Abstract
A 2-month-old infant who had undergone orthotopic liver transplantation at the age of2 weeks for carbamoyl phosphate synthetase deficiency developed infection of the CNS due to Aspergillus fumigatus. The patient was successfully treated with administration of a combination of antifungal agents (including intraventricular amphotericin B), drainage of the parletallobe abscess, and cessation of immunosuppression. An intraventricular catheter was used both to obtain ventricular fluid for microbiologic testing and to deliver amphotericin B during nearly 4 months of treatment. We review literature on aspergillosis in solid-organ transplant recipients, especially those in whom the disease involves the CNS, and discuss in particular clinical presentation, diagnosis, treatment, and outcome.
Superficial and systemic fungal infections relatively frequently occur after solid organ transplantation. Infections due to Aspergillus species, which are second in frequency only to infections caused by Candida species, account for a significant proportion of these infections. The outcome of infections due to Aspergillus species in solid-organ transplant recipients has been poor. Various clinical patterns of illness have been identified as prognostic markers; involvement of the CNS is usually fatal [1]. We report herein a favorable outcome in the case of a 6-month-old liver transplant recipient who developed aspergillosis of the CNS.
Case Report
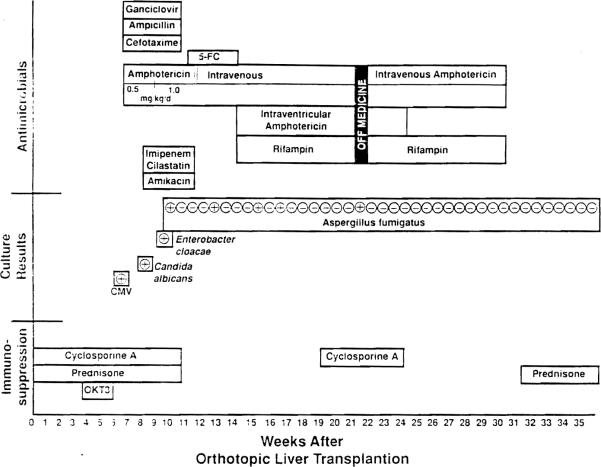
The patient was referred to Children's Hospital of Pittsburgh at 14 days of age; he had been diagnosed as having hyper-ammonemia secondary to carbamoyl phosphate synthetase deficiency and underwent orthotopic liver transplantation the same day. His hospital course is summarized in figure 1. Initial immunosuppressive therapy consisted of administration of cyclosporine and prednisone. His early postoperative course was complicated by an episode of steroid-resistant rejection, which subsequently resolved after treatment with OKT3 (monoclonal anti-CD3). On postoperative day 52 he developed fever, a petechial rash, and shock, and respiratory arrest subsequently occurred. Ampicillin, cefotaxime, and amphotericin B (the latter at a dosage of 0.5 mg[kg·d)) were administered empirically while the results of cultures were pending. After a rectal biopsy revealed evidence of cytomegalovirus (CMV), he was treated with ganciclovir for 21 days and showed gradual improvement. The dosage of amphotericin B was increased to 1.0 mg/(kg·d) when Candida albicans was isolated from his blood and urine on day 14 of treatment with ganciclovir. He received this increased dosage of amphotericin B for 14 days. During his convalescence from these infections, the patient experienced a generalized seizure that prompted the obtainment of a computed tomogram (CT), which showed a hemorrhage associated with hydrocephalus in the right parietal lobe. No specific explanation for the presence of the hemorrhage was identified at that time.
Figure 1.
The immunosuppressive and antimicrobial therapy administered throughout hospitalization and the results of cultures performed during the weeks after transplantation are outlined. 5-FC = 5-fluorocytosine.
On postoperative day 77 the child was diagnosed as having pneumonia and bacteremia due to Enterobacter cloacae. Therapy with imipenem-cilastatin in combination with amikacin was initiated, and administration of amphotericin Band ganciclovir was continued. Five days later he had a focal seizure; a lumbar puncture revealed 558 white blood cells (76% neutrophils, 7% lymphocytes, and 17% monocytes) and 6 red blood cells. In the eSF, the level of protein was 188 mg/dL, and that of glucose was <20 mg/dL. A second CT showed a 2-cm enhancing ring lesion in the right parietal lobe, at the site of the previously identified hemorrhage. Aspiration of the lesion was performed on postoperative day 84; a drain was left in situ. Histologic examination of the contents of the abscess cavity revealed branching hyphae that were consistent with the appearance of fungi, organisms which on culture were confirmed to be of the species Aspergillus fumigatus.
Administration of amphotericin B, which had been discontinued the previous day, was resumed at a dosage of 1 mg/(kg·d), and 5-fiuorocytosine (25 mg/kg every 6 hours) was added to the regimen. The drain was removed from the abscess cavity 2 days later. Maintenance therapy with cyclosporine and prednisone was discontinued. Intermittent iv boluses of steroid were utilized when rejection was biochemically evident. An intraventricular catheter was placed for worsening hydrocephalus on the fourteenth day of therapy with a combination of antifungal therapy. A culture of a specimen taken from the catheter 4 days later was positive for Aspergillus organisms. Daily intraventricular administration of amphotericin B, beginning at a total dose of 0.025 mg/d and increasing to a maximum total dose of 0.2 mg/d (0.05 mg/[kg·d]) over 5 days, was initiated, and rifampin administered orally at a single, daily dose of 20 mg/kg was added to the treatment regimen. Administration of the 5-fluorocytosine was discontinued.
He continued to receive this combination of iv and intraventricular amphotericin B, in addition to oral rifampin, for the next 48 days; cultures of the CSF were repeatedly negative during this interval. Antifungal therapy was discontinued, and specimens for surveillance cultures were obtained daily from his intraventricular drain. Cultures of specimens obtained 4 and 8 days after discontinuation of therapy were again positive for A. fumigatus; therapy with iv amphotericin B, intraventricular amphotericin B, and oral rifampin was resumed. A third CT revealed hydrocephalus but no evidence of new or residual abscess cavities. The intraventricular drain was replaced with an extraventricular drain 10 days later. A culture of the catheter tip was sterile. Therapy with intraventricular amphotericin B was continued for an additional 14 days, while iv amphotericin Band rifampin were administered for an additional 46 days.
The infant received iv amphotericin B for 111 days, intraventricular amphotericin B for 58 days, rifampin for 90 days, and 5-fluorocytosine for 19 days. Despite surveillance culturing of specimens from the extraventricular drain, no more cultures of the CSF yielded pathogens during the next 6 months. The patient began receiving low doses of prednisone several weeks after cessation of his antifungal therapy. Subsequently, his extraventricular drain was replaced with a ventricularperitoneal shunt as treatment for residual hydrocephalus. Therapy with cyc1osporine was restarted 6 months after the completion of his antifungal therapy. He remains asymptomatic and has excellent liver function, and no recurrent aspergillosis has become evident. Serial evaluations have revealed evidence of slow developmental progress; the delay in motor development has been more severe than that in cognitive development. At 20 months of age, he is functioning at the developmentallevel for ages 6–9 months. It is important to note that the neurologic insult in this infant was probably multifactorial; evidence of significant impairment had been recognized before transplantation. We believe that further insult occurred during the episode of shock associated with the CMV infection and during infection due to an Aspergillus species.
Discussion
Cases of invasive aspergillosis have been reported after transplantation of the liver [2–6], kidney [1, 7–11], and heart [12–15]. Aspergillosis was diagnosed in 9% of adult liver transplant recipients who received immunosuppressive therapy consisting of administration of azathioprine, prednisone, and antilymphocyte globulin [2]. Similarly, 6% of adult liver transplant recipients treated with cyclosporine and prednisone developed aspergillosis [3, 4]. All patients with aspergillosis from those three series of liver transplantations [2–4] died, as did an additional five patients from two smaller series [5, 6]. In 11 of 24 adult liver transplant recipients with aspergillosis, involvement of the CNS by the disease was identified during the course of infection or at autopsy. In contrast, only 2.7% of renal transplant recipients developed aspergillosis [1, 7–11]; in 26% of these patients, the disease involved the CNS [1, 8–10]. Weiland et al. [11 reported a 90% survival rate among renal transplant recipients with primary pulmonary aspergillosis who were treated with a combination of antifungal agents, resection. and. in some cases, cessation of immunosuppression; however, only one of 12 reported renal transplant recipients in whom involvement of the CNS by aspergillosis was documented [1, 7–11] has survived. Similar survival rates among heart transplant recipients and an occasional survivor of pulmonary disease have been reported, but none of such patients in whom the aspergillosis involved the CNS survived [12–15].
The diagnosis of aspergillosis of the CNS was made in our patient after he developed focal seizures. The acute development of focal neurologic deficits, such as focal seizure or hemiparesis, is common in patients with aspergillosis of the CNS [16]. These focal findings are thought to be the clinical expression of the invasion of blood vessels by Aspergillus organisms, which subsequently causes thrombosis and hemorrhagic infarction. An acute. unexplained infarct may be the earliest clue to the presence of aspergillosis of the CNS in a high-risk patient. The areas of infarction typically develop into either single or multiple abscesses involving the cerebrum, the cerebellum, and, rarely, the spinal cord. A CT is therefore a very important diagnostic tool; it can reveal evidence of both early and late aspergillosis of the CNS. Meningeal signs appear to be quite rare, although focal meningitis has been observed, especially in association with initial involvement of the sphenoid sinuses by aspergillosis and the subsequent contiguous spread to the CNS [16, 17]. Analysis of the spinal fluid may reveal complete normality or pleocytosis with increased protein. Uncommonly, CSF obtained by lumbar puncture has yielded an Aspergillus species when cultured.
Factors suspected to favor the development of invasive aspergillosis in renal transplant recipients include exposure to antilymphocyte globulin, history of bacterial sepsis or pneumonia (which perhaps exposes the patients to prolonged antibiotic usage), and CMV infection [1]. The characteristics of 50 % of patients reported by Weiland et al. [1] included at least two of these factors. Our patient, for whom all three of these risk factors were noted, might have been recognized as being at high-risk for aspergillosis.
Survival after aspergillosis of the CNS has been infrequent. Table 1 shows the characteristics of reported survivors of this disease. Five of the 11 survivors had no history of an underlying systemic condition predisposing to invasive aspergillosis. However, two of these patients had sinusitis. Aspergillus species are know to occasionally cause sinusitis and to subsequently involve the CNS in immunocompetent patients. The absence of any underlying immunologic defect may have been an important factor in the recovery of the aforementioned five patients. Seven of the survivors had a brain abscess due to an Aspergillus species. The abscesses were located in the frontal lobe and temporal lobe in two patients each and in the occipital lobe, parietal lobe, and an unspecified site in one patient each. In four of the seven patients, the abscesses were completely resected; six of the seven were treated with iv amphotericin B, which was used in combination with 5-fluorocytosine in three patients.
Table 1.
Characteristics of survivors of aspergillosis of the CNS.
| [Reference] | Age of patient (y) | Underlying condition | Aspergilius species isolated | Site and/or type of infection | Type of Treatment |
|
|---|---|---|---|---|---|---|
| Surgical | Medical (duration) | |||||
| [17] | 47 | None | Not specified | Sphenoid sinus; meningitis | None | iv AmB (NA) |
| [18] | 12 | ALL | A. fumigatus | Pulmonary: right occipital brain abscess | Resection of abscess | iv AmB and 5-FC (53 d). intracavitary AmB (4 d) |
| [7] | 24 | Renal transplantation | A. fumigatus | Pulmonary: endopthalmitis and brain abscess | Enucleation | iv AmB (1 y). intraocular AmB (NA) |
| [19] | 34 | Hx iv drug abuse | A. oryzae | Meningitis | None | iv AmB and 5-FC (3 mo), intraventricular AmB (3 mo) |
| [20] | 40 | None | A. versicolor | Left frontal brain abscess | Resection of abscess | None |
| [21] | 62 | None | A. fumigatus | Lung and T2 epidural abscesses | Resection of abscesses | iv AmB (15 d) |
| [22] | 27 | Sarcoid | A. fumigatus | Meningitis | None | 5-FC (3 mo) |
| [23] | 38 | Alcoholism | A. fumigatus | Right temporal brain abscess | Resection of abscess | iv AmB (NA) |
| [24] | 48 | None | Not specified | Maxillary sinus; right temporal brain abscess | None | iv AmB and 5-FC (NA) |
| [25] | 7 | None | A. fumigatus | Skin and ribs: lung and right frontal brain abscesses | Resection of ribs and abscesses | iv AmB (35 d) |
| [PR] | 0.2 | Liver transplantation | A. fumigatus | Right parietal brain abscess | Drainage of abscess | iv AmB (111 d), intraventricular AmB (58 d), 5-FC (19 d), rifampin (90 d) |
NOTE. AmB = amphotericin B: NA = not available; ALL = acute lymphoblastic leukemia; 5-FC = 5-fluorocytosine; Hx = history; PR = present report.
Of particular interest is the case reported by Burton et a1. [7] of the lone survivor of aspergillosis of the CNS after a renal transplantation. It is noteworthy that the renal graft also remained functional. Treatment in that case consisted of initial reduction of immunosuppression (the administration of azathioprine was discontinued, and that of prednisone was reduced to 15 mg/d) and initiation of a 12-month course of therapy with iv amphotericin B; no surgical excision was performed. During the last 9 months of treatment, therapy with azathioprine was restarted at a dosage of 125 mg/d, and that with prednisone was continued at a dosage of 15 mg/d. Amphotericin was administered at increasing intervals, eventually as infrequently as one dose every 2 weeks.
Whereas seven developed a brain abscess, three of the survivors described in table 1 developed meningitis due to an Aspergillus species. Although meningitis caused by an Aspergillus species is uncommon, it has been suggested that the outcome of patients with meningitis is superior to the outcome of patients with brain abscesses [17]. The remaining patient of the 11 survivors developed a spinal epidural abscess as a direct extension of a primary pulmonary lesion.
Chemotherapy with a combination of antifungal agents was utilized in our case as well as for the treatment of three of the survivors identified in the literature review. Initial support for the use of a combination of chemotherapeutic agents for the treatment of fungi originated from an in vitro evaluation of the synergistic action of amphotericin B and 5-fluorocytosine, which showed a tenfold increase in the killing activity when the efficacy of the combination of both drugs was compared to that of either drug alone [26]. Further evaluation of the in vitro activity of amphotericin B with 5-fluorocytosine and with rifampin against isolates of both A. fumigatus and Aspergillus flavus also demonstrated a synergy in inhibition but not killing [27]. It is unfortunate that results of antifungal susceptibility testing may be erratic [28], thereby raising doubts about the significance of the previously mentioned findings in regard to efficacy. However, an in vivo evaluation with use of a murine model of aspergillosis also demonstrated a significant improvement in survival rate when amphotericin B was used in combination with either 5-fluorocytosine or rifampin, in comparison with the survival rate when any of the drugs was used alone [29]. Numerous reports of successful treatments with use of a combination of chemotherapeutic agents have been published [30–34], although a recent review urged caution in the use of these combinations because of the lack of pertinent clinical trials [35]. Given the relative infrequency of aspergillosis of the CNS in transplant recipients and the uniformly poor outcome in such cases, utilization of a combination of antifungal agents for chemotherapy seems reasonable.
Concerns about the poor outcome of fungal infections of the CNS and the toxicity of amphotericin B have led to the search for alternative antifungal agents. The azole class of antifungal agents offers two possible alternatives. Itraconazole, a nitrogen-substituted triazole, has been found to be active in vitro against several species of Aspergillus [36, 37]. In vivo evaluation with use of a murine model revealed greater activity than that achieved by ketoconazole, but no comparison was made with the activity of amphotericin B [37, 38]. It is unfortunate that only a limited number of case repons have described the use of itraconazole for the treatment of aspergillosis in humans, with variable results [39–41]. Fluconazole is a newer triazole agent that has been extremely promising in the treatment of systemic fungal infection [42]. However, it was found to be less effective than amphotericin B in the treatment of experimental aspergillosis in a rabbit model [43], and experience in humans with invasive aspergillosis is lacking. Accordingly, administration of neither fluconazole nor itraconazole can be recommended as standard therapy for solid-organ transplant recipients with invasive aspergillosis.
To the best of our knowledge, our case represents the second reported cure of a solid-organ transplant recipient with aspergillosis of the CNS and the first reported survival of a liver transplant recipient with this disease. Treatment strategies included discontinuation of immunosuppression, surgical drainage of the abscess, and administration of a combination of antifungal agents (including intraventricular administration). The fact that the antifungal therapy was begun early (for other reasons) for this child may have contributed to the successful outcome of the infection. Treatment was continued for a total duration of 111 days because cultures were intermittently positive for pathogens during the first 2 months of therapy. The use of surveillance cultures of specimens from an intraventricular drain allowed follow-up of the patient for recurrences during his treatment and convalescence. His continued survival and slow but steady developmental progress 2 years after his infection support the fact that the aspergillus infection has been cured.
Acknowledgment
The authors thank Dr. Richard Simmons for his thoughtful review of the manuscript of this article.
References
- 1.Weiland D, Ferguson RM, Peterson PK, Snover DC, Simmons RL, Najarian JS. Aspergillosis in 25 renal transplant patients: epidemiology, clinical presentation. diagnosis and management. Ann Surg. 1983;198:622–9. doi: 10.1097/00000658-198311000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröter GPJ, Hoelscher M, Putnam CW, Porter KA, Starzl TE. Fungus infections after liver transplantation. Ann Surg. 1977;86:115–22. doi: 10.1097/00000658-197707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajszczuk CP, Dummer JS, Ho M, Van Thiel DH, Starzl TE, Iwatsuki S, Shaw B., Jr Fungal infections in liver transplant recipients. Transplantation. 1985;40:347–53. doi: 10.1097/00007890-198510000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusne S, Dummer JS, Singh N, Iwatsuki S, Makowka L, Esquivel C, Tzakis AG, Starzl TE, Ho M. Infections after liver transplantation: an analysis of 101 consecutive cases. Medicine. 1988;67:132–43. doi: 10.1097/00005792-198803000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brems JJ, Hiatt JR, Klein AS, Hart J, El-Khoury G, Winston D, Millis JM, Busuttil RW. Disseminated aspergillosis complicating orthotopic liver transplantation for fulminant hepatic failure refractory to corticosteroid therapy. Transplantation. 1988;46:479–81. doi: 10.1097/00007890-198809000-00038. [DOI] [PubMed] [Google Scholar]
- 6.Lie TS, Höfer M, Höhnke C, Krizek L, Kühnen E, Iwantscheff A, Köster O, Overlack A, Vogel J, Rommelsheim K. Aspergillosis after liver transplantation as a hospital infection. Dtsch Med Wochenschr. 1987;112:297–301. doi: 10.1055/s-2008-1068046. [DOI] [PubMed] [Google Scholar]
- 7.Burton JR, Zachery JB, Bessin R, Rathbun HK, Greenough WE, III, Sterioff S, Wright JR, Slavin RE, Williams GM. Aspergillosis in four renal transplant recipients. Diagnosis and effective treatment with amphotericin B. Ann Intern Med. 1972;77:383–8. doi: 10.7326/0003-4819-77-3-383. [DOI] [PubMed] [Google Scholar]
- 8.Gallis HA, Berman RA, Cate TR, Hamilton JD, Gunnells JC, Stickel DL. Fungal infection following renal transplantation. Arch Intern Med. 1975;135:1163–72. [PubMed] [Google Scholar]
- 9.Bach Me, Sahyoun A, Adler JL, Schlesinger RM, Breman J, Madras P, Pèng FK, Monaco AP. Influence of rejection therapy of fungal and nocardial infections in renal-transplant recipients. Lancet. 1973;1:180–4. doi: 10.1016/s0140-6736(73)90007-x. [DOI] [PubMed] [Google Scholar]
- 10.Eickhoff TC. Infectious complications in renal transplant recipients. Transplant Proc. 1973;5:1233–8. [PubMed] [Google Scholar]
- 11.Mills SA, Seigler HF, Wolfe WG. The incidence and management of pulmonary mycosis in renal allograft patients. Ann Surg. 1975;182:617–26. doi: 10.1097/00000658-197511000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stinson EB, Bieber CP, Griepp RB, Clark DA, Shumway NE, Remington JS. Infectious complications after cardiac transplantation in man. Ann Intern Med. 1971;74:22–36. doi: 10.7326/0003-4819-74-1-22. [DOI] [PubMed] [Google Scholar]
- 13.Brooks RG, Hofflin JM, Jamieson SW, Stinson EB, Remington JS. Infectious complications in heart-lung recipients. Am J Med. 1985;79:412–22. doi: 10.1016/0002-9343(85)90027-0. [DOI] [PubMed] [Google Scholar]
- 14.Montero CG, Martinez AJ. Neuropathology of heart transplantation: 23 cases. Neurology. 1986;36:1149–54. doi: 10.1212/wnl.36.9.1149. [DOI] [PubMed] [Google Scholar]
- 15.Green M, Wald ER, Fricker FJ, Griffith BP, Trento A. Infections in pediatric orthotopic heart transplant recipients. Pediatr Infect Dis J. 1989;8:87–93. [PubMed] [Google Scholar]
- 16.Walsh TJ, Hier DB, Caplan LR. Fungal infections of the central nervous system: comparative analysis of risk factors and clinical signs in 57 patients. Neurolog. 1985;35:1654–7. doi: 10.1212/wnl.35.11.1654. [DOI] [PubMed] [Google Scholar]
- 17.Beal MF, O'Carroll CP, Kleinman GM, Grossman RI. Aspergillosis of the nervous system. Neurology. 1982;32:473–9. doi: 10.1212/wnl.32.5.473. [DOI] [PubMed] [Google Scholar]
- 18.Henze G, Aldenhoff P, Stephani U, Grosse G, Kazner E, Staib F. Successful treatment of pulmonary and cerebral aspergillosis in an immunosuppressed child. Eur J Pediatr. 1982;138:263–5. doi: 10.1007/BF00441214. [DOI] [PubMed] [Google Scholar]
- 19.Gordon MA, Holzman RS, Senter H, Lapa EW, Kupersmith MJ. Aspergillus oryzae meningitis. JAMA. 1976;235:2122–3. [PubMed] [Google Scholar]
- 20.Venugopal PV, Venugopal TV, Thiruneelakantan K, Subramanian S, Shetty BMY. Cerebral aspergillosis: report of two cases. Sabouraudia. 1977;15:225–30. doi: 10.1080/00362177785380041. [DOI] [PubMed] [Google Scholar]
- 21.Seres JL, Hirohisa O, Benner EJ. Aspergillosis presenting as spinal cord compression: case report. J Neurosurg. 1972;36:221–4. doi: 10.3171/jns.1972.36.2.0221. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson OW, Israel HL. 5-Fluorocytosine treatment of meningeal and pulmonary aspergillosis. Am J Med. 1973;55:496–504. [Google Scholar]
- 23.Klein HJ, Richter HP, Schachenmayr W. Intracerebral Aspergillus abscess: case report. Neurosurgery. 1983;13:306–9. doi: 10.1227/00006123-198309000-00016. [DOI] [PubMed] [Google Scholar]
- 24.Mohandas S, Ahuja GK, Sood VP, Virmani V. Aspergillosis of the central nervous system. J Neurol Sci. 1978;38:229–33. doi: 10.1016/0022-510x(78)90069-2. [DOI] [PubMed] [Google Scholar]
- 25.Conen PE, Walker GR, Turner JA, Field P. Invasive primary aspergillosis of the lung with cerebral metastasis and complete recovery. Dis Chest. 1962;42:88–94. doi: 10.1378/chest.42.1.88. [DOI] [PubMed] [Google Scholar]
- 26.Medoff G, Comfort M, Kobayashi GS. Synergistic action of amphotericin B and 5-fluorocytosine against yeast-like organisms. Proc Soc Exp Biol Med. 1971;138:571–4. doi: 10.3181/00379727-138-35943. [DOI] [PubMed] [Google Scholar]
- 27.Kithara M, Seth VK, Medoff G, Kobayashi GS. Activity of amphotericin B, 5-fluorocytosine and rifampin against six clinical isolates of Aspergillus. Antimicrob Agents Chemother. 1976;9:915–9. doi: 10.1128/aac.9.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calhoun DL, Roberts GD, Galgiani JN, Bennett JE, Feingold DS, Jorgensen J, Kobayashi GS, Shadomy S. Results of a survey of antifungal susceptibility tests in the United States and interlaboratory comparison of broth dilution testing of flucytosine and amphotericin B. J Clin Microbiol. 1986;23:298–301. doi: 10.1128/jcm.23.2.298-301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arroyo J, Medoff G, Kobayashi GS. Therapy of murine aspergillosis with amphotericin B in combination with rifampin or 5-fluorocytosine. Antimicrob Agents Chemother. 1977;11:21–5. doi: 10.1128/aac.11.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Codish SD, Tobias JS, Hannigan M. Combined amphotericin B-flucytosine therapy in Aspergillus pneumonia. JAMA. 1979;241:2418–9. [PubMed] [Google Scholar]
- 31.Sekhar LN, Dujovny M, Rao GR. Carotid-cavernous sinus thrombosis caused by Aspergillus fumigatus: case report. J Neurosurg. 1980;52:120–5. doi: 10.3171/jns.1980.52.1.0120. [DOI] [PubMed] [Google Scholar]
- 32.Carrizosa J, Levison ME, Lawrence T, Kaye D. Cure of Aspergillus usrus endocarditis of a prosthetic valve. Arch Intern Med. 1974;133:486–90. [PubMed] [Google Scholar]
- 33.Ribner B, Keusch GT, Hanna BA, Perloff M. Combination amphotericin B–rifampin therapy for pulmonary aspergillosis in a leukemic patient. Chest. 1976;70:681–3. doi: 10.1378/chest.70.5.681. [DOI] [PubMed] [Google Scholar]
- 34.Beyt BE, Jr, Cannon RO, III, Tuteur PG. Successful treatment of invasive pulmonary aspergillosis in the immunocomprontised host. South Med J. 1978;71:1164–6. doi: 10.1097/00007611-197809000-00034. [DOI] [PubMed] [Google Scholar]
- 35.Drutz DJ. Newer antifungal agents and their use, including an update on amphotericin B and flucytosine. Curr Clin Top Infect Dis. 1982;3:97–135. [Google Scholar]
- 36.Espinel-Ingroff A, Shadomy S, Gebhart RJ. In vitro studies with R 51,211 (itraconazole) Antimicrob Agents Chemother. 1984;26:5–9. doi: 10.1128/aac.26.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Cutsem J, Van Gerven F, Van de Ven MA, Borgers M, Janssen PAJ. Itraconazole, a new triazole that is orally active in aspergillosis. Antimicrob Agents Chemother. 1984;26:527–34. doi: 10.1128/aac.26.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Cutsem J, Van Gerven F, Janssen PAJ. Activity of orally, topically, and parenterally administered itraconazole in the treatment of superficial and deep mycoses: animal models. Rev Infect Dis. 1987;9(Suppl 1):S15–32. doi: 10.1093/clinids/9.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 39.Van Cauteren H, Heykants J, De Coster R, Cauwenbergh G. Itraconazole: pharmacologic studies in animals and humans. Rev Infect Dis. 1987;9(Suppl 1):S43–50. doi: 10.1093/clinids/9.supplement_1.s43. [DOI] [PubMed] [Google Scholar]
- 40.Dupont B, Drouhet E. Early experience with itraconazole in vitro and in patients: pharmacokinetic studies and clinical results. Rev Infect Dis. 1987;9(Suppl 1):S71–6. doi: 10.1093/clinids/9.supplement_1.s71. [DOI] [PubMed] [Google Scholar]
- 41.Ganer A, Arathoon E, Stevens DA. Initial experience in therapy for progressive mycoses with itraconazole, the first clinically studied triazole. Rev Infect Dis. 1987;9(Suppl 1):S77–86. doi: 10.1093/clinids/9.supplement_1.s77. [DOI] [PubMed] [Google Scholar]
- 42.Galgiani JN. Susceptibility of Candida albicans and other yeasts to fluconazole: relation between in vitro and in vivo studies. Rev Infect Dis. 1990;12(Suppl 3):S272–5. doi: 10.1093/clinids/12.supplement_3.s272. [DOI] [PubMed] [Google Scholar]
- 43.Patterson TF, Miniter P, Andriole VT. Efficacy of fluconazoie in experimental invasive aspergillosis. Rev Infect Dis. 1990;12(Suppl 3):S281–5. doi: 10.1093/clinids/12.supplement_3.s281. [DOI] [PubMed] [Google Scholar]