Abstract
The doxycycline (DOX) -inducible gene expression systems allow tight temporal and spatial control of transgene expression, invaluable in studies of organ development and disease pathogenesis. Transgenic mice using the human Surfactant Protein C promoter to drive the expression of the reverse tetracycline transactivator (SP-C-rtTA) enabled functional analysis of essential gene function during lung development. Here we report that DOX-fed SP-C-rtTA mice during the period in which Type II cells differentiate results in cellular toxicity that may have confounded the interpretation of previous reports using this line. These effects included impaired alveologenesis, loss/reduction in expression of surfactant-associated proteins, and death. Severity was dependent on genetic background: outbred mice or those on a CD1 background are highly susceptible, whereas the C57BL/6 background appeared resistant by morphological criteria. However, quantitative analysis reveled that DOX fed, SP-C-rtTA C57BL/6 pups had reduced surfactant mRNA accumulation that could contribute to synthetic lethality when combined with other genetic alterations. We conclude that the combination of genetic backgrounds, length of DOX exposure and the presence of the SP-C-rtTA transgene contributed more than previously appreciated to the similarities seen in the phenotypes reported by investigators using the SP-C-rtTA, (tetO)7-Cre. These studies demonstrate the importance of using appropriate SP-C-rtTA only controls in all experiments.
Keywords: doxycycline, inducible conditional knockout, genetic background, toxicity of transgene, lung, airway, organogenesis, surfactant
Introduction
Conditional gene inactivation has evolved into a standard method used to decipher the function of essential genes in any desired organ. In principle, all methods rely on expression of Cre recombinase to remove “floxed” alleles, or genomic DNA fragments bracketed by loxP sites. The combination of cell type specific promoters, which provide spatial specificity for Cre expression, with inducible promoters, which endow the investigator with temporal control, contributed to the prevalence of this technology.
Cre toxicity is becoming appreciated by a growing number of investigators. The first reports came from the Berns (Akagi et al., 1997) and Capecchi (Schmidt-Supprian and Rajewsky, 2007) labs, which showed that somatic recombination can occur in the absence of loxP sites. This was thought to be mitigated by selection of tolerated Cre levels in the process of generating Cre-transgenic mice. However, another “Toxic alert” was recently been raised (Editorial, 2007). Lee et al. (Lee et al., 2006) have shown that RIP-Cre mice used for exploration of genetic pathways controlling pancreatic beta-cell development and function, display a phenotype of glucose intolerance even in the absence of any floxed allele. Results from studies of these mice were reported in 21 publications prior to this discovery. Others have also argued that toxicity can confound the results of experiments not controlling for Cre alone (Schmidt-Supprian and Rajewsky, 2007).
Bi-transgenic systems, driving expression of rtTA from tissue specific promoters and activating Cre with a doxycycline/rtTA-inducible promoter ((tetO)7-Cre transgene), has emerged as a leading system permitting tight temporal and spatial control of gene mutation (Branda and Dymecki, 2004). In the pulmonary research field, reverse tetracycline transactivator (rtTA) may be driven by the human Surfactant Protein C (SP-C) promoter in SP-C-rtTA transgenic mice (Perl et al., 2002a; Perl et al., 2002b). Cre expression is activated after animals also carrying (tetO)7-Cre are provided with doxycycline-containing food or water (Perl et al., 2002b). At present, the standard method to achieve gene loss in conduct airway and alveolar epithelial cells during desired developmental stages is to administer doxycycline (DOX) to the pregnant tri-transgenic dam which, in addition to SP-C-rtTA and (tetO)7-Cre, carries a floxed allele (Sauer, 1998).
In preparing to investigate the role of Notch signaling (Artavanis-Tsakonas et al., 1999; Ilagan and Kopan, 2007) in airway epithelial cells, we administered doxycycline to tri-transgenic SP-C-rtTA, (tetO)7-Cre, RBPjkflox/flox mice. To our surprise, we found that outbred controls carrying SP-C-rtTA died at birth with impaired alveologenesis and reduced surfactant production, independent of (tetO)7-Cre inheritance. We discovered that strain-dependent sensitivity of lung Type II epithelial cells to SP-C-rtTA, peaking between days 15.5 and 18.5 of gestation, is responsible for this lethality. CD1 mice were severely affected whereas the inbred C57BL/6 strain was affected to a lesser degree that might nonetheless synergize with a targeted allele. The considerable toxicity of the rtTA/DOX combination during the development of distal lung epithelial cells led us to review the literature in which SP-C-rtTA, (tetO)7-Cre transgenic mice were used. We noticed an alarming similarity in phenotypes that could be attributed at least in part to the toxic effects of rtTA, warranting re-examination of this popular targeting method.
Materials and methods
Generation of SP-C-rtTA, (tetO)7-Cre, RBPjkflox/flox mice and Doxycycline administration
RBPjkflox/flox mice were kindly provided by Dr. T. Honjo, Kyoto University (Tanigaki et al., 2002). SP-C-rtTA, (tetO)7-Cre mice were generated by Dr. J. Whitsett at the University of Cincinnati, provided to us in mixed background of C57BL6/CD1. PCR analysis confirmed that this rtTA gene contains the C-terminal fragment of the VP 16 transcription activator (data not shown). RBPjkflox/flox mice in a similar background were mated with SP-C-rtTA, (tetO)7-Cre mice to produce SP-C-rtTA, (tetO)7-Cre, RBPjk+/flox mice that were then mated with RBPjkflox/flox mice to obtain SP-C-rtTA, (tetO)7-Cre, RBPjkflox/flox males. These were mated with RBPjkflox/flox females to produce animals for analysis. Pregnant females were fed doxycycline food (600 mg/kg) (Bio-Serv) from E8.5 to E18.5 or E15.5. The mating strategy is illustrated in Supplemental Table 1A. Littermates were compared to each other in individual experiments. All animal procedures were performed according to NIH guidelines and maintained in the animal facility under Washington University animal care regulations.
Survival assay
We dissected doxycycline-fed mice at E18.5 to collect embryos. The umbilical cord was cut near the placenta with scissors to avoid excess bleeding. Amniotic fluid and membranes were removed from the mouth and nose using a cotton swab. Body temperature was kept elevated by maintaining the pups on a heat plate at 37 degrees. Pups chests were depressed with a cotton swab to stimulate breathing.
Histology and immunohistochemistry
The lungs were dissected and fixed in 4% paraformaldehyde (PFA), embedded in paraffin and sectioned at 6 μm. The sections were then stained with Hematoxylin and Eosin (H&E) for histological analysis. For immunohistochemistry, the sections were boiled in Trilogy (Cell Marque) or Antigen Unmasking Solution (Vector Laboratories, Inc) for antigen retrieval, pre-blocked with PBS supplemented with 1% BSA, 0.2% skimmed milk, and 0.3% Triton X-100. A rabbit primary anti-Pro-surfactant Protein C (1:2000; Chemicon) or Aquaporin5 antibody (1:200; Chemicon) was used, followed by a biotinylated anti-rabbit IgG (1:1000). Vectastain ABC kit (Vector Laboratory, Inc) and DAB Substrate Kit (Vector Laboratory, Inc) were used to detect the signal.
RNA isolation and quantitative analysis
Fresh lung samples were immediately immersed in RNAlater (Ambion, TX) and homogenized by electric homogenizer at 12,000rpm for 30 sec. Total RNA was collected by using RNeasy kit (QIAGEN Sciences) according to the manufacturer's specifications. The collected RNA was used for cDNA preparation using SuperScript II RT with random hexamers (Invitrogen). The cDNA was amplified with SYBR-Green PCR Master Mix (Applied Biosystems, CA) and gene-specific exon-exon junction-spanning primers using the ABI7700 sequence detection system (Applied Biosystems, CA). Normalizations across samples were performed using glyceraldehyde-3-phosphate dehydrogenase (Gapdh) primers. The differences between transgenic samples and RBPflox/flox or Wild Type as controls were calculated using the comparative CT(2-ΔΔCT) method. The histograms in the figures show the mean±s.e.m. of one to three independent experiments, each of which was performed in triplicate. All qRT-PCR primers were tested for the presence of non-specific primer dimers and for linear amplification. Primer sequences are below, Sftpa; AGGCAGACATCCACACAGCTT and ACCAGTGGTTTCTCCCAATCAC, Sftpb; GGAACACCAGTGAACAGGCTATG and AAACTGTTCACACTTTTGCCTGTCTA, Sftpc; ACCCTGTGTGGAGAGCTACCA and TTTGCGGAGGGTCTTTCCT, Sftpd; CCAACAAGGAAGCAATCTGACAT and CAAGACAAGCATGGAGAGAAAGG, ABCa3; AAGCCTGAACGAGTATGGTAGA and CTCCTCTAGGTCACCTAGCAC, Cebpa; ACAAGAACAGCAACGAGTACC and GTCAACTCCAGCACCTTCTG, GAPDH; AATGTGTCCGTCGTGGATCTGA and GATGCCTGCTTCACCACCTTCT.
Results and Discussion
SP-C-rtTA/DOX combination leads lethal defect
In order to generate conditional knockout mice in which the RBPjk gene, a core component of Notch signaling, is deleted from the lung epithelial cells, RBPjkflox/flox females were mated to SP-C-rtTA, (tetO)7-Cre, RBPjk+/flox males. The pregnant dams were maintained on DOX (600 mg/kg) diet from 8.5 days post coitum (dpc), a day before lung development starts in the developing embryos. To examine deletion efficiency in utero, and to record the consequences of RBP loss, litters were delivered by cesarean section at E18.5. We noticed that some pups died within one hour, displaying respiratory distress (Supplemental Table 1A). These pups failed in inflating their lungs while their littermates breathed normally (Supplemental Movie 1). Surprisingly, double-transgenic SP-C-rtTA, RBPjkflox/flox pups were found among the dead; in fact, all dead animal in this and five additional litters inherited SP-C-rtTA but only some inherited Cre or RBPjk alleles (Supplemental Table 1B). The observation that Cre inheritance was not a factor in lethality, but rtTA inheritance was, is a strong indicator of toxicity associated with the SP-C-rtTA transgene when the dams are provided with 600 mg/kg DOX in their diet.
Whereas RBPjk heterozygous mice generally do not display a phenotype, histological analysis revealed enlargement of the alveolar space in embryos inheriting the SP-C-rtTA transgene and exposed to Dox (Fig. 1A-D). Furthermore, the distributions of Type I and Type II cells in the alveolar epithelium was impaired as determined by immunohistochemistry with Aquaporin 5 (Fig. 1E-H) and ProSP-C antibodies (Fig. 1I-L). Since infant mortality is often associated with disruption of Surfactant-associated proteins (Sftp) secretion from Type II cells, we estimated the expression levels of Sftp A, B, C and D (Whitsett et al., 2005), their transporter ABCa3 (Ban et al., 2007; Cheong et al., 2007; Fitzgerald et al., 2007) and transcriptional regulator C/EBPα (Flodby et al., 1996; Sugahara et al., 2001) using qRT-PCR (Fig. 1M). In comparison with RBPjk+/flox, expression of these factors was reduced significantly in all Dox-fed embryos inheriting the SP-C-rtTA. This quantitative analysis confirmed that Cre inheritance did not contribute to the effect. These data therefore indicate that the presence of the SP-C-rtTA transgene is the genetic determinant causing abnormal alveolar development in embryos exposed to DOX, and that Type II cells are the main cell types affected. Since Type II cells are progenitors for Type I cells (Adamson and Bowden, 1974), the abnormal alveoli are likely the result of the Type II cell loss.
Figure 1.
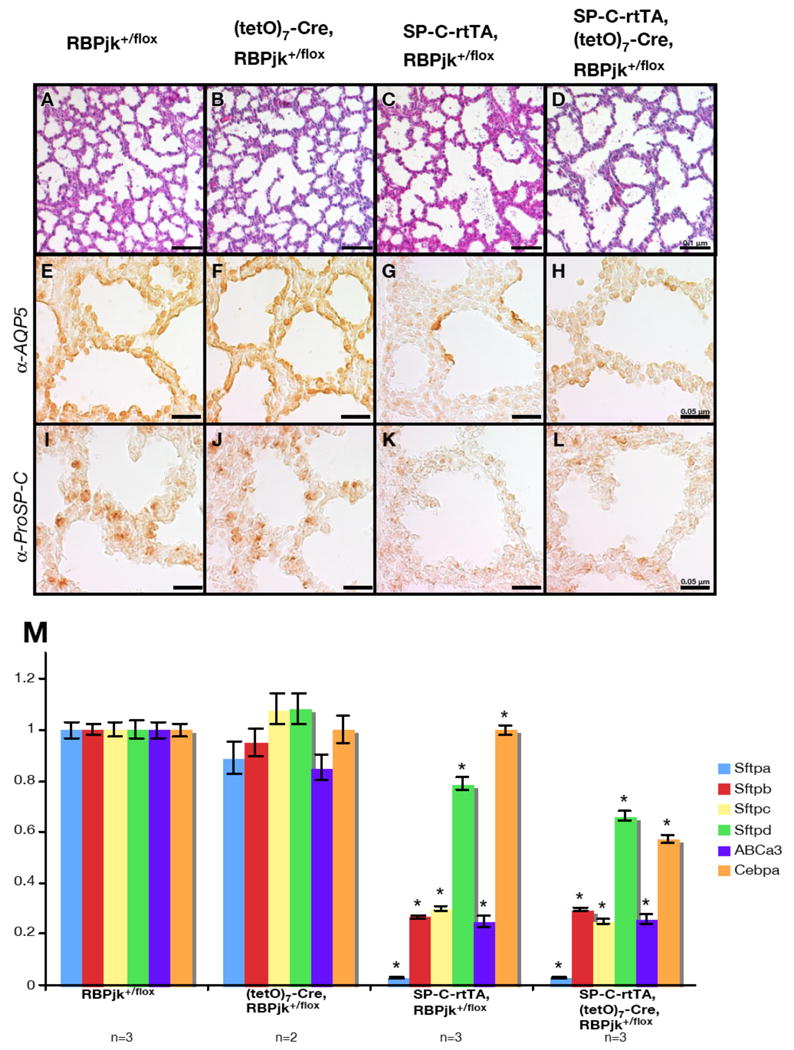
Doxycycline administration to the triple-transgenic mice leads to impaired alveologenesis whether or not the (tetO)7-Cre transgene was inherited. (A-D) Lung sections prepared at E18.5 and stained with the Hematoxylin and Eosin staining revealed alveolar enlargement in mice inheriting SP-C-rtTA (C and D) but not RBPjk+/flox or (tetO)7-Cre, RBPjk+/flox (A and B). Scale bar: 0.1 μm. Immunohistochemistry with anti-Aquaporin 5 (E-H) or anti-ProSP-C (I-L) antibody revealed defective differentiation of Type I cells (G and H) and Type II cells (K and L) in mice inheriting rtTA. Scale bar: 0.05 μm. (M) Quantitative RT-PCR analysis revealed expression levels of Surfactant-associated protein A to D (Sftpa to d) and their regulators ABCa3, C/EBPα in each genotype. Each value was normalized to the internal control GADPH and standardized to RBPjk+/flox. *P<0.05 versus RBPjk+/flox.
Lethality is due to tTA/DOX toxicity at late developmental stage
Type II cells appear in distal airways from E16.5, known as the canalicular stage, and produce surfactant proteins as they mature. Since exposing embryos to DOX from E8.5 to E15.5 my be sufficient to achieve complete deletion of floxed alleles, we wanted to ask if removing DOX during the period in which Type II cells differentiate would eliminate the toxicity associated with the rtTA/DOX complex. When we administered DOX from E8.5 to E15.5, we rescued double- and triple-transgenic inheriting SP-C-rtTA (Supplemental Table 1B). The pups born with this treatment grew to adulthood without any obvious respiratory problem. Histological analysis confirmed that Cre activity during the E8.5-E15.5 window was sufficient to remove the targeted alleles (Supplemental Fig. 1A).
At the histological level, double transgenic mice treated from E8.5 to E15.5 exhibited lung morphology indistinguishable from wild type mice at E18.5 (Fig. 2A-D). However, although the distribution of Type I and Type II cells was restored to normal (Fig. 2E-L), quantitative RT-PCR analysis revealed suppressed expression of Sftps and their regulators (Fig. 2M). Because SP-C-rtTA, RBPjkflox/flox double-transgenic mice displayed the same phenotype as their triple-transgenic, RBPjk-deficient littermates (Supplemental Fig. 1A), we conclude that neither Cre activity nor loss of Notch signaling in the distal airway contributes to the observed phenotype (the full description of the phenotype will be published elsewhere). These results reveled that although severe phenotypes are caused by exposure to rtTA/DOX between E15.5 to 18.5 (the canalicular and saccular stages), a moderate phenotype characterized by suppression of the Sftps and their regulators still occurs in the transgenic mice inheriting the SP-C-rtTA transgene. These observations confound the interpretation of studies using this line due to possible synergy between the toxic effects of SP-C-rtTA on Type II cells and the induced gene oblation.
Figure 2.
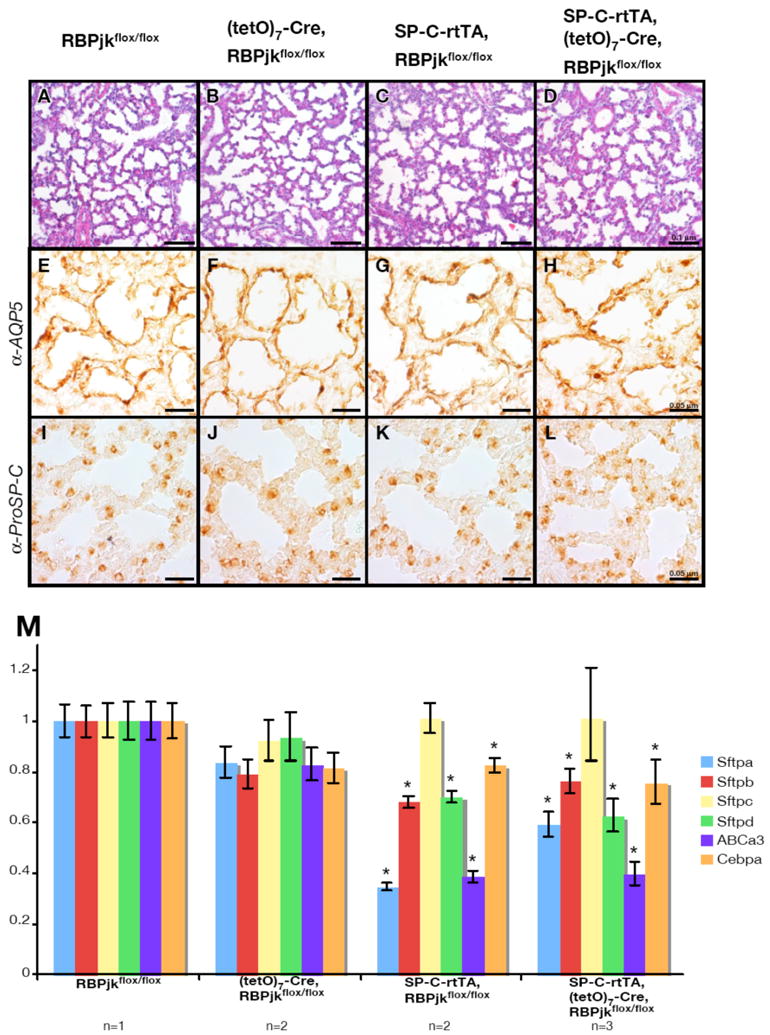
Rescue attained by suspension of doxycycline administration at E15.5. (A-D) Lung sections prepared at E18.5 and stained with the Hematoxylin and Eosin staining were morphologicaly normal. Scale bar: 0.1 μm. Normal distribution of Type I cells and Type II cells were observed with immunohistochemistry for Aquaporin 5 (E-H) and ProSP-C (I-L). Scale bar: 0.05 μm. (M) Expressions of Sftpa, b, d and ABCa3 were still reduced while Sftpc and Cebpa were partially restored in the rescued the SP-C-rtTA mice. Each value was normalized to the internal control GADPH and standardized to RBPjk f/f. *P<0.05 versus RBPjk f/f.
The particular genetic background appears resistant to the rtTA/DOX toxicity
Genetic background can be another confounding factor influencing the phenotype of a transgenic mouse. Like many studies involving multiple transgenes, we analyzed the behavior of SP-C-rtTA on a mixed background, here containing genetic contribution from C57BL/6 and CD1 with minimal contribution from FVB (the strain in which SP-C-rtTA and (tetO)7-Cre were made) and 129Sv (used to generate RBPjk+/flox). To ask if the genetic background contributed to rtTA/DOX toxicity, we mated an SP-C-rtTA, (tetO)7-Cre, RBPjk+/flox sire with wild type females of C57BL/6 or CD1 background. We fed the pregnant dams doxycycline diet from E8.5 to E18.5, delivered their fetuses by cesarean section at E18.5 and examined the phenotypes. Interestingly, alveolar morphology was normal in all SP-C-rtTA pups on the C57BL/6 background (Fig. 3C and D) whereas it was clearly abnormal on the CD1 background (Fig. 3G and H). Likewise, Type I and Type II cells were lost on the CD1 background but their distribution was normal on the C57BL/6 background (Fig. 3K, L, O, P, S, T, W and X). CD1 pups inheriting the SP-C-rtTA transgene exhibited reductions in the level of surfactants and their regulators comparable to that in mixed background animals (compare Fig. 1M, 2M and 4B). Although lung morphology with increased C57BL/6 background appeared similar to non-transgenic wild type (Fig. 4A), a significant reduction in the expression of SftpA, D and ABCa3 was still detected. These data indicate that the outbred CD1 background and the CD1; C57BL/6 mixed animals were susceptible to rtTA/DOX toxicity. It is important to note that even on the C57BL/6 background, the potential for confounding impact from rtTA/DOX toxicity exists.
Figure 3.
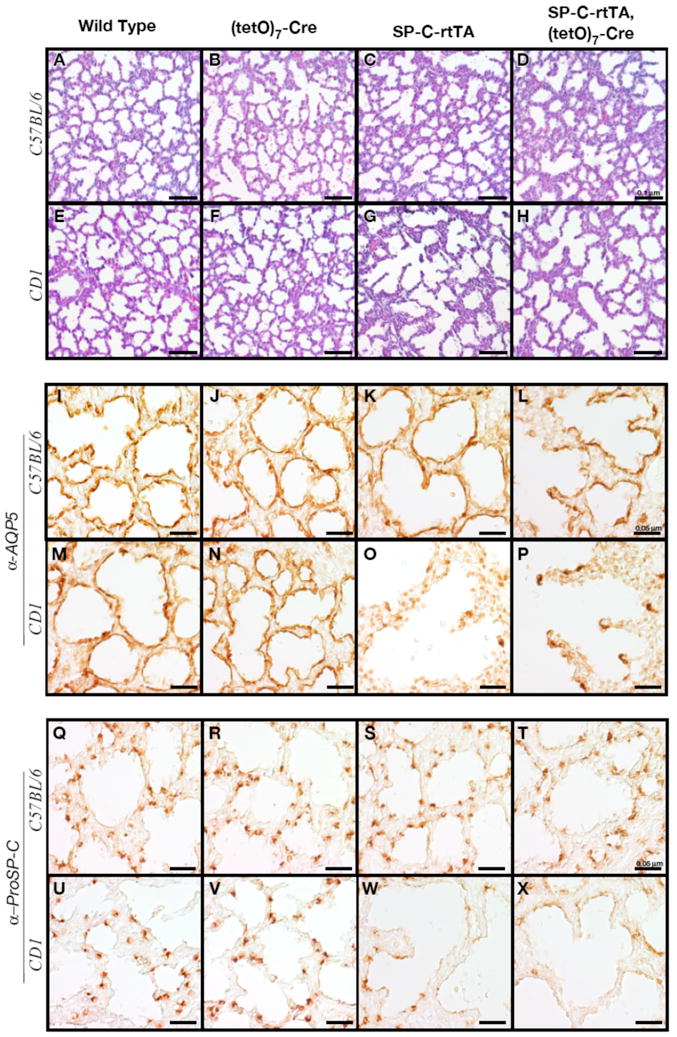
The sensitivity to the toxic effects of rtTA depends on genetic background. Lung sections were prepared from offspring of C57BL/6 (A-D, I-L, Q-T) or CD1 (E-H, M-P, U-X) dams. (A-H) The Hematoxylin and Eosin staining demonstrated alveolar enlargement in SP-C-rtTA transgenic mice on the CD1 genetic background (G and H) but not the C57BL/6 background (C and D). The impaired distribution of Type I cells (I-P) and Type II cells (Q-X) was observed by immunohistochemistry in only the CD1 genetic background (O, P, W and X) but not the C57BL/6 background (K, L, S and T).
Figure 4.
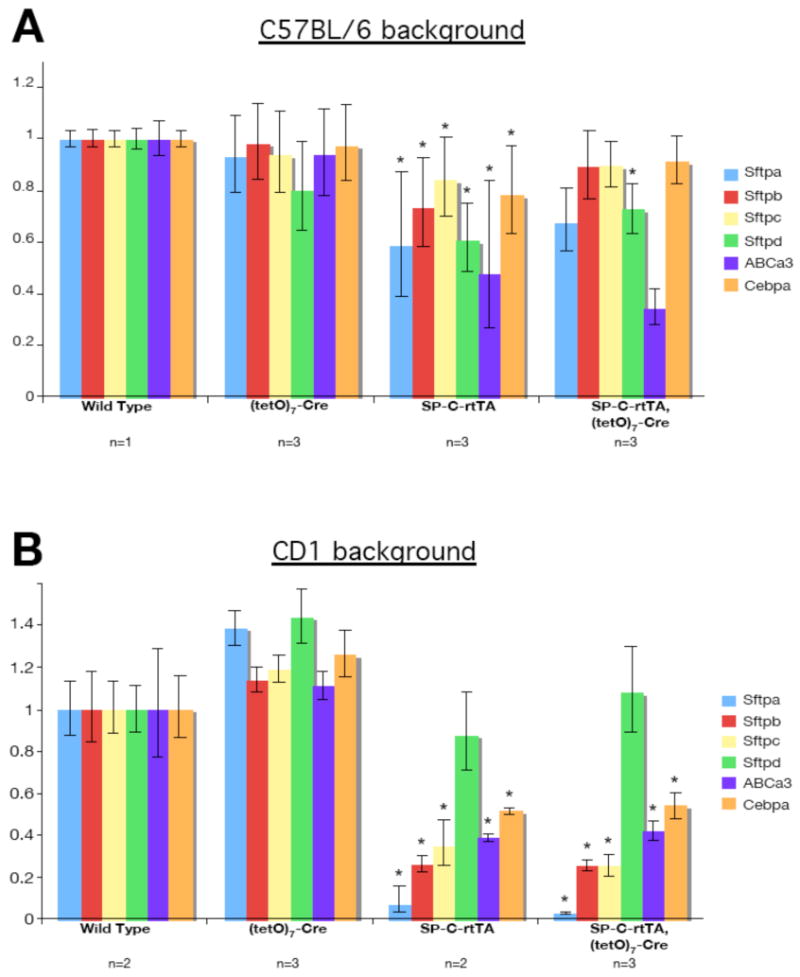
Genetic background influences surfactant production in SP-C-rtTA transgenic mice with doxycycline administration. (A) Mating with a wild type C57BL/6 female reduced the toxicity SP-C-rtTA transgenics from the severe suppression of the expression of the surfactants and surfactant-related factors. However, the expression of Sftpa, d and ABCa3 were still relatively repressed. (B) In the CD1 background, the SP-C-rtTA transgenic group showed suppression of gene expression similar to the previous experiments in Fig. 1M and 2M. Each value was normalized to the internal control GADPH and standardized to wild type. The SP-C-rtTA transgene is sufficient to reduce their expression. *P<0.05 versus wild type.
rtTA/DOX toxicity may have confounded the interpretation of previous reports
Identifying the precise mechanism behind SP-C-rtTA toxicity, or the genes conferring resistance, is outside the scope of this report and of questionable relevance to developmental biology or human health. However, several mechanistic assumptions can be made. The rtTA-VP16 used in these strain is known to bind to pseudo-tetO promoter sequence and may even bind non-specifically in a concentration and DOX dependent manner (Urlinger et al., 2000). Since global rtTA expression under CMV promoter does not impair mouse development in the presence of DOX (Furth et al., 1994), one can assume that although the strength of the SP-C promoter coupled with transgene copy number produce tolerable amount of this repressor, addition of DOX during the developmental window defined in our study represses genes essential for Type II cell development, resulting in toxicity and death.
It should be noted that others reported toxicity of rtTA to Clara cells in adult lungs when using the CCSP-rtTA transgene even in the absence of DOX (Sisson et al., 2006), leading to an emphysema-like phenotype and a caution regarding potential toxicity (Whitsett and Perl, 2006). If SP-C-rtTA were similarly toxic to mature Type II cells, dams would suffer respiratory distress; they did not. This would be consistent with toxicity following periods of maximal rtTA expression. Given the widespread use of these rtTA lines, it would be prudent to assume that other embryonic and adult cell types are sensitive to rtTA alone or in combination with DOX, requiring more detailed molecular control experiments then previously deemed necessary (Whitsett and Perl, 2006).
A case in point are studies using the SP-C-rtTA, (tetO)7-Cre system employed to date in at least nine publications (Basseres et al., 2006; Dave et al., 2006; Hokuto et al., 2004; Martis et al., 2006; Mucenski et al., 2003; Nguyen et al., 2005; Wan et al., 2005; Wan et al., 2004; Yanagi et al., 2007) (Table 1). These studies have yielded important new insights into genetic pathways controlling the development, function and maintenance of lung epithelial cells. However, in addition to phenotypes specific to the targeted allele, all studies reported similar reductions of surfactant-associated protein and Aquaporin-5 (with the exception of STAT3flox/flox, (Hokuto et al., 2004)). We established that loss of Type II cells and their products is the hallmark of rtTA/DOX poisoning. The authors of the STAT3 targeting study have not reported the genetic background of their animals but we expect that these mice were on a genetic background resistant to toxicity. Likewise, it is tempting to point out that the lethality reported in Table 1 might depend on genetic background and not on gene ablation since all mice with a CD1 contribution display some degree of perinatal mortality. Moreover, rtTA/DOX toxicity and background contribution could help explain how two independent studies targeting C/EBPα reported slightly different outcomes (Basseres et al., 2006; Martis et al., 2006).
Table 1.
Type II cell phenotypes and survival in published studies that have used the SP-C-rtTA, (tetO)7-Cre transgenic mice for gene targeting.
NA., information not available.
| Targeted gene | Alveolar morphology | Surfactant reduction | AQP5 defect | Survival at P1 | DOX administration | Genetic background | Ref. |
|---|---|---|---|---|---|---|---|
| b-catenin | Severe enlargement | Yes | N.A. | None | 625 mg/kg diet, E0.5∼ | C57BL/6; CD1 | Mucenski et al., 2003 |
| Foxa2 | Atelectasis | Yes | Yes | 46%Survival | 25mg/g diet, E0.5∼ | N.A. | Wan et al., 2005 |
| Foxa1-/-, Foxa2 | Abnormal | Yes | Yes | N.A. | 625 mg/kg diet, E0.5∼ | N.A. | Wan et al., 2004 |
| Laminin a5 | Enlargement | Yes | Yes | None | 1 mg/ml water, E6.5∼ | C57BL/6; CD1 | Nguyen et al., 2005 |
| Cebpa | Atelectasis | SftpC No SftpA,B Yes | Yes | 14%Survival | 625 mg/kg diet, E0.5∼ | N.A. | Basseres et al., 2006 |
| Cebpa | Atelectasis | Yes | Yes | None | 1 mg/ml water, E0.5∼ | C57BL/6; FVB/N | Martis et al., 2006 |
| STAT3 | Atelectasis | No | N.A. | 100%Survival | 625 mg/kg diet, E0.5∼ | N.A. | Hokuto et al., 2004 |
| Calcineurin b1 | Enlargement | Yes | Yes | 100%Survival (but all dead at P2) | N.A. | C57BL/6; FVB/N | Dave et al., 2006 |
| PTEN | Atelectasis | Yes | Yes | 10%Survival | 1 mg/ml water, E10.5-16.5 | C57BL/6; 129Ola | Yanagi et al., 2007 |
| RBPjk | Enlargement | Yes | Yes | 33%Survival | 625 mg/kg diet, E8.5∼ | C57BL/6; CD1 | Table S1 |
Conclusions
While interpretation of past reports pertaining to Type II cell development is confounded by the rtTA/DOX toxicity, additional controls should be able to identify the phenotypes obtained with SP-C-rtTA mice that are dependent on rtTA toxicity, gene oblation or interaction between the two. It is important to note that even when SP-C-rtTA littermates are morphologically indistinguishable from wild type, our data indicates that SP-C-rtTA transgenic mice are compromised- they have reduced mRNA levels for surfactants, their regulators, and an unknown number of other genes (Fig. 1, 2 and 4). The rtTA system in general, and the SP-C-rtTA transgene in particular, are powerful tools that will be difficult to replace. Until a perfect system is devised, proper controls must include paralleled molecular analysis of DOX-fed rtTA strains in matched background to their bi- and tri-transgenic littermates.
Supplementary Material
Acknowledgments
The authors thank Drs. Adrian Shifren, David Ornitz, Jeffery Miner for careful reading of the manuscript. We also thank Dr. Jeffery Whitsett for SP-C-rtTA, (tetO)7-Cre mice. This work was supported by Washington University MM was supported by a Toyobo Biotechnology Foundation Long-term Research Grant and the Japanese Society for the Promotion of Science. RK was supported by NIH grant HD 044056.
References
- Adamson IY, Bowden DH. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974;30:35–42. [PubMed] [Google Scholar]
- Akagi K, Sandig V, Vooijs M, Van der Valk M, Giovannini M, Strauss M, Berns A. Cre-mediated somatic site-specific recombination in mice. Nucleic Acids Res. 1997;25:1766–73. doi: 10.1093/nar/25.9.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Ban N, Matsumura Y, Sakai H, Takanezawa Y, Sasaki M, Arai H, Inagaki N. ABCA3 as a lipid transporter in pulmonary surfactant biogenesis. J Biol Chem. 2007;282:9628–34. doi: 10.1074/jbc.M611767200. [DOI] [PubMed] [Google Scholar]
- Basseres DS, Levantini E, Ji H, Monti S, Elf S, Dayaram T, Fenyus M, Kocher O, Golub T, Wong KK, Halmos B, Tenen DG. Respiratory failure due to differentiation arrest and expansion of alveolar cells following lung-specific loss of the transcription factor C/EBPalpha in mice. Mol Cell Biol. 2006;26:1109–23. doi: 10.1128/MCB.26.3.1109-1123.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- Cheong N, Zhang H, Madesh M, Zhao M, Yu K, Dodia C, Fisher AB, Savani RC, Shuman H. ABCA3 is critical for lamellar body biogenesis in vivo. J Biol Chem. 2007;282:23811–7. doi: 10.1074/jbc.M703927200. [DOI] [PubMed] [Google Scholar]
- Dave V, Childs T, Xu Y, Ikegami M, Besnard V, Maeda Y, Wert SE, Neilson JR, Crabtree GR, Whitsett JA. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. J Clin Invest. 2006;116:2597–609. doi: 10.1172/JCI27331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial. Nature. Vol. 449. 2007. Toxic alert; 378 pp. [Google Scholar]
- Fitzgerald ML, Xavier R, Haley KJ, Welti R, Goss JL, Brown CE, Zhuang DZ, Bell SA, Lu N, McKee M, Seed B, Freeman MW. ABCA3 inactivation in mice causes respiratory failure, loss of pulmonary surfactant, and depletion of lung phosphatidylglycerol. J Lipid Res. 2007;48:621–32. doi: 10.1194/jlr.M600449-JLR200. [DOI] [PubMed] [Google Scholar]
- Flodby P, Barlow C, Kylefjord H, Ahrlund-Richter L, Xanthopoulos KG. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J Biol Chem. 1996;271:24753–60. doi: 10.1074/jbc.271.40.24753. [DOI] [PubMed] [Google Scholar]
- Furth PA, St Onge L, Boger H, Gruss P, Gossen M, Kistner A, Bujard H, Hennighausen L. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A. 1994;91:9302–6. doi: 10.1073/pnas.91.20.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest. 2004;113:28–37. doi: 10.1172/JCI200419491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilagan MX, Kopan R. SnapShot: notch signaling pathway. Cell. 2007:128–1246. doi: 10.1016/j.cell.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–53. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- Martis PC, Whitsett JA, Xu Y, Perl AK, Wan H, Ikegami M. C/EBPalpha is required for lung maturation at birth. Development. 2006;133:1155–64. doi: 10.1242/dev.02273. [DOI] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–8. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–25. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002a;11:21–9. doi: 10.1023/a:1013986627504. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002b;99:10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–92. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–8. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- Sisson TH, Hansen JM, Shah M, Hanson KE, Du M, Ling T, Simon RH, Christensen PJ. Expression of the reverse tetracycline-transactivator gene causes emphysema-like changes in mice. Am J Respir Cell Mol Biol. 2006;34:552–60. doi: 10.1165/rcmb.2005-0378OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugahara K, Iyama KI, Kimura T, Sano K, Darlington GJ, Akiba T, Takiguchi M. Mice lacking CCAAt/enhancer-binding protein-alpha show hyperproliferation of alveolar type II cells and increased surfactant protein mRNAs. Cell Tissue Res. 2001;306:57–63. doi: 10.1007/s004410100420. [DOI] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–50. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A. 2000;97:7963–8. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Dingle S, Xu Y, Besnard V, Kaestner KH, Ang SL, Wert S, Stahlman MT, Whitsett JA. Compensatory roles of Foxa1 and Foxa2 during lung morphogenesis. J Biol Chem. 2005;280:13809–16. doi: 10.1074/jbc.M414122200. [DOI] [PubMed] [Google Scholar]
- Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A. 2004;101:14449–54. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett JA, Perl AK. Conditional control of gene expression in the respiratory epithelium: A cautionary note. Am J Respir Cell Mol Biol. 2006;34:519–20. doi: 10.1165/rcmb.F310. [DOI] [PubMed] [Google Scholar]
- Whitsett JA, Wert SE, Xu Y. Genetic disorders of surfactant homeostasis. Biol Neonate. 2005;87:283–7. doi: 10.1159/000084875. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Kishimoto H, Kawahara K, Sasaki T, Sasaki M, Nishio M, Yajima N, Hamada K, Horie Y, Kubo H, Whitsett JA, Mak TW, Nakano T, Nakazato M, Suzuki A. Pten controls lung morphogenesis, bronchioalveolar stem cells, and onset of lung adenocarcinomas in mice. J Clin Invest. 2007;117:2929–40. doi: 10.1172/JCI31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


