Abstract
Background
The societal costs and health benefits of tissue plasminogen activator (tPA) for ischemic stroke can be modeled and extended to the US population. Similarly, the societal impact of new thrombolytics with improved efficacy or safety or extending eligibility can also be modeled.
Methods
We previously modeled the impact of tPA on societal costs and health in the US. Pertinent publications on utilization, societal cost, and health impact were identified by systematic review, updated to include studies describing the impact of extending the tPA time window. Information on utilization of tPA was integrated with published per-use data on costs and health impact (converted to 2004 dollars) to generate annual projections for the US population. Model inputs were modified to reflect various characteristics of new thrombolytics.
Results
At its current price, tPA saves $6074 and adds 0.75 quality-adjusted life year (QALY) per use. If tPA were priced at $50,000/QALY, a standard benchmark for cost-effectiveness, it would cost $45,800 per dose and would be expected to generate $ 458 M in revenue annually for its manufacturer. Thrombolytics for stroke that extended the time window or improved efficacy could generate greater revenue if priced at an accepted level of cost-effectiveness. Given current clinical development costs, development of candidate drugs with 2.8% to 5.7% probability of ultimate FDA approval would be justified.
Conclusions
tPA produces substantial health and economic benefits in the US. Better thrombolytics for stroke could have substantial impact on society, and potential returns to developers would appear to justify greater investment in new candidates.
Keywords: Thrombolysis, health care, quality-adjusted life year, public health, cost-effectiveness
Introduction
Tissue plasminogen activator (tPA) reduces disability after ischemic stroke.1 Providing tPA acutely for patients with stroke results in additional costs to the healthcare system well beyond the cost of tPA itself. However, long-term disability is very expensive and more than offsets these costs.2 Cost-effectiveness analyses have suggested that tPA use results in substantial improvements in health—measured in quality-adjusted life years (QALYs)—while at the same time reducing costs to society.2–4 Although estimates of the value of tPA derived from cost-effectiveness analyses have varied based on model assumptions, all have found improvements in health and cost reductions with each use.
Pharmaceutical and biotechnology companies generally price drugs to optimize their profits. Many countries with universal health plans set cost-effectiveness criteria for drugs that are covered by their plans. This threshold is generally in the range of $50,000–$100,000 per QALY.5 Although such thresholds are not used in the US, healthcare systems often discourage use of agents that are less cost effective. Thus, it is not surprising that many drugs are priced to yield a net cost-effectiveness ratio of approximately $50,000/QALY. Competition can reduce prices, particularly when generics become available, while uncertainty about long-term benefits and acuity of illness will often raise them. Since use is not particularly sensitive to price below a threshold of cost-effectiveness, there is little incentive for a company to price a drug at a level at which society and the healthcare industry save money.
In this environment, the cost savings of tPA is exceptional, particularly given that it still has market exclusivity due to FDA regulations for biologic therapies. tPA was introduced first as a treatment for myocardial infarction.6 Its price was set for this indication, for which other agents were available. At its market price, tPA is cost effective but not cost saving for myocardial infarction, with one study finding a net cost of $32,678 per additional year of life when compared to streptokinase.7 The net benefits of tPA for treatment of stroke are much greater in terms of reduced disability and its associated costs but it is not feasible to have one price for myocardial infarction and another for stroke. In essence, Genentech foregoes some profit (perhaps involuntarily) to the benefit of society in general.
Pharmaceutical and biotechnology companies should be incentivized to produce more effective therapies for conditions with greater societal burden. Pricing that relates to the ultimate benefit to society provides this incentive. Development of new drugs is very expensive and risky, and can only be justified if the potential return is great enough. A gamble is worthwhile—in Vegas or in North Jersey—if the upfront cost is less than the expected return times the likelihood of that return. I sought to modify an economic model initially developed to assess the impact of clinical trials8 in order to estimate the potential incentives for society and industry to enhance use of tPA or to create better thrombolytics for ischemic stroke.
Methods
In a previous publication, we described in detail a model developed to estimate the impact of specific clinical trials on societal costs and health.8 In this analysis, societal impact for a new intervention was estimated as the cost and health benefits associated with each use—derived from published cost-utility and other economic models—multiplied by the number of uses. For this analysis, we applied the prior model to tPA and a series of hypothetical thrombolytics. For simplicity, we assumed that the price of a drug represented a true cost to society.
To estimate the impact of tPA, usage data and estimates of the impact of the intervention on health and costs were derived from the literature using standard methods of systematic review.9 Methods for identifying publications of pertinent cost-utility analyses and usage of tPA were derived from previously validated, highly sensitive search strategies.10 When multiple publications on cost, health implications, or usage were identified, articles were reviewed independently by three physician clinical researchers, who were asked to select the most pertinent reference based on predefined criteria. To estimate the impact of extending the time window for thrombolysis, we assumed a similar benefit and projected greater usage based on actual arrival times and treatment rates in a large cohort study.11 Since the selected cost-utility model was published prior to publication of the ECASS III trial, showing efficacy of tPA in a 0–4.5 window after stroke onset,12 we did not attempt to account for current usage and benefits in the 3–4.5 hour window. Model inputs are shown in Table 1.
Table 1.
Model inputs
| Input | Value | Source | References |
|---|---|---|---|
| tPA cost | $2,200 | Fagan | 2 |
| Net cost to society per use | −$6,074 | Fagan | 2 |
| Net benefit in health per use | 0.75 QALY | Fagan | 2 |
| Annual uses in US | 10,000 | American Heart Association, Reeves | 13, 14 |
| Value of a QALY | $40,300 | Bureau of Labor Statistics | 15 |
| Impact of altering time window | Variable | California Acute Stroke Pilot Registry | 11 |
For tPA and other hypothetical thrombolytics, we utilized a health economic model designed to estimate US aggregate treatment costs or savings and impact on public health, measured in QALYs and societal costs (defined as total related net expenditures, including healthcare and productivity costs, regardless of the payer). Annual utilization was projected for 10 years beginning in the first year after development completion, remaining stable at the rate described in the most recent publications meeting review criteria.13, 14 For hypothetical thrombolytics, utilization rates were modified to account for differences in modeled indications, such as time window. Annual net costs and benefits were calculated by multiplying per-use estimates by net changes in utilization of tPA or a hypothetical thrombolytic.
Total net value of a thrombolytic was calculated at annual time points after funding completion by combining trial costs and treatment costs with a monetary value for a QALY, derived by valuing a QALY at per-capita US gross domestic product ($40,310),15 which estimates the average annual economic productivity of a US resident, regardless of employment or age.16 All costs and benefits were converted to 2004 dollars using the Medical-U portion of the Consumer Price Index.17 Overall societal impact of a drug was estimated as the total net health benefit in QALYs multiplied by gross domestic product, minus the increase in total costs related to the tested interventions minus the total cost of the development program. A “fair” price was defined as one associated with a net cost to society of $50,000/QALY, a common standard for cost effectiveness.5
To evaluate the business case for drug development, we calculated the minimum probability for ultimate drug approval necessary to justify clinical development costs given the potential 10-year returns in revenue. We estimated development costs of $171 M per drug candidate based on expenditures for phase I–III trials of 1682 drugs developed at 182 public companies.18 Given that basic and translational studies of thrombolytic drug candidates are often justified by development for cardiac indications, we did not include estimates of these pre-clinical costs. We estimated that drug production, distribution, and marketing costs accounted for 30% of sales revenue.19 We also assumed that new drugs would replace tPA use in the 0–3 hour window.
Relationship with the Sponsor
The original study was funded through a contract form NINDS. The current study was performed without additional financial support and was designed, data analyzed, and final version of the manuscript prepared independent of NINDS. There are no other conflicts of interest.
Results
Based on model input derived from systematic review including current utilization of tPA for stroke, the net costs and health benefits of its use, and the value of a QALY, tPA use in the US is associated annually with $60 million in direct cost savings to society and an additional 7510 QALYs, for a total annual societal benefit of $363 million. Its manufacturer receives approximately $22 million in annual revenue.
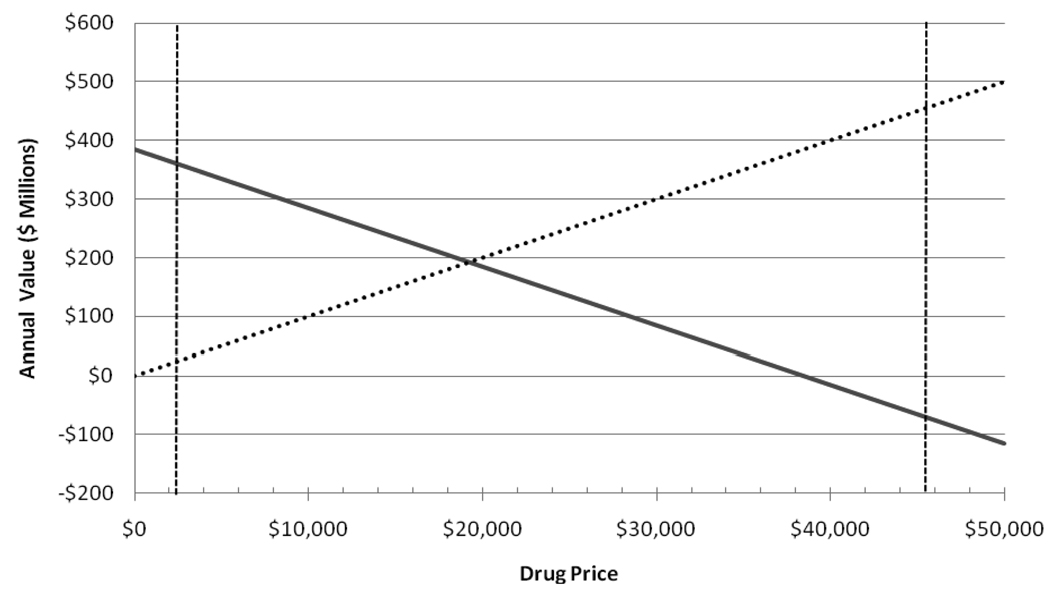
Although the price of tPA was determined primarily by its usage for myocardial infarction, a company developing a new thrombolytic for stroke—perhaps only marginally superior—would be free to set a price. For such a drug, setting a price equivalent to $50,000/QALY, a common standard for acceptable “fair” pricing, a manufacturer could generate annual revenues of $458 million at a price per dose of $45,800, although this would result in a net loss to society of $73 million annually (Figure, Table 2). Thus, the 10-year return to a manufacturer would be $4.58 billion. Such a return would justify a development cost of $171 M with a 5.3% likelihood of success.
Figure.
Annual total returns to society (solid line) and to the developer (dashed line) for tPA for stroke at prices ranging from free to $50,000 per dose in millions of 2004 dollars. Societal return includes a valuation of QALYs at $40,310. Vertical lines indicate the actual price of tPA ($2,200) and a price equivalent to a valuation of $50,000/QALY ($45,800).
Table 2.
Modeled annual impact of hypothetical thrombolytics in the US market
| Thrombolytic characteristic | QALY gained per use |
Treatable Population |
Total Societal Value |
"Fair" price* |
Developer Revenue at "Fair" Price |
Success Probability Required for Even Bet** |
|---|---|---|---|---|---|---|
| tPA equivalent | 0.751 | 10,000 | $ 385 M | $45,800 | $ 458 M | 5.3% |
| 30% more effective than tPA | 0.976 | 10,000 | $ 476 M | $57,100 | $ 571 M | 4.3% |
| 3–6 hour window | 0.751 | 9,300 | $ 358 M | $45,800 | $ 426 M | 5.7% |
| 0–6 hour window | 0.751 | 19,300 | $ 743 M | $45,800 | $ 884 M | 2.8% |
Fair price was defined as the price associated with a net cost to society of $50,000/QALY.
The probability that a drug candidate will ultimately receive FDA approval necessary to justify a typical investment for clinical development given modeled 10-year returns.
For other hypothetical thrombolytics of varying efficacy and time windows for use, modeled total annual benefits to society (equivalent to health and financial benefits at a modeled price of $0) were substantial (Table 2). Such benefits supported high “fair” prices and large annual revenues to manufacturers. Expected 10-year returns justified typical clinical developments costs for success probabilities ranging from 2.8% to 5.7% (Table 2).
Discussion
In this model of the impact of tPA and hypothetical thrombolytics for acute ischemic stroke, the annual value of stroke thrombolytics to society was very high, driven primarily by substantial health improvements as measured in QALYs. Better thrombolytics could justify even higher prices or be extended to larger populations. Investment in the development of new thrombolytics appears warranted, with modest expectations of success still justifying clinical development based on modeled rates of return. A relative paucity of new thrombolytics in development for stroke belies this potential.
Society benefits from a relatively low price for tPA. Very few drugs are cost saving, with prices generally set at the maximum society will bear given the benefits of a drug on health or tolerability. The economic and health benefits of tPA would justify a price of $45,800 per dose if the drug were priced at $50,000/QALY, a standard benchmark of cost-effectiveness. The price of tPA was set for its initial use in acute coronary syndrome, an indication with other available treatments and for which the benefit of tPA was more modest than after stroke. This accident of history has benefited the health care system, with tPA one of the few proprietary drugs associated with cost savings. Given this opportunity, efforts to extend use of tPA to more patients with acute stroke could result in major health and financial benefits to society and would justify expensive interventions to improve implementation.
The results of this study are based on a model of models, and findings should be interpreted cautiously. Although we took great care in selecting most model inputs, results were largely dependent on a single cost-utility analysis that required a number of uncertain assumptions.2 Furthermore, we integrated results of that study into a model with its own assumptions. Newer evidence on the utility and costs of tPA (particularly in the 3–4.5-hour window) and the systems of care required for its use were not integrated into the model since this would have required revisiting a complex systematic review.
Acknowledgements
None.
Grant Support: This work was funded by a contract from the National Institutes of Health/NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Fagan SC, Morgenstern LB, Petitta A, Ward RE, Tilley BC, Marler JR, Levine SR, Broderick JP, Kwiatkowski TG, Frankel M, Brott TG, Walker MD. Cost-effectiveness of tissue plasminogen activator for acute ischemic stroke. NINDS rt-PA Stroke Study Group. Neurology. 1998;50:883–890. doi: 10.1212/wnl.50.4.883. [DOI] [PubMed] [Google Scholar]
- 3.Mar J, Begiristain JM, Arrazola A. Cost-effectiveness analysis of thrombolytic treatment for stroke. Cerebrovasc Dis. 2005;20:193–200. doi: 10.1159/000087204. [DOI] [PubMed] [Google Scholar]
- 4.Sandercock P, Berge E, Dennis M, Forbes J, Hand P, Kwan J, Lewis S, Lindley R, Neilson A, Wardlaw J. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke. 2004;35:1490–1497. doi: 10.1161/01.STR.0000126871.98801.6E. [DOI] [PubMed] [Google Scholar]
- 5.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 6.An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. The GUSTO investigators. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001. [DOI] [PubMed] [Google Scholar]
- 7.Mark DB, Hlatky MA, Califf RM, Naylor CD, Lee KL, Armstrong PW, Barbash G, White H, Simoons ML, Nelson CL. Cost effectiveness of thrombolytic therapy with tissue plasminogen activator as compared with streptokinase for acute myocardial infarction. N Engl J Med. 1995;332:1418–1424. doi: 10.1056/NEJM199505253322106. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SC, Rootenberg JD, Katrak S, Smith WS, Elkins JS. Effect of a US National Institutes of Health programme of clinical trials on public health and costs. Lancet. 2006;367:1319–1327. doi: 10.1016/S0140-6736(06)68578-4. [DOI] [PubMed] [Google Scholar]
- 9.Petitti DB. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis. New York: Oxford University Press; 1994. [Google Scholar]
- 10.Search strategies for MEDLINE in Ovid Syntax and the PubMed translation. [Accessed Nov. 8, 2005];Health Information Research Unit, McMaster University. Available at http://hiru.mcmaster.ca/hedges/
- 11.California Acute Stroke Pilot Registry Investigators. Prioritizing interventions to improve rates of thrombolysis for ischemic stroke. Neurology. 2005;64:654–659. doi: 10.1212/01.WNL.0000151850.39648.51. [DOI] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, Schneider D, von Kummer R, Wahlgren N, Toni D. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 13.American Heart Association. Heart Disease and Stroke Statistics --2004 Update. Dallas: American Heart Association; 2004. [Google Scholar]
- 14.Reeves MJ, Arora S, Broderick JP, Frankel M, Heinrich JP, Hickenbottom S, Karp H, LaBresh KA, Malarcher A, Mensah G, Moomaw CJ, Schwamm L, Weiss P. Acute stroke care in the US: results from 4 pilot prototypes of the Paul Coverdell National Acute Stroke Registry. Stroke. 2005;36:1232–1240. doi: 10.1161/01.STR.0000165902.18021.5b. [DOI] [PubMed] [Google Scholar]
- 15.Comparative real Gross Domestic Product per capita and per employed person, fifteen countries, 1960–2004 (page 9) 2005:1–15. [Google Scholar]
- 16.Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Introduction. Osteoporos Int. 1998;8 Suppl 4:S7–S80. [PubMed] [Google Scholar]
- 17.Bureau of Labor Statistics Data: Prices and Living Conditions. [Accessed Sept. 6, 2005];U.S. Department of Labor Web site. Available at: http://www.bls.gov/data/
- 18.Adams CP, Brantner VV. Spending on new drug development1. Health Econ. 2008;19:130–141. doi: 10.1002/hec.1454. [DOI] [PubMed] [Google Scholar]
- 19.Silverman E. [Accessed July 6, 2010];Which drugs have the biggest pre-tax margins? Available at http://www.pharmalot.com/2010/01/which-drugs-have-the-biggest-pre-tax-margins/