Abstract
The ultimate goal of diagnostic research is a blood test detecting the risk of Alzheimer disease (AD) before neuronal damage develops. Current amyloid-β (Aβ) tests do not detect the process leading to neurodegeneration. Novel immunologic and proteomics tests are based on aberrant appearance of inflammatory cytokines in the CSF and other protein biomarkers in the CSF or blood, and immune biomarkers of peripheral blood mononuclear cells (PBMC's). Cytokines, chemokines, complement factors, serum amyloid P component, and signaling proteins in the CSF or blood may be a rich source of diagnostic biomarkers, but the power of these tests will need to be examined in prospective studies. Recently-described flow cytometric test of defective Aβ phagocytosis detects patients with AD with a high sensitivity and specificity in distinct populations (confirmed AD patients vs. active University professors), but further experience is necessary for its use in general population at risk of AD. The analysis of the transcriptome of peripheral blood mononuclear cells “stressed” by Aβ is beginning to unravel the relations between specific pathways and AD. Thus novel diagnostic tests may provide biomarkers for pre-clinical detection, clarification of progression from MCI to AD, and follow-up of patients in clinical trials of immunostimulating therapies.
Keywords: Alzheimer disease biomarkers, amyloid-beta, macrophage, microglia, astrocyte, phagocytosis, inflammation, serum amyloid P, complement, neuron
1. Introduction
The quest for effective prevention of AD requires detection of the risk of future dementia with high sensitivity and specificity in the pre-clinical phase before neuronal damage develops. This period may be several decades, as suggested by the Nunn study (Riley, et al., 2005). The search for biological biomarkers has established cerebrospinal fluid (CSF) biomarkers, low Aβ 1-42 and high phosphorylated-τ 181 (P-τ) as markers of the state, but not the process, of neurodegeneration (Sunderland, et al., 2003) (Blennow and Hampel, 2003). Low Aß and high τ or P-τ in CSF have been confirmed in many studies as reliable biomarkers of AD (Craig-Schapiro, et al., 2008) but are infrequently used in the management of individual patients, although they serve differential diagnosis and follow-up in clinical trials. Imaging studies are useful in evaluating the risk of mild cognitive impairment (MCI) progression to AD. In patients with MCI, structural magnetic resonance imaging (MRI) predicted future dementia (Killiany, et al., 2000). PET scanning using the Pittsburgh compound B showed brain Aβ in MCI patients progressing to AD (Fagan, et al., 2007). However, imaging studies are not ideal for diagnosing AD in younger patients without atrophy detectable by magnetic resonance imaging (MRI) (Schoonenboom, et al., 2008). Moreover, in a follow-up study of Aβ vaccine trial, MRI brain volume dissociated from cognitive function (Fox, et al., 2005). Low CSF Aβ and high τ predicted faster progression of cognitive deficits, but the correlation with 2-year future brain atrophy in preclinical subjects was only 0.30 (Fagan, et al., 2009). Consequently, to diagnose the process leading to neurodegeneration in preclinical subjects, novel biomarkers of preclinical AD additive, with or preceding Aβ, and imaging biomarkers are necessary.
Abnormal innate immune responses to certain aggregated proteins, such as occurs in AD to Aβ and in amyotrophic lateral sclerosis (ALS) to superoxide dismutase-1 (SOD-1), appear to be the critical immune mechanisms of neurodegenerative diseases. Although inflammation has a long history as the hallmark of AD (Akiyama, et al., 2000), Aβ phagocytosis has also garnered interest as a diagnostic technique (Fiala, et al., 2007) (Avagyan, et al., 2009). Immunopathological studies of the AD brain revealed upregulation of complement, cytokines, and acute phase reactants (such as alpha1-antichymotrypsin) in the CNS of AD patients (Akiyama, et al., 2000). Complement activation products C1q, C4b, C3b co-localize with Aß plaques and may determine local recruitment and activation of glial cells and degree of removal of Aß (Eikelenboom and Veerhuis, 1996). Early neuropathological changes preceding the neurodegenerative changes of AD, i.e. the presence of amyloid- associated factors and clustering of activated cytokine-secreting microglia, together with the role of polymorphism of certain cytokines (IL-1, IL-6, TNF-α) and acute-phase proteins (α1-antichymotrypsin) as the genetic risk factors for AD, have led to the concept that pro-inflammatory cytokines released by activated microglia are the driving force in AD pathology (Akiyama, et al., 2000).
The innate responses to Aβ, inflammation and defective Aβ phagocytosis, are both promising as a basis of new tests for early detection. These tests require traditional and new methods for Aβ, fluorescence microscopy, flow cytometry, ELISA, and enzyme assays, as well as microarray hybridization, real-time-PCR, high-throughput sequencing, and proteomics techniques to clarify the pathological pathways of AD. In this review, we consider diagnostic testing:: first by cytokine and proteomics fluid biomarkers of AD, second by novel biomarkers related to inflammation, third by those related to phagocytosis, and fourth by transcriptional responses in Aβ-stressed macrophages.
2. Diagnostic testing by cytokine and proteomics CSF and plasma tests
Although CSF levels of most cytokines cannot be used to diagnose or to differentiate dementias, they might be useful for monitoring therapeutic effects of anti-inflammatory drugs. Moreover, CSF TNFα has been implicated as a modulator of synaptic activity and long term potentation and was found to be significantly higher in MCI cases, especially in those that progress to AD (Tarkowski, et al., 2003). CSF levels of the anti-inflammatory cytokine transforming growth factor (TGF)-ß1, a regulator of brain responses to inflammation and injury, are consistently found to be increased in AD cases compared to controls (Rota, et al., 2006). MCI cases progressing to AD had lower levels of TGF-ß1 at baseline than controls or nonprogressors, suggesting a propensity to a pro-inflammatory state at the time of progression from MCI to AD (Tarkowski, et al., 2003). Immunohistochemical studies of post mortem brain indicate a gradual increase in activated microglia and a decrease of COX-2-activated neurons in parallel with the Braak score (i.e. the score for neurofibrillary changes) (Hoozemans et al, 2005).
Other potential biomarkers may be acute phase proteins or other amyloid associated proteins, markers of lipid peroxidation and early markers for neurodegeneration and regeneration. A number of amyloid associated factors (cytokines, chemokines, complement factors and acute phase proteins) have been found to be altered in the CSF in some studies. C-reactive protein (CRP) and IL-6 were significantly higher in the CSF of MCI patients compared to AD patients (p < 0.01), suggesting that inflammatory processes are already ongoing and detectable in CSF, even before Aβ decline and τ increases in CSF, which was especially noticeable in those MCI cases with a low risk AD profile (Aβ 42 >495 pg/ml and total τ< 356 pg/ml or P-τ< 54 pg/ml) (Schuitemaker, et al., 2008) (Fig.1). Serum amyloid P component (SAP) may be another biomarker of progression to AD. SAP is mainly produced in the liver, but also locally in the brain. SAP binds to amyloid and may protect Aß from proteolysis. In addition, SAP may be directly neurotoxic. In a recent cross-sectional study, no differences in CSF SAP levels between controls, AD patients and MCI cases were observed. However, after a 2-3 year follow-up, the MCI patients who progressed to AD had significantly lower CSF SAP levels in comparison to non-progressors (median 13 and 20 mg/L; p< 0.05). After correction for sex, age and MMSE, low SAP levels were associated with a two-fold increased risk of progression to AD (Hazard ratio =2.2 (95% CI 0.9 to 4.6)), which suggests that SAP may serve as a CSF biomarker to distinguish progressors from non-progressors (Verwey, et al., 2008). We speculate that the non-progressor MCI cases have normal CSF SAP levels, because of low amounts of fibrillar Aß deposits and “normal” SAP synthesis, whereas the progressor MCI cases already have fibrillar Aß deposits that bind SAP and consequently less SAP draining to CSF. After MCI progression to AD, activated glial cells produce cytokines and consequently acute phase proteins including SAP and, consequently, SAP production increases and, despite consumption by binding to fibrillar amyloid, CSF SAP levels are restored. A dicarboxylic acid, pyrrolidone ring-containing compound (CPHPC), was shown to cross-link SAP pentamers leading to their clearance from the circulation and diminished amyloid deposits in systemic amyloidosis patients. In a recent study, CPHPC was shown to lower plasma as well as CSF levels of SAP in AD patients (Kolstoe, et al., 2009), opening new avenues for treatment.
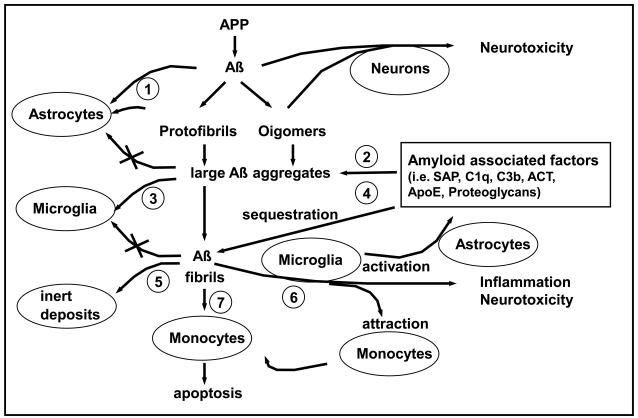
Figure 1. Diagram of putative events associated with extracellular Aß deposition and clearance (Adapted from Veerhuis et al, 2005).
(1) Monomeric and small oligomeric Aß are prevented from neurotoxic action through astrocytic uptake; (2) Amyloid associated factors (AAF's) enhance formation of Aß aggregates indigestible by astrocytes; (3) Microglia move in to clear the aggregated Aß; (4) AAF's enhance Aß fibrillization; (5) In late stage, AAF's lead to Aß deposit encapsulation and sequestration, and subsidence of the inflammatory response; (6) In intermediate stage, AAF's activate microglia to secrete neurotoxic and inflammatory agents, which stimulate synthesis of APP and AAF's, and are chemotactic; (7) Monocyte/macrophages ingest larger Aß aggregates but eventually die and contribute to vascular amyloid formation.
In the post-mortem brain, increased cyclooxygenase-2 (COX-2), cell cycle protein expression and activation of the unfolded response were detected in mid-temporal neurons in Braak stage III -IV in association with diffuse (low-fibrillar) Aß plaques. Neuronal COX-2 and cell cycle protein expression again decreased in stages V and VI, when profound τ-related neurofibrillary changes were observed (Hoozemans, et al., 2005). Thus, the levels of inflammatory cytokines, including prostaglandin E2 (PGE2), appear to be increased in early, compared to late AD stages. F2-isoprostanes, the end-products of lipid peroxidation, were augmented in the CSF of AD cases and increased F2-isoprostane levels predicted progression from MCI to AD, and possibly from pre-clinical state to MCI (de Leon, et al., 2007).
Transcriptional microarray studies of AD hippocampal tissues showed that the main alterations in early AD cases included decreases in neurotrophic support and activation of apoptotic and neuroinflammatory signaling (Colangelo, et al., 2002).To identify new biomarkers in the serum or CSF, global pattern of proteins is analyzed using either 2-D gel electrophoresis followed by matrix-assisted laser desorption/ionization- time of flight (MALDI TOF) or protein chip arrays on which bound proteins are identified by surface enhanced laser desorption ionization (SELDI)- time-of-flight (TOF)- mass spectrometry (MS). A multiplex quantitative proteomics method identified a panel of unique AD vs. Parkinson disease vs. dementia with Lewy body (DLB) protein markers in the CSF (Abdi, et al., 2006). In another study, complement factor H (CFH) precursor and alpha-2-macroglobulin were identified as significantly different in patients compared to age-matched controls (Hye, et al., 2006). Using the SELDI TOF approach, 15 biomarkers, including cystatin C, N-terminally truncated cystatin C, Aß1-40, and complement anaphylatoxin C3a desArg, were selected that, combined with t and Aß1-42 ELISA data, could distinguish patients with stable MCI from patients with MCI progressing to AD (Simonsen, et al., 2007). Another study produced a panel of signaling plasma proteins related to hematopoiesis, immune responses, apoptosis and neuronal support, which was predictive of progression of MCI to AD and confirmatory of AD diagnosis (Ray, et al., 2007). Taken together, a number of factors that are either up or down regulated or exist in certain isoforms or splice products have been reported, but these findings seem to vary in different stages of AD (for a comprehensive overview, see (Zetterberg, et al., 2008)).
PBMC's of AD patients have crucial transcriptional defects of MGAT-III and other genes detected by microarray hybridization (Fiala, et al., 2007). In addition, we have recently observed in AD PBMC's defective expression of another glycan transferase MGAT-II using Affymetrix manufactured custom oligo glycan array (Fiala and Head, unpublished data). We have also shown by 2D-DIGE electrophoresis of differentially-labeled proteins of AD PBMC's before and after exposure to Aß differences in the expression of 8 proteins (Fiala, Avliyakulov and Haykinson, unpublished observations) (Fig. 2). These proteomics techniques will be helpful to clarify the pathways responsible for defective phagocytosis in AD patients.
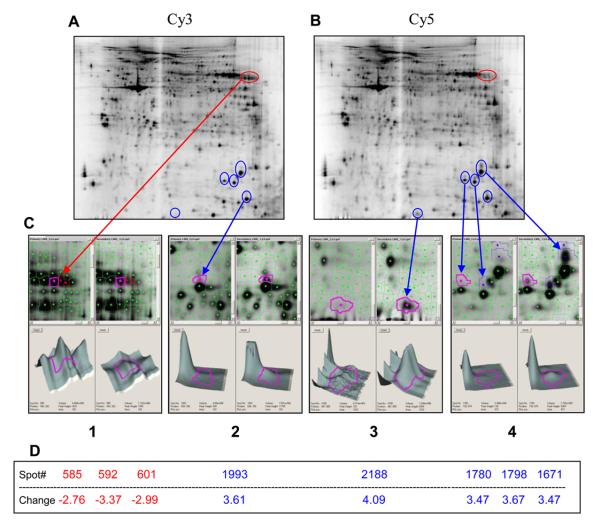
Figure 2. Differentially-expressed proteins in Aβ-stimulated or not stimulated PBMC's of an AD patient.
Proteins from PBMC's cultured without Aβ were labeled with Cy3 (panel A), those cultured with Aβ were labeled with Cy5 (panel B), and both were examined by 2D-DIGE electrophoresis. Protein spots showing significant changes of more than 2.5-fold are shown. Proteins showing a decreased expression after incubation with Aβ are labeled red and those showing an increased expression after incubation are labeled blue. There were 8 spots showing at least 2.5-fold change in protein expression, five of them showing an increased expression upon Aβ treatment and 3 spots (red) showing a decreased expression (panels C and D). Although the proteins have not yet been identified, the observed characteristic spot pattern may be due to a shift in post-translational modification of these proteins.
3. Aβ clearance from the brain and tests of Aβ phagocytosis
The immunopathology of neurodegenerative diseases involves molecular perturbations embroiling both the nervous and immune systems dealing with disposal of extra- and intracellular waste-products with propensity to aggregation, such as Aβ in AD and superoxide dismutase-1 (SOD-1) in ALS. In addition, autophagic degradation of intracellular components, such as mitochondria, via the lysosomal pathway, is abnormal in AD neurons (Boland, et al., 2008). The characteristic pathology of AD, accumulation of Aβ, may be initially caused by defects in physiological mechanisms of clearance and degradation of Aβ in the brain. Physiological clearance of Aβ may occur through drainage to perivascular spaces, or actively through uptake and processing of the Aß by astrocytes, microglia or monocyte-derived macrophages. Fibrillar deposits are probably accessible only to macrophages.
3.1 Physiological clearance of Aβ
Studies in mouse models showed that physiological clearance of Aβ across the blood-brain barrier (BBB) depends upon Aβ binding to low-density lipoprotein receptor related protein-1 (LRP1) and receptor for advanced glycation end products (RAGE). In the plasma of AD patients, LRP1 is decreased (Deane, et al., 2009). In the AD brain, RAGE, CD36, and low-density lipoprotein receptor (LDLR) are associated with cerebral amyloid angiopathy. LRP-1 and LDLR are involved in Aβ internalization and Aβ-mediated cell death of cerebral perivascular cells (Wilhelmus, et al., 2007). In addition, megalin/low-density lipoprotein receptor-related protein-2 (LRP2) mediates insulin-like growth factor-1 -induced clearance of Aβ through the choroid plexus (Carro, et al., 2005). Physiological degradation of Aβ involves degradation by neprilysin (NEP) , insulin-degrading enzyme, endothelin-converting enzyme, plasmin, and cathepsin B (Morelli, et al., 2005). Differences in matrix metalloproteinase-9 (MMP-9) activity contribute to the apo E isoform-specific differences in Aβ degradation by macrophages (Zhao, et al., 2009).
The physiological clearance is clearly not sufficient in the AD brain, where Aβ assemblies accumulate both extra- and intraneuronally: Oligomeric Aβ accumulates in neurons and fibrillar Aβ in plaques and congophilic vessels (Zaghi, et al., 2009). APP transgenic animal models do not reproduce the immune transcriptional defects of human patients (Fiala, et al., 2007). Thus the pathways of immune clearance in human brain must be assembled piecemeal from immunochemical studies of post-mortem brains and in vitro studies of astrocytes, microglia and blood macrophages.
3.2 Role of astrocytes
In the human brain, Aß accumulates inside astrocytes in areas remote from fibrillar Aß deposits, suggesting that astrocytes can ingest Aβ at early stages of Aß accumulation. Primary adult as well as fetal astrocytes bind and ingest fluorescent Aβ when examined by flow cytometry and microscopy (Nielsen, et al., 2009). No significant difference in Aβ1-42 uptake between AD and non-AD astrocytes and no influence of ApoE genotype on Aβ1-42 uptake were observed. Because Aß containing astrocytes were only found in areas with nonfibrillar Aß, it is possible that astrocytes may be the first in line to clear Aß from the interstitial space, but the Aß peptide readily aggregates and forms fibrils, which may escape the reach of astrocytes. Various amyloid associated proteins, such as SAP, complement factors, apolipoproteins and various glycosaminoglycans, may enhance the aggregation process.
3.3 Role of microglia
Microglia may be the second in line to clear the Aß deposits, but probably are also unable to ingest large deposits because of their poor phagocytic function, as judged from flow cytometric testing of the uptake of fluorescently labeled Aß1-42 by adult human microglia (Familian, et al., 2007). Since neuroinflammation in AD is characterized by both degeneration and regeneration that occurs in a spatial and temporal specific pattern (Hoozemans, et al., 2006), microglia may change their activation state in time. In comparison to monocyte-derived macophages, microglia from adult human brain were found to be less eager to ingest fluorescent labeled Aß1-42 in vitro but to produce higher levels of the cytokines TNF- and IL-6 in response to Aß1-42 in combination with complement factor C1q and SAP (Familian, et al., 2007) (Fig.1). The CD68- immunoreactive, ramified and also the amoeboid, activated microglia seen associated with fibrillar Aß plaques in post mortem temporal cortex specimens from AD patients were COX-2 negative, CD14-dim (25% isolated microglia (~10 days after isolation) were positive by flow cytometry) but to some extent exhibiting Toll-like receptor 4 (TLR-4) immunopositive vesicles.
Increased TLR4 immunostaining can be detected in glial cells surrounding plaques in AD patients (Walter et al 2007). Although a fully functional receptor complex of TLR4, MD-2 and CD14 is required for full activation of microglia, TLR4 has been found to mediate mouse microglia and human monocyte activation by aggregated Aß, resulting in release of inflammatory products and in microglial neurotoxicity (Walter et al 2007).
Immune cells in the AD brain appear to exhibit a hybrid activation state that includes characteristics of classical (inflammatory) and alternative (repair and extracellular matrix remodeling) activation (Colton, et al., 2006). Repair processes mediated by alternative activation genes, however, can also lead to fibrosis and in AD brain possibly to activation of astrocytes with concomitant production of factors that enhance encapsulation of the fibrillar Aß deposits. This would, in turn, lead to a subsiding of the inflammatory response and to microglia returning to a resting state, a phenomenon observed in later Braak stages (Veerhuis, et al., 2005)
3.4 Role of macrophages
The lion share of Aβ clean-up is probably reserved for blood-derived macrophages, which are effective phagocytes able to surround and ingest even large chunks of Aβ when tested in vitro. However, only macrophages of control subjects are fully functional, whereas macrophages or monocytes of AD patients are poorly phagocytic for Aβ, which is retained on cell surface, and are susceptible to apoptosis (Avagyan, et al., 2009) (Zaghi, et al., 2009). Transcription of Toll-like receptors (TLR's) 1-10 is decreased in macrophages of AD patients compared to macrophages of control subjects (Fiala, et al., 2007).
Immunohistochemical examination of AD brains with Braak stages III to V showed increased numbers of macrophages in perivascular spaces and the neuropil compared to age-matched control subjects (Fiala, et al., 2002). In addition, large macrophages distinct from microglia by their large size were noted to migrate across the vessel wall into the neuropil and invade and partially clear neuritic plaques. These CD68-positive macrophages included COX-2-positive (up to 56%) and iNOS-positive (40%) subsets. In the experience of the authors, only perivascular macrophages were CD14-positive (Fiala, et al., 2001) (Veerhuis, unpublished data). In contrast, microglia in the parenchyma (even microglia in neuritic plaques) were, only occasionally, faintly CD14-immunoreactive (Veerhuis, unpublished data). In vitro, 80% of monocyte-derived macrophages isolated from blood of healthy donors were positive by flow cytometry (Veerhuis, unpublished data). Since AD patients have an increased percentage of CD14+/CD69+ monocytes in peripheral blood (Kusdra, et al., 2000), the expression of CD14 on macrophages in the brain may depend upon the stage of the disease as well as the process of monocyte immigration. Ramified microglia were on the periphery of neuritic plaques and appeared Aß-negative (Fiala, et al., 2002). Brain microglia, astrocytes and neurons secrete chemokines, which are responsible for attracting monocytes to migrate across the blood-brain barrier from microvessels into the neuropil (Fiala, et al., 2005).
3.5 Flow cytometric test of Aβ phagocytosis
Phagocytosis of Aβ can be tested using in vitro differentiated macrophages exposed to fluorescent Aβ or using a “tissue assay” with monocytes incubated on AD brain tissues in vitro for 2-4 days (monocytes are labeled using CellTracker (InVitrogen) or detected by immunofluorescent staining with anti-CD68) (Fiala, et al., 2007). Macrophages and monocytes of AD patients are generally poorly phagocytic for Aβ, which is retained on cell surface, whereas control subjects' macrophages robustly phagocytize Ab without undergoing apoptosis. In the AD brain, a subset of endogenous macrophages is phagocytic for oligomeric Ab and probably migrates to congophilic vessels, but these macrophages suffer apoptosis spilling oligomeric Ab into congophilic deposits (Zaghi, et al., 2009). In the tissue assay, monocytes of control subjects up load Ab from neurons and degrade it, whereas monocyte/ macrophages of AD patients suffer apoptosis.
Based on these considerations of the role of macrophages in clearance of Aβ, we have developed a flow cytometric assay of Aβ phagocytosis using peripheral blood monocytes. The assay was tested in a pilot study of 18 AD patients (mean age 77.4 years) and 14 control subjects, active professors and businessmen (mean age 74.2 years) (Avagyan, et al., 2009). The test was positive, i.e. showing defective phagocytosis < 400 MFI Units in 94% patients, whereas 0% active age-matched control subjects had defective phagocytosis. However, 75 % caregivers were positive in this assay. The caregivers are at risk of AD due to exposure to a high stress of care giving and lack of mental and physical exercise. Future prospective studies are necessary to determine the value of the test for predicting the risk of dementia, which was suggested in a small sample of MCI patients by 60% positive results. The test may be useful for follow-up of the immune status of patients with AD or risk of AD on various immunomodulating therapies, such as curcuminoids (Fiala, et al., 2007) or vitamin D3 recently shown to improve phagocytosis of Aβ in AD macrophages in vitro (Masoumi, et al., 2009).
4. Transcriptome of macrophages stressed by Aβ
To understand the molecular basis of AD patients' macrophage defects, we tested the transcriptome of human PBMC's stressed with Aβ using the Operon Human Genome Oligo Set Version 3.0 array. Thirty five genes were up regulated >2.2 fold in normal PBMC's stressed with Ab compared to AD PBMC's stressed with Aβ, including the critical gene, MGAT-III, which was up regulated 327 fold. The product of MGAT-III may have a critical role in phagocytosis since transfection of normal monocytes and macrophages by MGAT-III siRNA blocked Aβ phagocytosis. In addition, all TLR's were downregulated in PBMC's of AD patients during phagocytosis (Fiala, et al., 2007). In microglial cell lines and transgenic mice, individual TLR's have been investigated for their role in inflammation (TLR4), Aβ clearance (TLR2) and neuronal apoptosis. However, in AD patients' monocytes, no single gene defects but a web of transcriptional (Fiala, et al., 2007) and signaling (Fiala, unpublished observation) defects appear to lead to poor differentiation and maturation of macrophages, decreased phagocytosis and degradation, and increased inflammation.
5. Conclusions
Aβ/P- τ-based CSF tests and MR and PET imaging tests are reliable tests of Aβ pathology in the brain. Novel tests based on phagocytosis and/or inflammation may be useful in predicting future risk of AD, but the power of these tests will need to be examined in prospective studies. Positive prediction of the risk of AD in aged retired subjects is a daunting task. The collusion between the pathways of aging per se and those of AD renders a difficult comparison of AD patients to age-matched controls from general population (especially in stressed caregivers). However, a comparison of AD patients with active age-matched professors and businessmen by the flow cytometric test in our pilot study showed significant differences with a clear break-point between patients and controls. The flow cytometric test is based on Aβ pathways specific for AD and might have a high specificity for AD even in very old age.
Peripheral blood-derived macrophages from patients and control subjects have distinct properties in vitro and in vivo. AD macrophages show increased propensity to apoptosis and decreased ability of Aβ phagocytosis in vitro and in vivo, whereas control macrophages are fully functional. The underlying transcriptional pathologies of AD patients are complex and will need to be resolved by molecular techniques but already provide diagnostic biomarkers for AD and future risk of AD. These biomarkers may be useful for the follow-up of individual patients on various immunomodulating therapies.
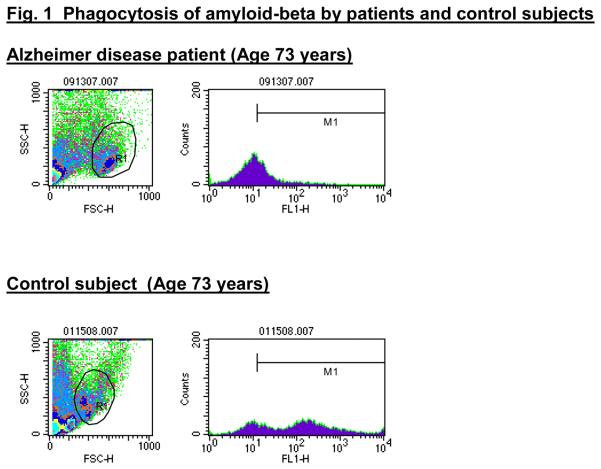
Figure 3. Flow cytometric test results in AD patients and control subjects.
PBMC's were incubated overnight with FAM-Aβ and, after staining with anti CD14-PE, were tested by flow cytometry as described (Avagyan, et al., 2009). Monocytes of control subject bind more FAM-Aβ compared to patients.
Acknowledgements
The development of a flow cytometric test has been supported by MPBiomedicals, LLC (M.F.). Glycan array hybridization was supported by a grant from National Institute of General Medical Sciences GM62116 to the Consortium for Functional Glycomics. 2D-DIGE electrophoresis was performed by the Functional Proteomics and Imaging Laboratory of the UCLA Biological Chemistry Department. Recent work on adult human glial cells discussed in this review was financially supported by Stichting Dioraphte (R.V.) and by the Internationale Stichting Alzheimer Onderzoek (ISAO; grant 06517 to R.V.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdi F, Quinn JF, Jankovic J, McIntosh M, Leverenz JB, Peskind E, Nixon R, Nutt J, Chung K, Zabetian C, Samii A, Lin M, Hattan S, Pan C, Wang Y, Jin J, Zhu D, Li GJ, Liu Y, Waichunas D, Montine TJ, Zhang J. Detection of biomarkers with a multiplex quantitative proteomic platform in cerebrospinal fluid of patients with neurodegenerative disorders. J Alzheimers Dis. 2006;9:293–348. doi: 10.3233/jad-2006-9309. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avagyan H, Goldenson B, Tse E, Masoumi A, Porter V, Wiedau-Pazos M, Sayre J, Ong R, Mahanian M, Koo P, Bae S, Micic M, Liu PT, Rosenthal MJ, Fiala M. Immune blood biomarkers of Alzheimer disease patients. J Neuroimmunol. 2009;210:67–72. doi: 10.1016/j.jneuroim.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Blennow K, Hampel H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 5.Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carro E, Spuch C, Trejo JL, Antequera D, Torres-Aleman I. Choroid plexus megalin is involved in neuroprotection by serum insulin-like growth factor I. J Neurosci. 2005;25:10884–10893. doi: 10.1523/JNEUROSCI.2909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–473. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- 8.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig-Schapiro R, Fagan AM, Holtzman DM. Biomarkers of Alzheimer's disease. Neurobiol Dis. 2008 doi: 10.1016/j.nbd.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Leon MJ, Mosconi L, Li J, De Santi S, Yao Y, Tsui WH, Pirraglia E, Rich K, Javier E, Brys M, Glodzik L, Switalski R, Saint Louis LA, Pratico D. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol. 2007;254:1666–1675. doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- 11.Deane R, Bell RD, Sagare A, Zlokovic BV. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eikelenboom P, Veerhuis R. The role of complement and activated microglia in the pathogenesis of Alzheimer's disease. Neurobiol Aging. 1996;17:673–680. doi: 10.1016/0197-4580(96)00108-x. [DOI] [PubMed] [Google Scholar]
- 13.Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 15.Familian A, Eikelenboom P, Veerhuis R. Minocycline does not affect amyloid beta phagocytosis by human microglial cells. Neurosci Lett. 2007;416:87–91. doi: 10.1016/j.neulet.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 16.Fiala M, Lin J, Ringman J, Kermani-Arab V, Tsao G, Patel A, Lossinsky AS, Graves MC, Gustavson A, Sayre J, Sofroni E, Suarez T, Chiappelli F, Bernard G. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. J Alzheimers Dis. 2005;7:221–232. doi: 10.3233/jad-2005-7304. discussion 255-262. [DOI] [PubMed] [Google Scholar]
- 17.Fiala M, Liu NQ, Reddy S, Graves MC. Macrophages infiltrate brain and express COX-2 and iNOS in Alzheimer's disease and AIDS. Alzheimer's Reports. 2001;4:1–7. [Google Scholar]
- 18.Fiala M, Liu PT, Espinosa-Jeffrey A, Rosenthal MJ, Bernard G, Ringman JM, Sayre J, Zhang L, Zaghi J, Dejbakhsh S, Chiang B, Hui J, Mahanian M, Baghaee A, Hong P, Cashman J. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer's disease patients are improved by bisdemethoxycurcumin. Proc Natl Acad Sci U S A. 2007;104:12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV. Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer's disease brain and damage the blood-brain barrier. Eur J Clin Invest. 2002;32:360–371. doi: 10.1046/j.1365-2362.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 20.Fox NC, Black RS, Gilman S, Rossor MN, Griffith SG, Jenkins L, Koller M. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 21.Hoozemans JJ, van Haastert ES, Veerhuis R, Arendt T, Scheper W, Eikelenboom P, Rozemuller AJ. Maximal COX-2 and ppRb expression in neurons occurs during early Braak stages prior to the maximal activation of astrocytes and microglia in Alzheimer's disease. J Neuroinflammation. 2005;2:27. doi: 10.1186/1742-2094-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Hye A, Lynham S, Thambisetty M, Causevic M, Campbell J, Byers HL, Hooper C, Rijsdijk F, Tabrizi SJ, Banner S, Shaw CE, Foy C, Poppe M, Archer N, Hamilton G, Powell J, Brown RG, Sham P, Ward M, Lovestone S. Proteome-based plasma biomarkers for Alzheimer's disease. Brain. 2006;129:3042–3050. doi: 10.1093/brain/awl279. [DOI] [PubMed] [Google Scholar]
- 24.Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, Albert MS. Use of structural magnetic resonance imaging to predict who will get Alzheimer's disease. Ann Neurol. 2000;47:430–439. [PubMed] [Google Scholar]
- 25.Kolstoe SE, Ridha BH, Bellotti V, Wang N, Robinson CV, Crutch SJ, Keir G, Kukkastenvehmas R, Gallimore JR, Hutchinson WL, Hawkins PN, Wood SP, Rossor MN, Pepys MB. Molecular dissection of Alzheimer's disease neuropathology by depletion of serum amyloid P component. Proc Natl Acad Sci U S A. 2009;106:7619–7623. doi: 10.1073/pnas.0902640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kusdra L, Rempel H, Yaffe K, Pulliam L. Elevation of CD69+ monocyte/macrophages in patients with Alzheimer's disease [In Process Citation] Immunobiology. 2000;202:26–33. doi: 10.1016/S0171-2985(00)80049-2. [DOI] [PubMed] [Google Scholar]
- 27.Masoumi A, Goldenson B, Ghirmai S, Avagyan H, Zaghi J, Abel K, Zheng X, Espinosa-Jeffrey A, Mahanian M, Liu PT, Hewison M, Mizwicki M, Cashman J, Fiala M. 1alpha,25-dihydroxyvitamin D3 Interacts with Curcuminoids to Stimulate Amyloid-beta Clearance by Macrophages of Alzheimer's Disease Patients. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2009-1080. [DOI] [PubMed] [Google Scholar]
- 28.Morelli L, Bulloj A, Leal MC, Castano EM. Amyloid beta degradation: a challenging task for brain peptidases. Subcell Biochem. 2005;38:129–145. doi: 10.1007/0-387-23226-5_6. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HM, Veerhuis R, Holmqvist B, Janciauskiene S. Binding and uptake of A beta1-42 by primary human astrocytes in vitro. Glia. 2009;57:978–988. doi: 10.1002/glia.20822. [DOI] [PubMed] [Google Scholar]
- 30.Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 31.Riley KP, Snowdon DA, Desrosiers MF, Markesbery WR. Early life linguistic ability, late life cognitive function, and neuropathology: findings from the Nun Study. Neurobiol Aging. 2005;26:341–347. doi: 10.1016/j.neurobiolaging.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Rota E, Bellone G, Rocca P, Bergamasco B, Emanuelli G, Ferrero P. Increased intrathecal TGF-beta1, but not IL-12, IFN-gamma and IL-10 levels in Alzheimer's disease patients. Neurol Sci. 2006;27:33–39. doi: 10.1007/s10072-006-0562-6. [DOI] [PubMed] [Google Scholar]
- 33.Schoonenboom NS, van der Flier WM, Blankenstein MA, Bouwman FH, Van Kamp GJ, Barkhof F, Scheltens P. CSF and MRI markers independently contribute to the diagnosis of Alzheimer's disease. Neurobiol Aging. 2008;29:669–675. doi: 10.1016/j.neurobiolaging.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA, Jonker C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.01.014. doi:10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Simonsen AH, McGuire J, Hansson O, Zetterberg H, Podust VN, Davies HA, Waldemar G, Minthon L, Blennow K. Novel panel of cerebrospinal fluid biomarkers for the prediction of progression to Alzheimer dementia in patients with mild cognitive impairment. Arch Neurol. 2007;64:366–370. doi: 10.1001/archneur.64.3.366. [DOI] [PubMed] [Google Scholar]
- 36.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 37.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veerhuis R, Boshuizen RS, Familian A. Amyloid associated proteins in Alzheimer's and prion disease. Curr Drug Targets CNS Neurol Disord. 2005;4:235–248. doi: 10.2174/1568007054038184. [DOI] [PubMed] [Google Scholar]
- 39.Verwey NA, Schuitemaker A, van der Flier WM, Mulder SD, Mulder C, Hack CE, Scheltens P, Blankenstein MA, Veerhuis R. Serum amyloid p component as a biomarker in mild cognitive impairment and Alzheimer's disease. Dement Geriatr Cogn Disord. 2008;26:522–527. doi: 10.1159/000178756. [DOI] [PubMed] [Google Scholar]
- 40.Wilhelmus MM, Otte-Holler I, van Triel JJ, Veerhuis R, Maat-Schieman ML, Bu G, de Waal RM, Verbeek MM. Lipoprotein receptor-related protein-1 mediates amyloid-beta-mediated cell death of cerebrovascular cells. Am J Pathol. 2007;171:1989–1999. doi: 10.2353/ajpath.2007.070050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaghi J, Goldenson B, Inayathullah M, Lossinsky AS, Masoumi A, Avagyan H, Mahanian M, Bernas M, Weinand M, Rosenthal MJ, Espinosa-Jeffrey A, de Vellis J, Teplow DB, Fiala M. Alzheimer disease macrophages shuttle amyloid-beta from neurons to vessels, contributing to amyloid angiopathy. Acta Neuropathol. 2009;117:111–124. doi: 10.1007/s00401-008-0481-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zetterberg H, Ruetschi U, Portelius E, Brinkmalm G, Andreasson U, Blennow K, Brinkmalm A. Clinical proteomics in neurodegenerative disorders. Acta Neurol Scand. 2008;118:1–11. doi: 10.1111/j.1600-0404.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhao L, Lin S, Bales KR, Gelfanova V, Koger D, Delong C, Hale J, Liu F, Hunter JM, Paul SM. Macrophage-mediated degradation of beta-amyloid via an apolipoprotein E isoform-dependent mechanism. J Neurosci. 2009;29:3603–3612. doi: 10.1523/JNEUROSCI.5302-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]