215. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Shirasawa T., Esaki Y., Yoshiki S., Hirokawa K. Osteopontin mRNA is expressed by smooth muscle-derived foam cells in human atherosclerotic lesions of the aorta. J Clin Invest. 1993 Dec;92(6):2814–2820. doi: 10.1172/JCI116901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janat M. F., Argraves W. S., Liau G. Regulation of vascular smooth muscle cell integrin expression by transforming growth factor beta1 and by platelet-derived growth factor-BB. J Cell Physiol. 1992 Jun;151(3):588–595. doi: 10.1002/jcp.1041510319. [DOI] [PubMed] [Google Scholar]
- Leavesley D. I., Ferguson G. D., Wayner E. A., Cheresh D. A. Requirement of the integrin beta 3 subunit for carcinoma cell spreading or migration on vitronectin and fibrinogen. J Cell Biol. 1992 Jun;117(5):1101–1107. doi: 10.1083/jcb.117.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavesley D. I., Schwartz M. A., Rosenfeld M., Cheresh D. A. Integrin beta 1- and beta 3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993 Apr;121(1):163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw L., Almeida M., Hart C. E., Schwartz S. M., Giachelli C. M. Osteopontin promotes vascular cell adhesion and spreading and is chemotactic for smooth muscle cells in vitro. Circ Res. 1994 Feb;74(2):214–224. doi: 10.1161/01.res.74.2.214. [DOI] [PubMed] [Google Scholar]
- Loeser R. F. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheum. 1993 Aug;36(8):1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- Miyauchi A., Alvarez J., Greenfield E. M., Teti A., Grano M., Colucci S., Zambonin-Zallone A., Ross F. P., Teitelbaum S. L., Cheresh D. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991 Oct 25;266(30):20369–20374. [PubMed] [Google Scholar]
- Naito M., Hayashi T., Funaki C., Kuzuya M., Asai K., Yamada K., Kuzuya F. Vitronectin-induced haptotaxis of vascular smooth muscle cells in vitro. Exp Cell Res. 1991 May;194(1):154–156. doi: 10.1016/0014-4827(91)90145-k. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D., Pierschbacher M., Ruoslahti E. Identification of a bone sialoprotein receptor in osteosarcoma cells. J Biol Chem. 1988 Dec 25;263(36):19433–19436. [PubMed] [Google Scholar]
- Panetti T. S., McKeown-Longo P. J. The alpha v beta 5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993 Jun 5;268(16):11492–11495. [PubMed] [Google Scholar]
- Perez-Reyes N., Halbert C. L., Smith P. P., Benditt E. P., McDougall J. K. Immortalization of primary human smooth muscle cells. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1224–1228. doi: 10.1073/pnas.89.4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Influence of stereochemistry of the sequence Arg-Gly-Asp-Xaa on binding specificity in cell adhesion. J Biol Chem. 1987 Dec 25;262(36):17294–17298. [PubMed] [Google Scholar]
- Ross F. P., Chappel J., Alvarez J. I., Sander D., Butler W. T., Farach-Carson M. C., Mintz K. A., Robey P. G., Teitelbaum S. L., Cheresh D. A. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993 May 5;268(13):9901–9907. [PubMed] [Google Scholar]
- Skinner M. P., Raines E. W., Ross R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994 Nov;145(5):1070–1081. [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. Integrin (alpha v beta 3)-ligand interaction. Identification of a heterodimeric RGD binding site on the vitronectin receptor. J Biol Chem. 1990 Feb 5;265(4):2168–2172. [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique alpha and common beta subunits. J Cell Biol. 1987 Oct;105(4):1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Carter W. G., Piotrowicz R. S., Kunicki T. J. The function of multiple extracellular matrix receptors in mediating cell adhesion to extracellular matrix: preparation of monoclonal antibodies to the fibronectin receptor that specifically inhibit cell adhesion to fibronectin and react with platelet glycoproteins Ic-IIa. J Cell Biol. 1988 Nov;107(5):1881–1891. doi: 10.1083/jcb.107.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. Role of the integrin alpha v beta 6 in cell attachment to fibronectin. Heterologous expression of intact and secreted forms of the receptor. J Biol Chem. 1994 Mar 4;269(9):6940–6948. [PubMed] [Google Scholar]
- Zhang Z., Morla A. O., Vuori K., Bauer J. S., Juliano R. L., Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol. 1993 Jul;122(1):235–242. doi: 10.1083/jcb.122.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk S., D'Errico J. A., Somerman M. J., Farach-Carson M. C., Butler W. T. Evidence that a non-RGD domain in rat osteopontin is involved in cell attachment. J Bone Miner Res. 1993 Dec;8(12):1499–1506. doi: 10.1002/jbmr.5650081213. [DOI] [PubMed] [Google Scholar]