Abstract
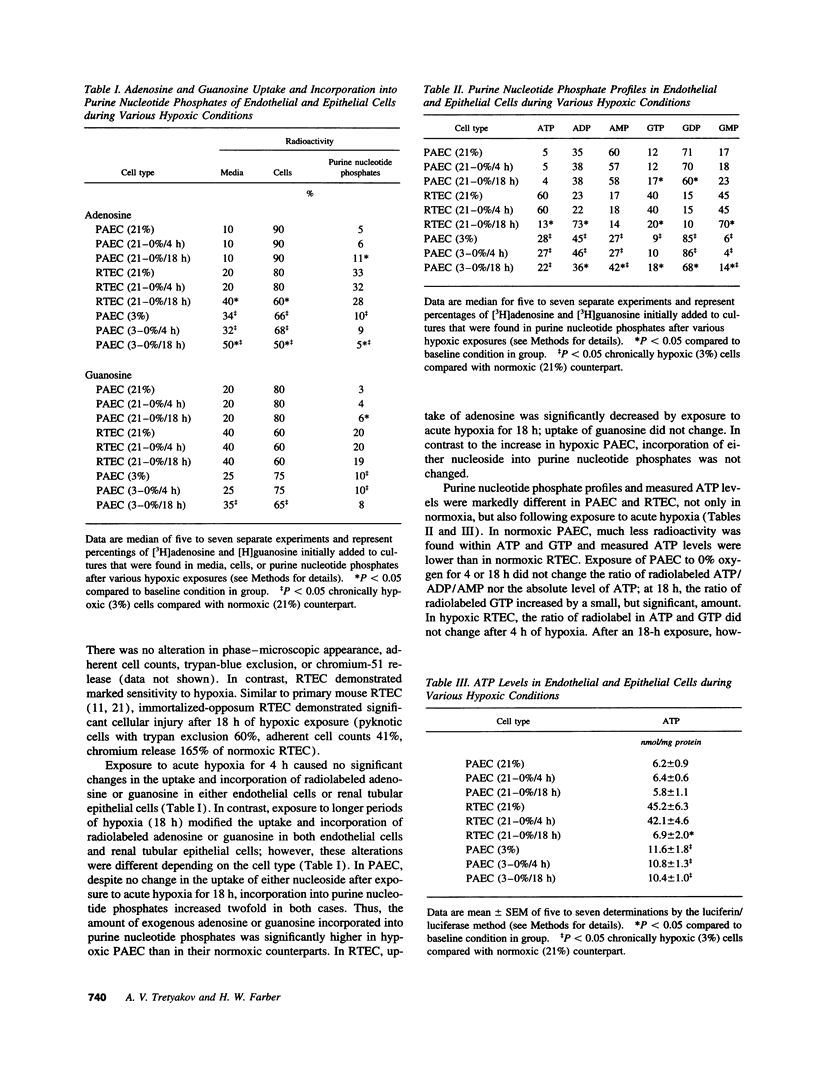
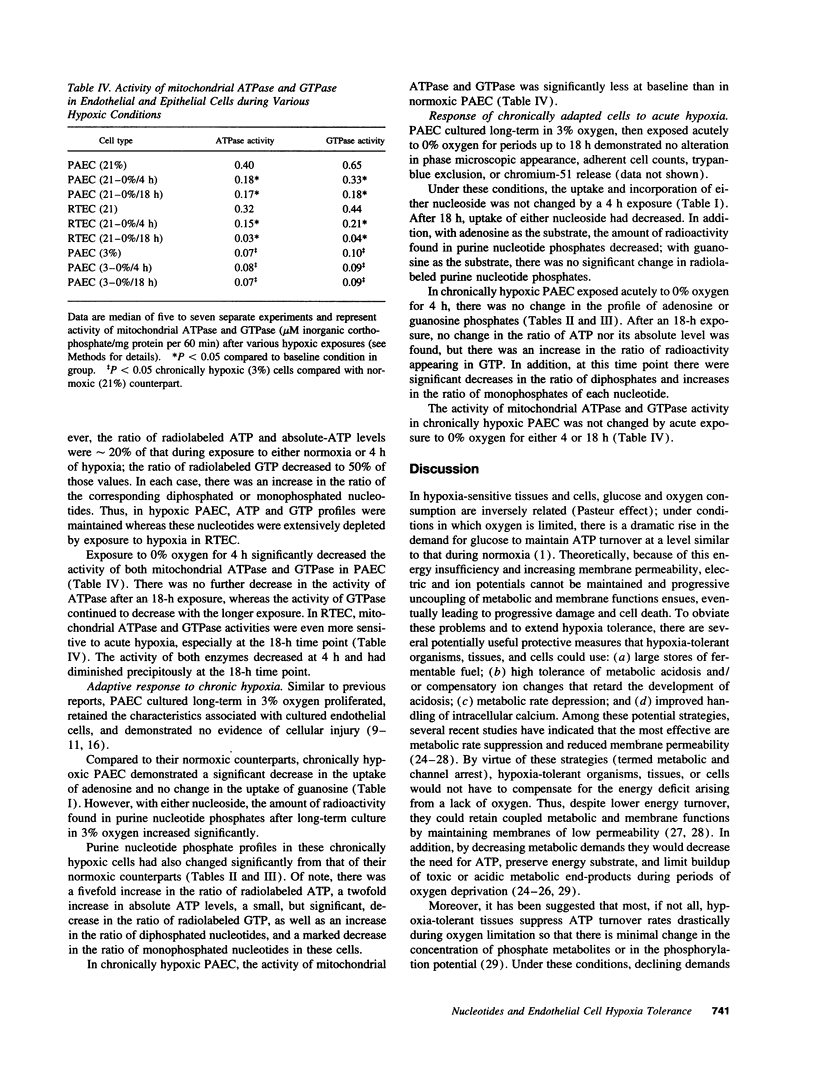
The ability of cells to tolerate hypoxia is critical to their survival, but varies greatly among different cell types. Despite alterations in many cellular responses during hypoxic exposure, pulmonary arterial endothelial cells (PAEC) retain their viability and cellular integrity. Under similar experimental conditions, other cell types, exemplified by renal tubular epithelial cells, are extremely hypoxia sensitive and are rapidly and irreversibly damaged. To investigate potential mechanisms by which PAEC maintain cellular and functional integrity under these conditions, we compared the turnover of adenine and guanine nucleotides in hypoxia tolerant PAEC and in hypoxia-sensitive renal tubular endothelial cells under various hypoxic conditions. Under several different hypoxic conditions, hypoxia-tolerant PAEC maintained or actually increased ATP levels and the percentage of these nucleotides found in the high energy phosphates, ATP and GTP. In contrast, in hypoxia-sensitive renal tubular endothelial cells, the same high energy phosphates were rapidly depleted. Yet, in both cell types, there were minor alterations in the uptake of the precusor nucleotide and its incorporation into the appropriate purine nucleotide phosphates and marked decreases in ATPase and GTPase activity. This maintenance of high energy phosphates in hypoxic PAEC suggests that there exists tight regulation of ATP and GTP turnover in these cells and that preservation of these nucleotides may contribute to the tolerance of PAEC to acute and chronic hypoxia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat G. B., Block E. R. Effect of hypoxia on phospholipid metabolism in porcine pulmonary artery endothelial cells. Am J Physiol. 1992 May;262(5 Pt 1):L606–L613. doi: 10.1152/ajplung.1992.262.5.L606. [DOI] [PubMed] [Google Scholar]
- Buck L. T., Hochachka P. W. Anoxic suppression of Na(+)-K(+)-ATPase and constant membrane potential in hepatocytes: support for channel arrest. Am J Physiol. 1993 Nov;265(5 Pt 2):R1020–R1025. doi: 10.1152/ajpregu.1993.265.5.R1020. [DOI] [PubMed] [Google Scholar]
- Buck L. T., Hochachka P. W., Schön A., Gnaiger E. Microcalorimetric measurement of reversible metabolic suppression induced by anoxia in isolated hepatocytes. Am J Physiol. 1993 Nov;265(5 Pt 2):R1014–R1019. doi: 10.1152/ajpregu.1993.265.5.R1014. [DOI] [PubMed] [Google Scholar]
- Buck L. T., Land S. C., Hochachka P. W. Anoxia-tolerant hepatocytes: model system for study of reversible metabolic suppression. Am J Physiol. 1993 Jul;265(1 Pt 2):R49–R56. doi: 10.1152/ajpregu.1993.265.1.R49. [DOI] [PubMed] [Google Scholar]
- Dwyer B. E., Nishimura R. N., Brown I. R. Synthesis of the major inducible heat shock protein in rat hippocampus after neonatal hypoxia-ischemia. Exp Neurol. 1989 Apr;104(1):28–31. doi: 10.1016/0014-4886(89)90005-8. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Barnett H. F. Differences in prostaglandin metabolism in cultured aortic and pulmonary arterial endothelial cells exposed to acute and chronic hypoxia. Circ Res. 1991 May;68(5):1446–1457. doi: 10.1161/01.res.68.5.1446. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Center D. M., Rounds S. Effect of ambient oxygen on cultured endothelial cells from different vascular beds. Am J Physiol. 1987 Oct;253(4 Pt 2):H878–H883. doi: 10.1152/ajpheart.1987.253.4.H878. [DOI] [PubMed] [Google Scholar]
- Farber H. W., Rounds S. Effect of long-term hypoxia on cultured aortic and pulmonary arterial endothelial cells. Exp Cell Res. 1990 Nov;191(1):27–36. doi: 10.1016/0014-4827(90)90031-5. [DOI] [PubMed] [Google Scholar]
- Futai M., Noumi T., Maeda M. ATP synthase (H+-ATPase): results by combined biochemical and molecular biological approaches. Annu Rev Biochem. 1989;58:111–136. doi: 10.1146/annurev.bi.58.070189.000551. [DOI] [PubMed] [Google Scholar]
- Graven K. K., Troxler R. F., Kornfeld H., Panchenko M. V., Farber H. W. Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J Biol Chem. 1994 Sep 30;269(39):24446–24453. [PubMed] [Google Scholar]
- Graven K. K., Zimmerman L. H., Dickson E. W., Weinhouse G. L., Farber H. W. Endothelial cell hypoxia associated proteins are cell and stress specific. J Cell Physiol. 1993 Dec;157(3):544–554. doi: 10.1002/jcp.1041570314. [DOI] [PubMed] [Google Scholar]
- Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Induction characteristics of oxygen regulated proteins. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1287–1290. doi: 10.1016/0360-3016(86)90155-0. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W., Castellini J. M., Hill R. D., Schneider R. C., Bengtson J. L., Hill S. E., Liggins G. C., Zapol W. M. Protective metabolic mechanisms during liver ischemia: transferable lessons from long-diving animals. Mol Cell Biochem. 1988 Nov;84(1):77–85. doi: 10.1007/BF00235195. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hochachka P. W. Molecular mechanisms of defense against oxygen lack. Undersea Biomed Res. 1989 Sep;16(5):375–379. [PubMed] [Google Scholar]
- Jackson D. C. Metabolic depression and oxygen depletion in the diving turtle. J Appl Physiol. 1968 Apr;24(4):503–509. doi: 10.1152/jappl.1968.24.4.503. [DOI] [PubMed] [Google Scholar]
- Jiang C., Xia Y., Haddad G. G. Role of ATP-sensitive K+ channels during anoxia: major differences between rat (newborn and adult) and turtle neurons. J Physiol. 1992 Mar;448:599–612. doi: 10.1113/jphysiol.1992.sp019060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junod A. F., Jornot L., Petersen H. Differential effects of hyperoxia and hydrogen peroxide on DNA damage, polyadenosine diphosphate-ribose polymerase activity, and nicotinamide adenine dinucleotide and adenosine triphosphate contents in cultured endothelial cells and fibroblasts. J Cell Physiol. 1989 Jul;140(1):177–185. doi: 10.1002/jcp.1041400121. [DOI] [PubMed] [Google Scholar]
- Kelly D. A., Storey K. B. Organ-specific control of glycolysis in anoxic turtles. Am J Physiol. 1988 Nov;255(5 Pt 2):R774–R779. doi: 10.1152/ajpregu.1988.255.5.R774. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisberg J. I., Mills J. W., Jarrell J. A., Rabito C. A., Leaf A. Protection of cultured renal tubular epithelial cells from anoxic cell swelling and cell death. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5445–5447. doi: 10.1073/pnas.77.9.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land S. C., Buck L. T., Hochachka P. W. Response of protein synthesis to anoxia and recovery in anoxia-tolerant hepatocytes. Am J Physiol. 1993 Jul;265(1 Pt 2):R41–R48. doi: 10.1152/ajpregu.1993.265.1.R41. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Glycolytic activity and enhancement of serotonin uptake by endothelial cells exposed to hypoxia/anoxia. Circ Res. 1987 May;60(5):653–658. doi: 10.1161/01.res.60.5.653. [DOI] [PubMed] [Google Scholar]
- Lee S. L., Fanburg B. L. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol. 1986 May;250(5 Pt 1):C766–C770. doi: 10.1152/ajpcell.1986.250.5.C766. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Cao L., Brett J., Ogawa S., Silverstein S. C., Stern D. Hypoxia induces glucose transporter expression in endothelial cells. Am J Physiol. 1992 Aug;263(2 Pt 1):C326–C333. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- Mertens S., Noll T., Spahr R., Krützfeldt A., Piper H. M. Energetic response of coronary endothelial cells to hypoxia. Am J Physiol. 1990 Mar;258(3 Pt 2):H689–H694. doi: 10.1152/ajpheart.1990.258.3.H689. [DOI] [PubMed] [Google Scholar]
- Nilsson G. E., Alfaro A. A., Lutz P. L. Changes in turtle brain neurotransmitters and related substances during anoxia. Am J Physiol. 1990 Aug;259(2 Pt 2):R376–R384. doi: 10.1152/ajpregu.1990.259.2.R376. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Gerlach H., Esposito C., Pasagian-Macaulay A., Brett J., Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990 Apr;85(4):1090–1098. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESSMAN B. C. Intramitochondrial nucleotides. I. Some factors affecting net interconversions of adenine nucleotides. J Biol Chem. 1958 Jun;232(2):967–978. [PubMed] [Google Scholar]
- Richards J. M., Gibson I. F., Martin W. Effects of hypoxia and metabolic inhibitors on production of prostacyclin and endothelium-derived relaxing factor by pig aortic endothelial cells. Br J Pharmacol. 1991 Jan;102(1):203–209. doi: 10.1111/j.1476-5381.1991.tb12154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll D. E., Murphy B. J., Laderoute K. R., Sutherland R. M., Smith H. C. Oxygen regulated 80 kDa protein and glucose regulated 78kDa protein are identical. Mol Cell Biochem. 1991 May 15;103(2):141–148. doi: 10.1007/BF00227480. [DOI] [PubMed] [Google Scholar]
- Schwartz P., Piper H. M., Spahr R., Spieckermann P. G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol. 1984 Jun;115(3):349–361. [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan A. M., Schwartz J. H., Kroshian V. M., Tercyak A. M., Laraia J., Masino S., Lieberthal W. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol. 1993 Sep;265(3 Pt 2):F342–F350. doi: 10.1152/ajprenal.1993.265.3.F342. [DOI] [PubMed] [Google Scholar]
- Suarez R. K., Doll C. J., Buie A. E., West T. G., Funk G. D., Hochachka P. W. Turtles and rats: a biochemical comparison of anoxia-tolerant and anoxia-sensitive brains. Am J Physiol. 1989 Nov;257(5 Pt 2):R1083–R1088. doi: 10.1152/ajpregu.1989.257.5.R1083. [DOI] [PubMed] [Google Scholar]
- Tretyakov A. V., Farber H. W. Endothelial cell phospholipid distribution and phospholipase activity during acute and chronic hypoxia. Am J Physiol. 1993 Sep;265(3 Pt 1):C770–C780. doi: 10.1152/ajpcell.1993.265.3.C770. [DOI] [PubMed] [Google Scholar]
- Tsai I. H., Murthy S. N., Steck T. L. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982 Feb 10;257(3):1438–1442. [PubMed] [Google Scholar]
- Wang C. S., Alaupovic P. Glyceraldehyde-3-phosphate dehydrogenase from human erythrocyte membranes. Kinetic mechanism and competitive substrate inhibition by glyceraldehyde 3-phosphate. Arch Biochem Biophys. 1980 Nov;205(1):136–145. doi: 10.1016/0003-9861(80)90092-2. [DOI] [PubMed] [Google Scholar]
- Webster K. A., Discher D. J., Bishopric N. H. Induction and nuclear accumulation of fos and jun proto-oncogenes in hypoxic cardiac myocytes. J Biol Chem. 1993 Aug 5;268(22):16852–16858. [PubMed] [Google Scholar]
- Zimmerman L. H., Levine R. A., Farber H. W. Hypoxia induces a specific set of stress proteins in cultured endothelial cells. J Clin Invest. 1991 Mar;87(3):908–914. doi: 10.1172/JCI115097. [DOI] [PMC free article] [PubMed] [Google Scholar]


