Abstract
The hepatic stimulatory substance (HSS) extracted from weanling rat livers was purified 381,000-fold using chromatographic techniques including nondissociating polyacrylamide gel electrophoresis (nondenaturing PAGE). The activity of this highly purified HSS, named Acr-F4, was assessed in two in vivo models. In 40% hepatectomized rats, it produced a fivefold increase in the proliferative rate normally seen following this partial hepatectomy. In Eck fistula dogs, the level of base increase in hepatocyte renewal was amplified threefold by an infusion of Acr-F4 (50 ng/kg/day). Acr-F4 had no influence on the regenerative response of the kidney following a unilateral nephrectomy or of the bowel following a 40% resection of the small bowel. On the basis of these findings, it can be concluded that HSS (Acr-F4) has a high biological activity and is organ specific.
Keywords: hepatic stimulatory substance, purification, gel electrophoresis
That liver regeneration could be augmented by extracts prepared from livers in an active proliferative state has been demonstrated by several investigators (1–4). However, all attempts focused on purification and characterization of the putative growth factor in these extracts have produced few and generally inconclusive results. A partially purified factor, hepatic stimulatory substance (HSS), capable of stimulating in vivo hepatocyte proliferation (5, 6) has been isolated from weanling rat liver homogenates.
In this paper, data concerning further purification and characterization of HSS is reported, made possible by the use of new laboratory techniques and the introduction of a very sensitive and reliable animal model for assessing HSS activity in vivo. Specifically, nondissociating polyacrylamide gel electrophoresis allowed us to obtain a 381,000-fold purification of HSS, eliminating almost all contaminating proteins in the preparation. In addition, the dog with a portacaval shunt coupled with the availability of specific antibodies against HSS made it possible to achieve this level of purification.
MATERIALS AND METHODS
Chemicals and Materials
Type-V neuraminidase, trypsin, aprotinin, and proteins used as molecular weight markers were purchased from Sigma Chemical Company, St. Louis. Missouri, [methyl-3H]Thymidine (50–80 Ci/mmol) was obtained from New England Nuclear, Boston, Massachusetts. l-l-Tosylamido-2-phenylethyl chlorome-thyltrypsin ketone was purchased from Worthington Biochemical Corporation, Boston, Massachusetts. Amicon ultrafiltration membrane filters were purchased from Amicon Corporation, Danvers, Massachusetts. The chemicals required for electrophoresis were purchased from Bio-Rad Laboratories, Richmond, California.
Animals
Adult male Fischer (F344) rats (180–200 g), weanling male rats (60–90 g), and male mongrel dogs (15–20 kg) were purchased from Hilltop Lab Animals, Scottsdale, Pennsylvania. They were maintained in temperature- and light- (6 am to 6 pm) controlled rooms until used. They were given food and water ad libitum.
Surgical Procedures
Adult rats underwent either a 40% hepatectomy or a sham operation consisting of a laparotomy and manual manipulation of the liver between 7:30 and 9:30 am using the method of Higgins and Anderson (7). Unilateral nephrectomy and 40% resections of the small bowel were performed as described previously (8).
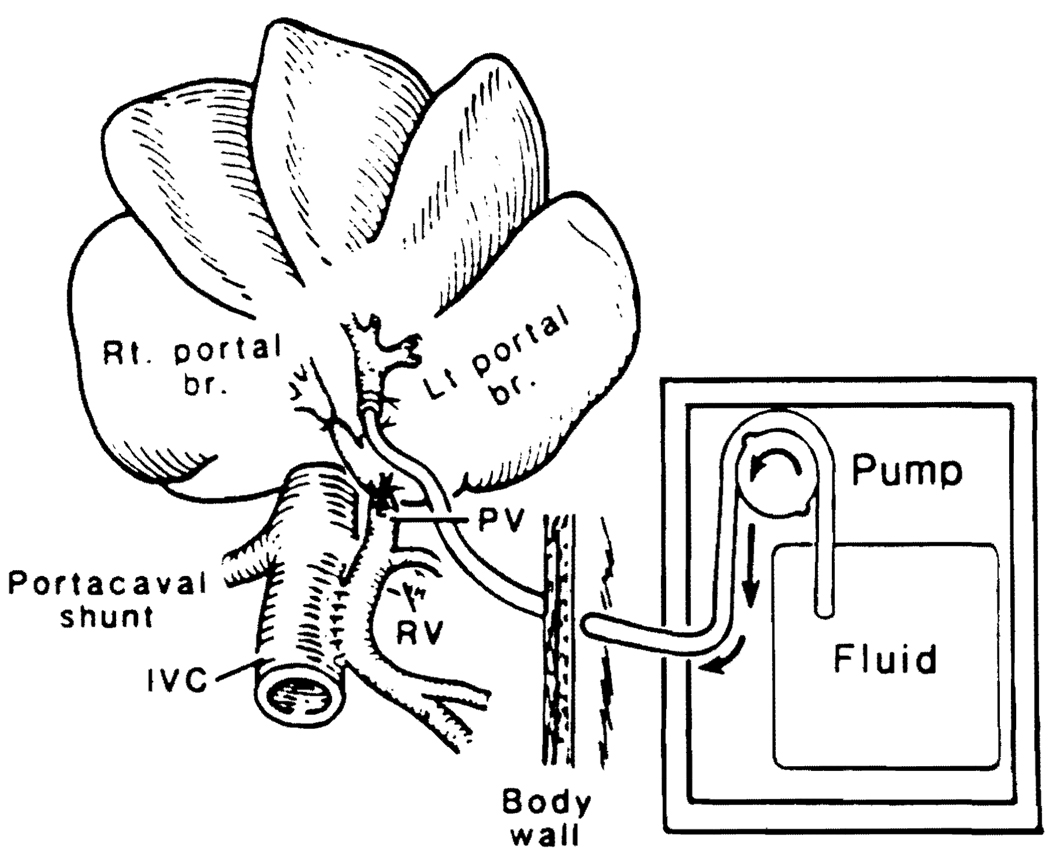
In dogs, large side-to-side portacaval shunts were constructed with an excision of an ellipse of tissue from both the portal vein and the inferior vena cava and anastomosing the two vessels side to side (Figure 1). The shunts were made completely diverting by individually ligating the main right and left portal trunks distal to the anastomosis of the portal vein to the IVC (9). The tip of a small infusion catheter was placed into the ligated left portal branch within the liver and led through the body wall and via a long subcutaneous tunnel to a small calibrated finger pump that was placed into a dog jacket (Figure 1).
Fig 1.
Portacaval shunt model in dogs.
HSS Preparation and Purification
The steps for HSS preparation and purification are summarized in Table 1. These methods (6) yielded an active fraction that has been identified as F150 because it elutes from the column with a 150 mM NaCl gradient.
TABLE 1.
Preparation of HSS
| Purification steps | Product | |
|---|---|---|
| 1. | Remove the liver, immediately after killing by guillotine, between 7:00 and 8:00 am | |
| 2. | Mince and then homogenize the liver in 150 mM sodium acetate buffer, pH 4.65 (35:100 w/v) | |
| 3. | Ultracentrifuge homogenate at 24,000 g for 30 min at 4° C | Cytosol fraction (Cyt-F) |
| 4. | Heat at 65° C for 15 min | |
| 5. | Centrifuge at 30,000 g for 20 min at 4° C, collect supernatant and add to it 6 vol of cold ethanol (1:6, v/v) | |
| 6. | Stir at 2–8° C for 2 hr | |
| 7. | Centrifuge 30,000 g for 20 min at 4° C | |
| 8. | Resuspend precipitate in 0.150 mM ammonium acetate, pH = 6 | Alcohol fraction (OH-F) |
| 9. | Filter OH-F through an Amicon membrane with a molecular weight cutoff of 30,000 Da | |
| 10. | Collect the filtrate and concentrate it by a 500-Da cutoff Amicon membrane | Mr 30,000 fraction (30 kDa-F) |
| 11. | Lyophilize 30 kDa-F | |
| 12. | Resuspend lyophilized 30 kDa-F in phosphate buffer 5 mM, pH 6, and perform chromatography using mono Q HR 5/5 column with a linear 0–200 mM NaCl gradient in phosphate buffer | |
| 13. | Collect the chromatographic peak at 150 mM NaCl gradient | 150 fraction (F150) |
Nondissociating PAGE
An aliquot of 0.6 mg lyophilized fraction F150 resuspended in Tris buffer 0.025 M, pH 8.3, underwent electrophoresis using nondissociating PAGE (10, 11) on 8% acrylamide. With this technique, F150 generates several distinct bands, and the gel can be divided in four zones from which its proteins can be eluted. The eluates, acrylamide fractions 1–4 (Acr F1–F4) are dialyzed against 150 mM ammonium acetate, lyophilized, and stored at −70° C until being tested further.
In Vivo Determination of Activity of HSS and Its Fractions
In rats, 6 hr after a 40% partial hepatectomy, control rats were given intraperitoneal injections of 2 ml of 5 mM phosphate buffer, pH 7.4, whereas HSS-treated rats received either F150 or an acrylamide fraction dissolved in 2 ml phosphate buffer, 5 mM, pH 7.4, at the protein concentrations indicated in the tables. Seventeen hours later, 50 µCi [3H]thymidine were injected intraperitoneally, and the animals were sacrificed 1 hr later. Six hours after surgery, the rats that had received a unilateral nephrectomy or 40% resection of the small bowel were treated as described above for the 40% hepatectomized rats.
[3H]Thymidine incorporation and mitotic index determinations were made as described previously (9, 12). An augmentation of all parameters, beyond the modest response that is present after sham surgery, was considered to be indicative of biologic activity of the liver extracts.
In dogs, the active electrophoretic fraction, Acr-F4 was infused in the left hepatic lobes through the left portal branch as described in Table 2. At the time of sacrifice, liver tissue was obtained from the left and right hepatic lobes and shunt patency and catheter position were verified. The labeling index was determined as described earlier (9).
TABLE 2.
Protocol for Acr-F4 Infusion and % of Labeled Nuclei Determination in Eck Fistula Dogs
| Beginning of infusion | 6 hr after surgery |
| Duration of infusion | 4 days |
| Infused material | Acr-F4 (50 ng/kg body wt/day) in saline containing NH4 acetate 5 mM and bovine serum albumin 0.5 mg/100 ml |
| Volume infused | 25 ml/day |
| Treatment for labelling nuclei | 200 µCi of [3H]thymidine (82.2 Ci/mmol)/kg body wt injected IV two hours before killing |
Determination of DNA Synthesis in Organs Other Than Liver
DNA synthesis in kidney and small intestine was determined as described previously (8).
Analyses of Physical and Chemical Properties
F150 and Acr-F4 were tested for trypsin and chymotrypsin sensitivity (13), heat stability, and neuroaminidase sensitivity (14).
SDS-PAGE
SDS-polyacrylamide gradient slab gel, using 7.5–20% gel with a 5% stacking gel, was prepared and developed according to the method of Laemmli (15). Both F150 and Acr-F4 undergo electrophoresis under these conditions. Protein bands were visualized using Coomassie blue R250 according to the method of Weber and Osborn (16).
Monoclonal Antibody (Ab)
Murine monoclonal antibodies against Acr-F4 were raised using PHC 43 and PHC 67 cells (17). The cells were cultured in serum-free medium and the monoclonal Abs were separated by protein-A chromatography. Activity was assessed by ELISA and found to be in the IgG fraction.
Protein Determination
Protein content was determined by the method of Lowry et al (18). Submicrogram quantities were measured using the method of McK-night (19).
Statistical Analysis
The unpaired Student's t test was used for the statistical analysis of all data.
RESULTS
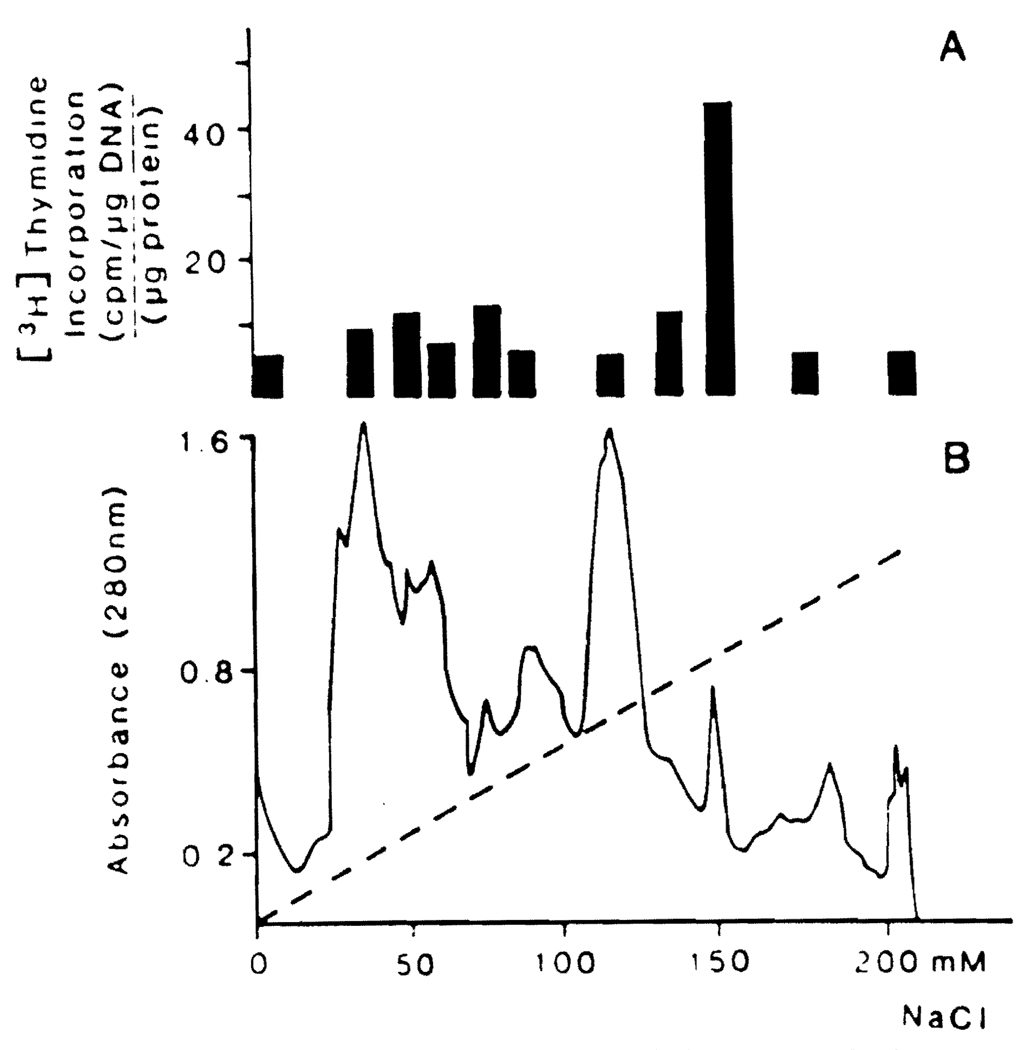
In earlier investigations (6), the greatest degree of purification of HSS was obtained using fast protein liquid chromatography. By this technique, F150 (Figure 2), which was the most active fraction in 40% hepatectomized rats, was prepared. The use of nondissociating PAGE made it possible to further purify F150 (Figure 3).
Fig 2.
Elution and activity profile of HSS from FPLC. Stimulatory activity of FPLC fractions (A). Elution profile of 30 kDa (B). The amount of protein injected for each fraction was 3 µg. Each bar expresses [3H]thymidine average values from six rats. The statistical analysis shows that only the value of F150 was significant (P < 0.0001).
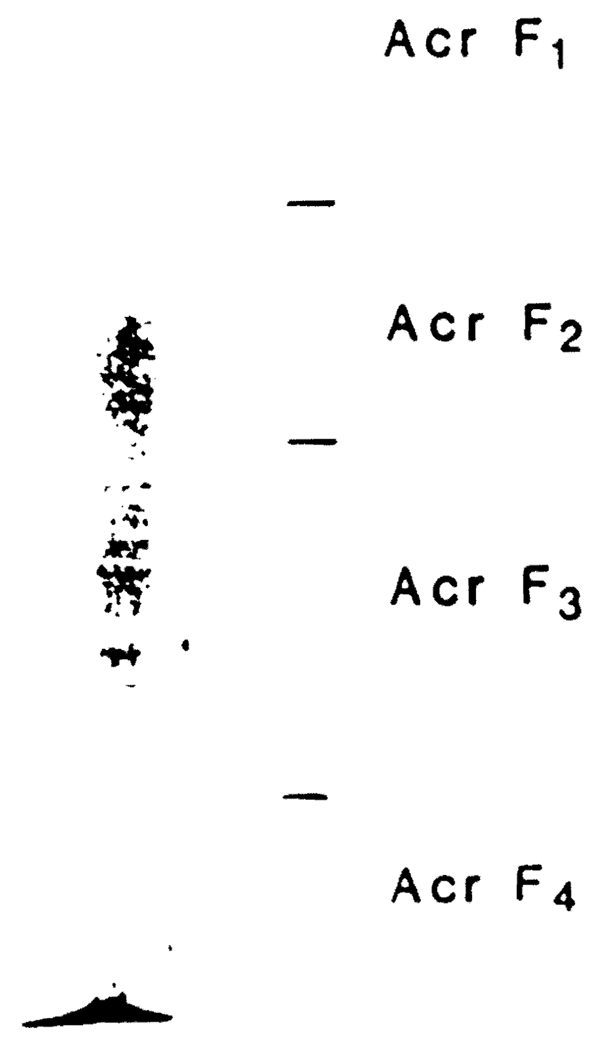
Fig 3.
Nondissociating PAGE of F150. For our nondissociating continuous system. we used Tris HCI 0.375 M as resolving gel buffer and Tris HCI 0.025 M glycine 0.192 M as reservoir buffer. The acrylamide gel concentration was 8%. The material used for this run was obtained as pool of ten F150.
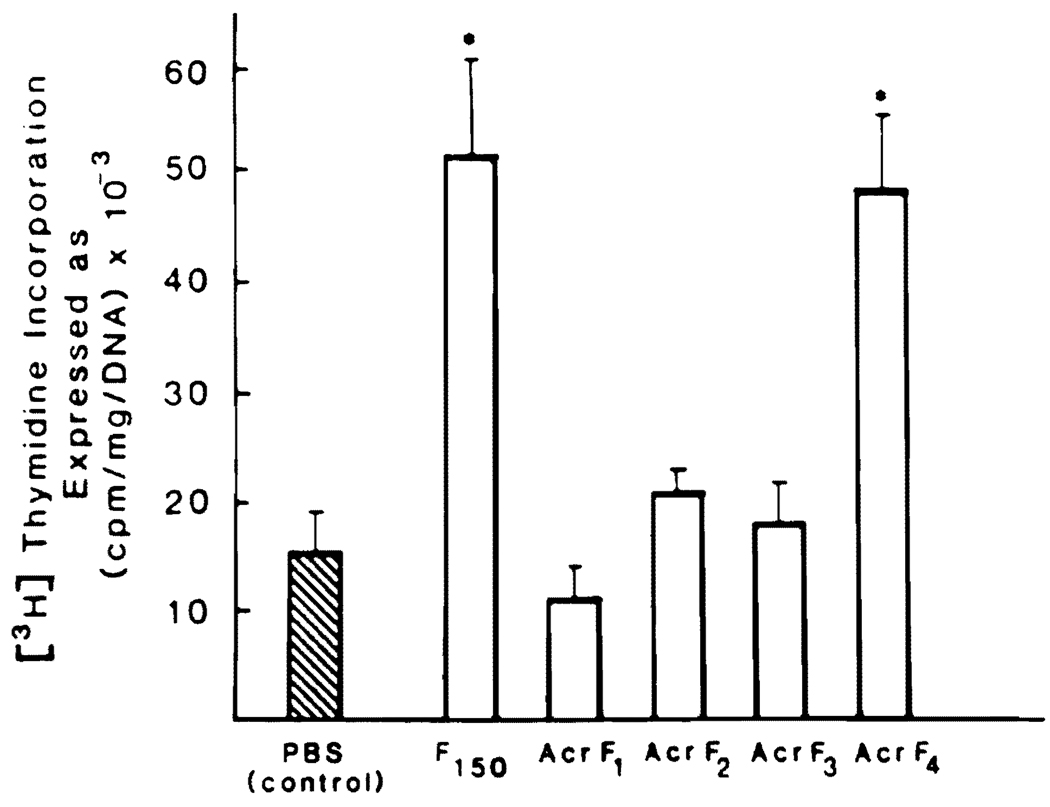
The activity of F150 and its PAGE fractions were compared using 40% hepatectomized rats (Figure 4). The administration of 150 (3 µg/rat) produced results as previously reported (6). When fractions of Acr F1–F4 were tested, the only fraction with stimulatory activity similar to that of F150 was found in Acr-F4.
Fig 4.
Effect of F150 and PAGE fractions (Acr-F1–F4) on DNA synthesis in 40% hepatectomized rats. The experimental conditions are described in Materials and Methods. The values expressed by the bars are averages ± SD from no less than 15 rats. All HSS fraction were dissolved in 2 ml PBS at the following concentrations: F150 1.5 µg/m1; Acr-F1 0.05 µg/m1; and Acr-F2–F4 0.15µg/m1. *P < 0.01 versus control.
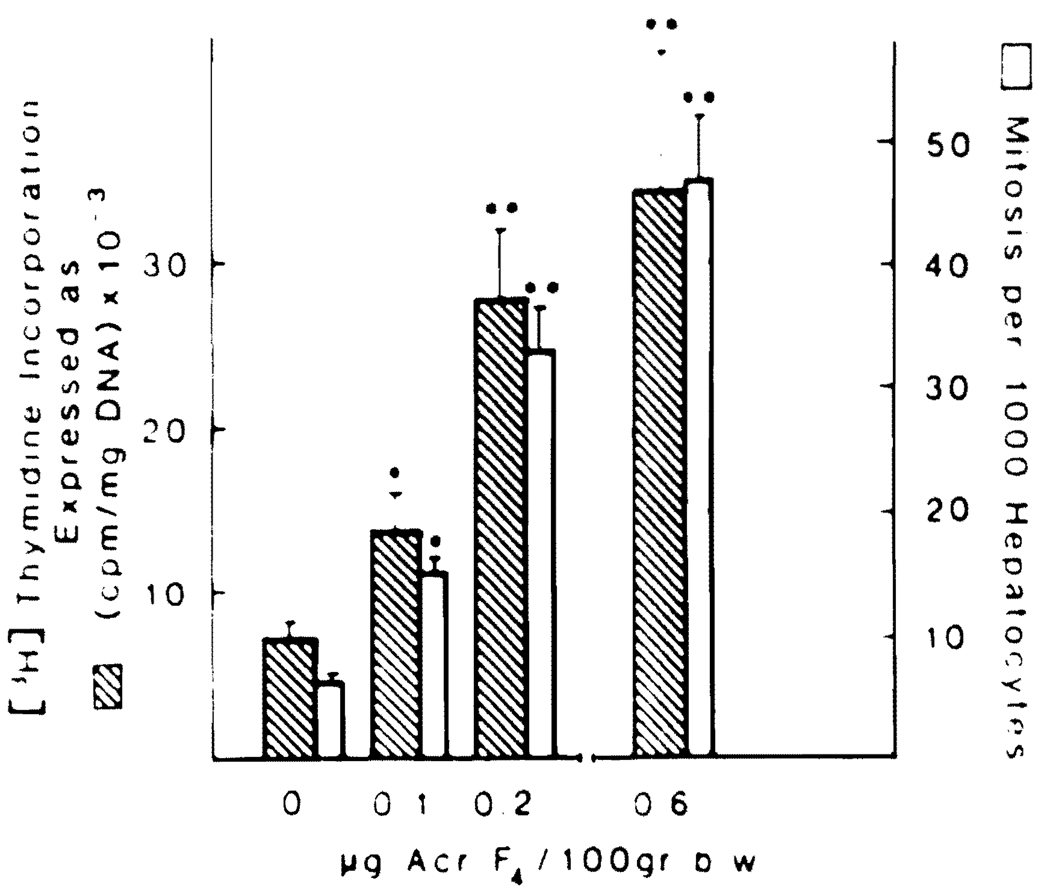
The results shown in Figure 5 demonstrate a dose–effect relation between the amount of Acr-F4 injected and the resultant increase in hepatocyte DNA synthesis and the number of mitoses enumerated. In addition, with the highest dose of Acr-F4 (0.6 µg/100 g body wt), the activity achieved was fivefold greater than the background response in control animals.
Fig 5.
[3H]Thymidine incorporation and mitotic index in 40% hepatectomized rats treated with different doses of Acr-F4. The experimental conditions are described in Materials and Methods. The values expressed by the bars are averages ± SD from no less than 15 rats. Acr-F4 doses were dissolved in 2 ml PBS.
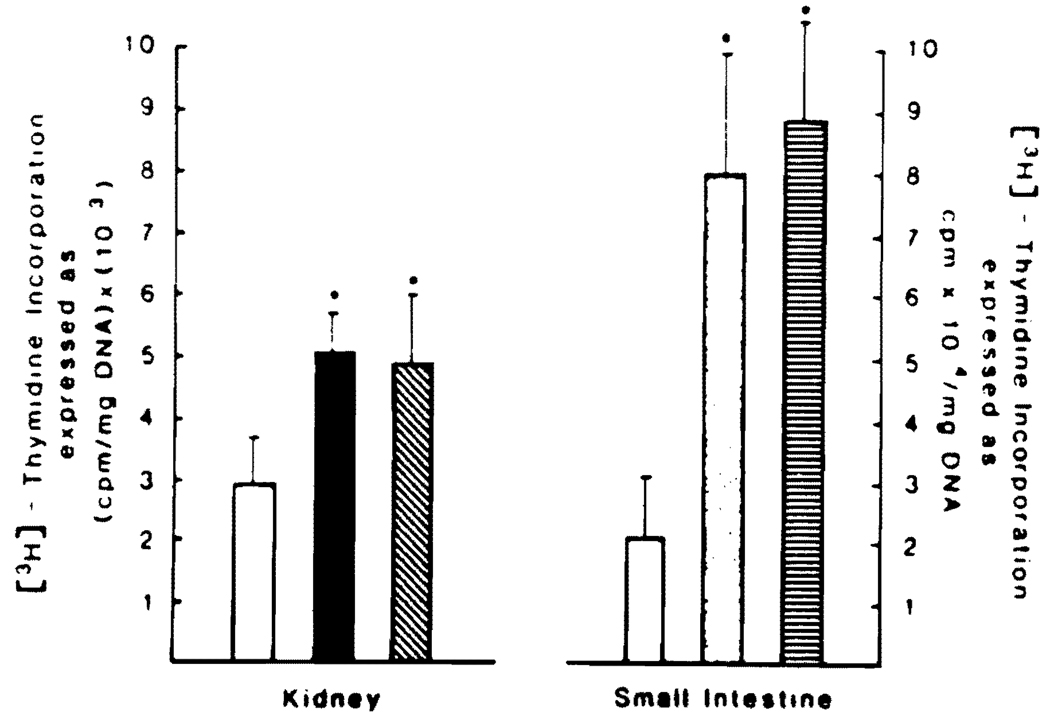
To evaluate the organ specificity of Acr-F4, rats with either a unilateral nephrectomy or a 40% resection of the small bowel were tested. No increase in DNA synthesis was found in the contralateral residual kidney or in the remaining small intestine (Figure 6).
Fig 6.
[3H]Thymidine incorporation in kidney and small intestine from normal rats and Acr F4-treated or untreated rats with unilateral nephrectomy or 40% resected small intestine. The values represented by the different bars (▭ controls, ▬ unilateral nephrectomy, ▧ Acr F4 treatment + unilateral nephrectomy, □ partial enterectomy, ▤ Acr F4 treatment + partial enterectomy), are the means ± SD from 10 rats.
The experiments performed in dogs demonstrate that when Acr-F4 was administered as a continuous infusion beginning 6 hr after portacaval shunt in the left portal vein, the mitotic rate tripled in the left liver lobe while no effect was seen in the right side of the liver. This effect was completely eliminated with the addition of anti-Acr-F4 monoclonal antibody to the infusion fluid (Table 3). The monoclonal antibody vehicle was inert when tested alone.
TABLE 3.
Effect in Dogs of Left Portal Branch Infusion for 4 Days of Acr-F4, Beginning 6 hr after Portacaval Shunt
| Labeled nuclei/1000 hepatocytes |
|||
|---|---|---|---|
| Substance | No. animals | Right lobe | Left lobe |
| Vehicle | 3 | 4.4 ± 0.6 | 4.8 ± 0.4 |
| Acr-F4 | 3 | 4.4 ± 0.5 | 12.2 ± 1.0† |
| Acr-F4 + Monoclonal Ab* | 4.1 ± 0.2 | 4.3 ± 0.3 | |
A mixture of active IgG(s), adding up to 150 µg of protein daily, was added to the vehicle containing Acr-F4 and incubated for 2 hr at 37 ° C before infusion.
Significantly different from control (P < 0.001 compared to all other groups).
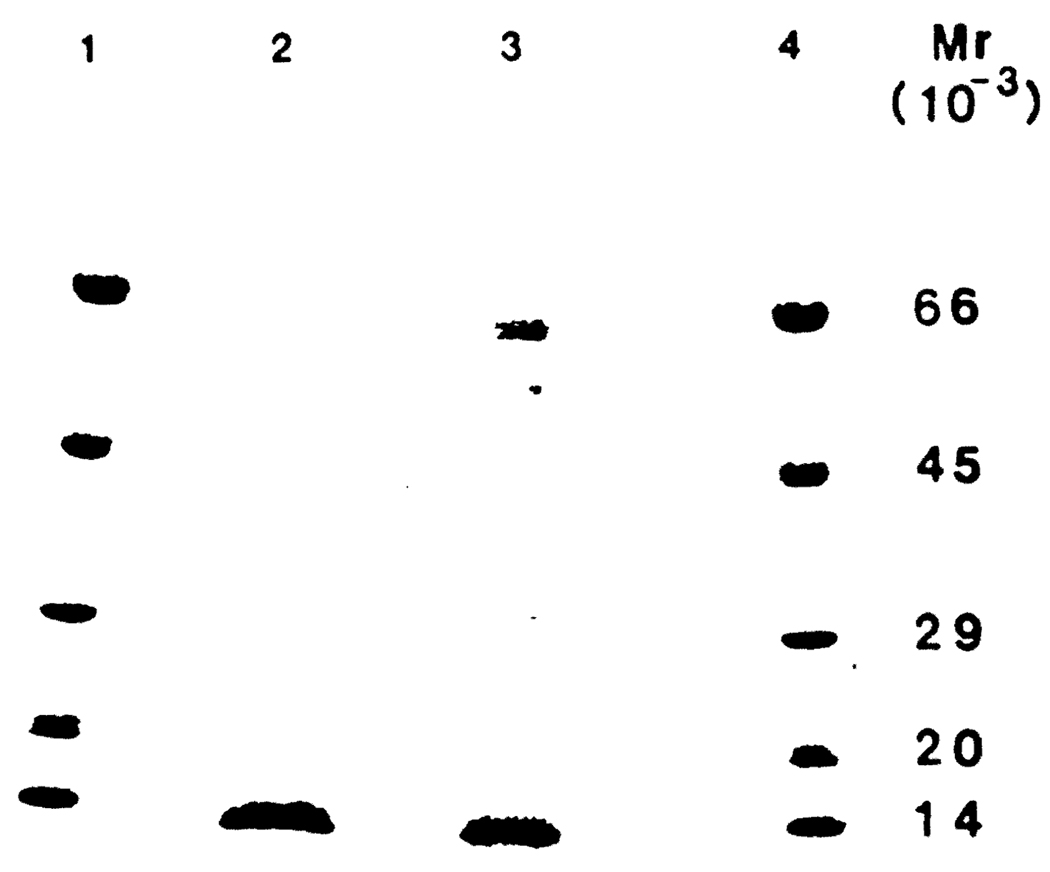
Table 4 summarizes the physicochemical characteristics of Acr-F4. It contains one major protein band with a molecular weight of about 14,000 (Figure 7). Experiments conducted in 40% hepatectomized rats demonstrated that Acr-F4 is heat-resistant and is not digested by neuroaminidase, whereas it is sensitive to proteolitic enzymes (data not shown).
TABLE 4.
Physicochemical Characteristics of Acr-F4
| Mol wt (Da) | 14,000 |
| Heat resistance | + |
| pH stability | 4.5–7.5 |
| Alcohol stability | + |
| Resistance to | |
| Trypsin | − |
| Chymotrypsin | − |
| Neuroaminidase | + |
Fig 7.
SDS-PAGE of F150 and Acr-F4. The gel preparation is described in Materials and Methods. Lanes 1 and 4 = standard mixture (molecular weights are multiplied by 10−3); slot 2 = Acr-F4; slot 3 = F150.
DISCUSSION
The idea of a specific intrinsic liver growth factor was conceived almost 40 years ago when Teir and Ravanti (20) and Blomqvist (21) first reported a growth stimulatory activity in crude mesh extracts of weanling and regenerating rat liver but not in extracts from normal adult rat liver. Since then, a large number of studies (1–6) have suggested that regenerating liver is a source of a growth stimulator which is specific for the liver (Table 5).
TABLE 5.
Summary of the Literature
| Resistance to |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Investigator | Time period |
Biological source |
Name of substance |
Biological assay system |
Heat | Try- psin |
Chymo- trypsin |
Neuro- aminidase |
Response organ/ species |
Purification (%) |
Mr* |
| Blomqvist | 1957 | NbReL† | in vivo: NR | ||||||||
| LaBrecque | 1975–1987 | WRL-ReRL | HSS | in vitro: HTC cells | + | − | − | + | Yes/No | 110,000 | 14,000–15,000 |
| in vivo: 40% HeR | |||||||||||
| Hatase | 1979 | NL | in vitro: L-929 fibroblast | − | − | 30,000 | |||||
| in vivo: N and 34% HeR | |||||||||||
| Starzl | 1979 | ReL from 70% He dog | in vivo: dog with portacaval shunt | Yes | |||||||
| Goldberg | 1980–1985 | ReL from 70% He rat | Hepato-poietin | in vivo: NR | + | − | − | + | Yes/No | 13,000 | 38,000 |
| Terblanche | 1980 | Re dog L | in vivo: NR | ||||||||
| Francavilla | 1984–1985 | WRL | HSS | in vivo: 40% HeR | + | − | − | + | Yes/No | 38,000 | 15,000–50,000 |
| Schwarz | 1985 | ReL from 70% He R and pig | in vivo: 34% He female R and WR | + | No | 14,000–25,000 | |||||
| in vitro: hepatocyte cells | |||||||||||
| Lieberman | 1984 | Mouse plasma membrane | in vitro: NR-6 line fibroblast | − | + | − | − | ||||
| Fleig | 1986 | ReL from 60% He rabbit | in vivo: NR | − | − | ||||||
Determined by SDS-PAGE.
L, liver; N, normal; Nb, newborn; R, rat; Re, regenerating; He, hepatectomized; W, weanling.
Among the substances proposed and studied, HSS has been studied most extensively by LaBrecque and coworkers (1, 2), Starzl et al (3), and Francavilla et al (5, 6).
Data reported previously (6) and the new data in this paper regarding Acr-F4 are summarized in Table 6. A 381,000-fold increase in activity over the original material was achieved using the 40% hepatectomized rat model as the test system. The activity present in this fraction (Acr-F4) is not species-specific, as demonstrated by the results obtained in dogs as well as rats and produced a dose–response that was specific for the liver (Figure 6).
TABLE 6.
Steps of Purification and Biological Activity of F150and Acr-F4 from Weanling Rat Livera
| Material Injected | Protein (µg/rat) | DNA synthesis (cpm/mg DNA) | Specific activity (units/mg protein) | Purification-fold |
|---|---|---|---|---|
| Cytosol | 7.5 × 104 | 43,350 ± 8,820 | 0.02 | |
| OH-F | 1.0 × 104 | 66,350 ± 11,350 | 0.30 | 15 |
| 30 kDa-F | 0.27 ± 104 | 63,520 ± 13,220 | 1.05 | 52 |
| F150 | 3 | 54,380 ± 10,200 | 762 | 38,100 |
| Acr-F4 | 3 × 10−1 | 49,350 ± 7,084 | 7620 | 381,000 |
All data, with the exception of the ones regarding Acr-F4 (see figure 4), are from our previous publication (6). The purification scheme of HSS has been described in Table 1. The [3H]thymidine incorporation in a 40% hepatectomized rat given an injection of PBS was 16,550 ± 3000 cpm/mg DNA. The numbers are averages from no less than 20 different rats ± SD.
The HSS found in weanling rat liver also has a powerful regenerating or growth effect on dog liver as assessed by the Eck fistula model. The degree of stimulation achieved with 50 ng/kg/day was as potent as gram quantities of crude cytosol (3, 4) and was as pronounced as the most potent well-recognized hepatotrophic substance currently available: insulin (9). In common with insulin (9) and crude cytosol (4), purified HSS affects only the directly infused liver tissue with little spillover to the uninfused liver. This suggests that it is largely degraded or consumed within a single pass through the liver, leaving little or none available to effect the contralateral hepatic lobes.
In an earlier report (6), it was shown that HSS prepared under these conditions loses its in vitro activity while retaining its in vivo activity. Thus it has not been possible to compare the HSS purified by LaBrecque et al (22) and Fleig and Hoss (23), which remained active in vitro with Acr-F4. The explanation for the disparities between in vivo and in vitro growth stimulation seen with Acr-F4 and these other fractions will not be resolvable until these substances are known.
In conclusion, the retention of in vivo activity of a highly purified HSS fraction, the ability to abolish the stimulatory activity of this fraction with specific monoclonal antibodies, and the organ specificity of Acr-F4 suggests that its complete identification should be close at hand.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant DK 29961 from the National Institutes of Health, Bethesda, Maryland, and grant 87/01291-44 from Consiglio Nazionale delle Ricerche, Italy.
Footnotes
Presented at the Proceedings of the International Meeting on Normal and Neoplastic Growth in Hepatology, Bari, Italy, June 1989.
REFERENCES
- 1.LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaBrecque DR, Bachur NR. Hepatic stimutator substance Physicochemical characteristics and specificity. Am J Physiol 242 (Gastrointest Liver Physiol 5) 1982:G281–G288. doi: 10.1152/ajpgi.1982.242.3.G281. [DOI] [PubMed] [Google Scholar]
- 3.Starzl TE, Terblanche J, Porter KA, Jones AF, Usui S, Mazzoni G. Growth-stimulating factor in regenerating canine liver. Lancet. 1979;2:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terblanche J, Porter KA, Starzl TE, Moore J, Patzelt L, Hayashida N. Stimulation of hepatic regeneration after partial hepatectomy by infusion of a cytosot extract from regenerating dog liver. Surg Gynecol Obstet. 1980;151:538–544. [PMC free article] [PubMed] [Google Scholar]
- 5.Francavilla A, DiLeo A, Polimeno L, Gavaler J, Pellicci R, Todo S, Kam I, Prelich J, Makowka L, Starzl TE. The effect of hepatic stimulatory substance (HSS) isolated from regenerating hepatic cytosol and 50,000 and 300,000 subfractions in enhancing survival in experimental acute hepatic failure in rats treated with d-glactosamine. Hepatology. 1986;6:1346–1351. doi: 10.1002/hep.1840060621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francavilla A, Ove P, Polimeno L, Coetzee M, Makowka L, Rose J, Van Thiel DH, Starzl TE. Extraction and partial purification of a hepatic stimulatory substance in rats, mice, and dogs. Cancer Res. 1987;47:5600–5605. [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 8.Francavilla A, Barone M, Zeevi A, Scotti C, Carrier G, Mazzaferro V, Prelich J, Todo S, Hiras G, Fung J, Starzl TE. FK506 as a growth control factor. Transplant Proc. 1990;23:90–92. [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Porter KA, Watanabe K, Putnam CW. Effects of insulin/glucagon infusions on liver morphology and cell division after complete portacaval shunt in dogs. Lancet. 1976;1:821. doi: 10.1016/s0140-6736(76)90477-3. [DOI] [PubMed] [Google Scholar]
- 10.Ornstein L. Disc electrophoresis. I. Background and theory. Ann NY Acad Sci. 1964;121(2):321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- 11.Davis BJ. Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121(2):404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 12.Cotzee ML, Short J, Klein K, Ove P. Correlation and circulating levels of a serum protein with triodothyronine levels and hepatoma growth. Cancer Res. 1982;42:155–160. [PubMed] [Google Scholar]
- 13.Thaler Fl, Michalopoulos GK, Hepatopoietin A. Partial characterization and trypsin activation of a hepatocyte growth factor. Cancer Res. 1985;45:2545–2549. [PubMed] [Google Scholar]
- 14.Goldberg M. Purification and partial characterization of a liver cell proliferation factor called hepatopoietin. J Cell Biochem. 1985;27:291–302. doi: 10.1002/jcb.240270310. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 17.Andrzejewski C, Jr, Rauch J, Lafer E, Stollar BD, Schwartz RS. Antigen-binding diversity and idiotypic cross-reactions among hybridoma autoantibodies to DNA. J Immunol. 1980;126:226–231. [PubMed] [Google Scholar]
- 18.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 19.McKnight GS. A colorimetric method for the determination of submicrogram quantities of protein. Anal Biochem. 1979;78:86–92. doi: 10.1016/0003-2697(77)90011-2. [DOI] [PubMed] [Google Scholar]
- 20.Teir H, Ravanti K. Mitotic activity and growth factors in the liver of the whole rat. Exp Cell Res. 1953;5:500–507. doi: 10.1016/0014-4827(53)90236-5. [DOI] [PubMed] [Google Scholar]
- 21.Blomqvist K. Growth stimulation in the liver and tumor development following intraperitoneal injections of liver homogenates in the rat. Acta Pathol Microbiol Scand. 1957 Suppl:121. [PubMed] [Google Scholar]
- 22.LaBrecque DR, Steele G, Fogerty S, Wilson M, Barton J. Purification and physical-chemical characterization of hepatic stimulator substance. Hepatology. 1987;7:100–106. doi: 10.1002/hep.1840070121. [DOI] [PubMed] [Google Scholar]
- 23.Fleig WE, Boss G. Partial purification of rat hepatic stimulator substance and characterization of its action on hepatoma cells and normal hepatocytes. Hepatology. 1989;9:240–248. doi: 10.1002/hep.1840090213. [DOI] [PubMed] [Google Scholar]