Abstract
Prostate cancer (PCa) is a heterogeneous disease with regard to molecular alterations and clinical course. The investigation of genetic alterations associated with PCa pathogenesis is highly challenging. Genome-wide analyses and epidemiological studies have identified only a handful of candidate genes possibly associated with hereditary or sporadic PCa. Cancer cells often rely for survival on common biochemical pathways such as enhanced anaerobic glycolysis and lipogenesis. The lipogenic enzyme fatty acid synthase seems to play a crucial part in PCa by conferring growth and survival advantages to cancer cells. We summarize the current understanding of the molecular events in PCa, and highlight the importance of altered lipid metabolism in the development and progression of prostate malignancy.
Keywords: Prostate cancer, risk stratification, genetic mutations, lipid metabolism, FASN, therapeutic approaches
The clinical problem
Epidemiology
Among the male population of the USA, prostate cancer is the most prevalent non-cutaneous neoplasm (1 in 6 men will be diagnosed with PCa cancer during their lifetime) and a leading cause of cancer-related death, second only to lung and colon cancer.1 Its frequency varies widely, with the highest rates reported in Western countries, and the lowest rates in Asia.2 Multiple factors contribute to the high incidence and prevalence of PCa. Risk factors include age, family history and race, but also a diet high in fat, and obesity. Higher body mass index (BMI) and adult weight gain increase the risk of dying from prostate cancer.3
Stage migration and risk stratification
The early 1990s saw a dramatic increase in the incidence of prostate cancer with the introduction of the prostate-specific antigen (PSA) blood screening test. As screening became more widespread in the USA, there was a significant stage migration toward detection of prostate cancer at earlier stages. For example, results from the American Surveillance, Epidemiology, and End Results (SEER) database showed that the percentage of prostate cancer patients with metastatic disease at the time of diagnosis dropped from 16% in the 1980s to 4% in 2003.4 In 2004, results from the Prostate Cancer Prevention trial showed that even men with PSA less than the traditional cut-off of 4.0 ng/ml had a substantial risk of harbouring prostate cancer (15.2% for all patients with PSA <4.0; 26.9% for patients with PSA 3.1–4.0), which led many clinicians to use variations in PSA measurement as an indication to biopsy of men with PSA<4.0, thereby further augmenting the continued stage migration effect .5
Among men with clinically localized prostate cancer, treatment decisions of whether to pursue a local-only therapy (e.g. surgery, radiation, brachytherapy) versus a more aggressive combined method therapy (radiation plus hormones) is typically made based on the risk of recurrence. The commonest stratification system was developed by D’Amico et al and uses clinical T-stage, PSA, and Gleason score to divide patients into low, intermediate, and high risk.6 Due to stage migration, the proportion of people with low-risk disease increased from 30% in 1990 to 45% in 2000, and it is likely that low-risk disease represents most diagnoses of prostate cancer.7
For patients with low-risk disease, it is likely that a large proportion do not need treatment. The risk stratification system is not good enough to discern, based on clinical and pathological parameters, which low-risk patients have disease that will never progress from those who will die from their disease if left untreated (even though these represent the minority of low-risk patients). The net result is that many patients who do not need treatment may choose to be treated and suffer side effects unnecessarily, while others who need treatment may forgo therapy and miss a crucial opportunity to be cured. There is therefore a significant need for an improved risk-stratification system that incorporates novel biomarkers from relevant pathways to better determine the risk of recurrence and allow patients to make more informed decisions about treatment. Patients who recur and then develop metastases may be treated with androgen deprivation therapy for a while, but nearly all will eventually develop androgen-independent disease if they do not die of other causes. Median survival in the androgen-independent state is only ~18 months, and few treatment options are available which have shown benefit at this stage besides docetaxel, which extends life expectancy by only ~2–3 months over the prior standard of care.8 There is an equally urgent need to investigate relevant pathways in prostate cancer for new biomarkers and risk stratification, and for the development of novel agents that can prolong survival.
This review discusses genetic markers and molecular pathways in prostate cancer with a particular emphasis on fatty acid synthase (FASN) and its possible role as a biomarker and therapeutic target.
Genetic mutations and epigenetic changes in prostate cancer
Analyses of polymorphic microsatellites have shown that multiple foci of cancer arise independently within the same prostate.9 An increasing number of studies prove that prostate cancer can be genetically classified into different subgroups, exemplifying the extensive phenotypic and molecular heterogeneity of this disease. Genetic profile analyses have detected only a few, potentially significant mutations in hereditary and sporadic prostate cancer.
Hereditary PCa
The difficulty in the identifying genes that are highly penetrant in PCa can be attributed to:
the advanced age of onset of PCa (median age: 60 years) makes the collection and molecular analyses of samples from more than two generations almost impossible
the high frequency of PCa makes it difficult to distinguish sporadic cases from hereditary cases in families with high rates of disease
hereditary PCas do not have specific clinical (except perhaps an earlier age of onset) or pathological characteristics that differentiate them from sporadic cases.
Several studies have shown a familial predisposition for PCa. Over the last two decades, genome-wide analyses and genetic epidemiologic studies of PCa have led to the identification of high-risk alleles at several genetic.10 Among these, only a few candidate genes may be promising.
RNAse L expresses an endoribonuclease related to the antiviral and pro-apoptotic interferon-induced activities and maps to the HPC1 locus. MSR1 encodes for a macrophage-specific receptor that binds polyanionic ligands (including bacteria, oxidized serum lipoproteins, apoptotic cells) and is located on 8p22-23. Alterations in these two genes appear to be associated with progression and severity of PCa. Unlike breast cancer, for which familiar predisposition is strongly linked to polymorphisms in the genes BCRA 1 and BCRA 2, alterations in the RNAse L and/or MSR1_genes do not account for most hereditary PCa cases. Freedman et al, using a whole-genome single nucleotide polymorphism analysis (SNP), identified a new locus at 8q24 that increases the risk for PCa in African-Americans and is associated with early onset of the malignancy.11 They also showed that the alleles previously identified in this locus12 are insufficient to explain the frequency of 8q24 amplification, pointing out that unmapped risk alleles in this region are yet be identified. This line of investigation appears to hold the most promise to shed light on the pathogenesis of hereditary PCa.
Sporadic PCa
Sporadic PCa often shows heterogeneous patterns of oncogene activation, and is rarely associated with mutations in classic oncogenes or tumour suppressor genes; the investigation of oncogene expression profile correlated to disease development and progression is highly challenging. Recently, genome-wide tools (e.g. comparative genome hybridization, spectral karyotyping, SNP analysis) have provided insight into common PCa chromosomal alterations13 As for hereditary PCa, only a handful of candidate genes have shown promise in having a causative role.14 These genes can be distinguished into those playing an active part in the early and those in the late phases of carcinogenesis. Genes having a putative role in tumour initiation encode for:
the tumour suppressor proteins Pten, p27, Nkx3.1and Rb
the transcription factor Myc
glutathione S-transferase-π (GSTP1) which has a role on damage-related stress prevention
hepsin (cell-surface serine protease)
Alpha-methylacyl-CoA racemase (AMACR), an enzyme involved in β-oxidation of branched-chain fatty acids.
The genes aforesaid have been found to be overexpressed in most gene expression profiling studies.15 KLF6, a zinc finger transcription factor, the polycomb protein EZH2 (enhancer of zeste homolog 2) and telomerase have also been implicated as well in the early phases of prostate carcinogenesis.
In contrast, genes involved in cancer progression and metastases include the androgen receptor (AR) p53, Bcl2, ETV1 and ERG1. The role in tumour development and progression for most of the genes mentioned above has been supported by the generation of relevant mouse models generated in the last two decades. These often mimic many of the characteristics of the human cancer.16 Most models result in focal areas of prostatic intraepithelial neoplasia (PIN), but not in infiltrating tumours,17 suggesting that individual alteration/deletion of these genes is not sufficient to produce invasive PCa and may require other secondary events for cancer progression. Other models rapidly progress to local invasive adenocarcinoma followed by metastasis, as c-myc transgenic mice, prostate conditional Pten knockout, and the compound mice Pten +/−:p27 null, and p53- Rb- knock-out mice.16
Along with mutations in individual genes, a chromosomal translocation has recently been identified in >50% of PCa. By using a bioinformatics approach able to discover candidate oncogenic chromosomal aberrations on the basis of outlier gene expression (COPA), strong and mutually exclusive outlier profiles for ERG and ETV1 (both members of ETS_transcription factor family) were identified in PCa patients18 This imbalance resulted from the fusion of TMPRSS2 (21q22.2), coding for an androgen-regulated transmembrane serine protease, with ERG_(21q22.3) or ETV1 (7p21.2) (and rarely with other members of the_ETS_family) and represents the commonest rearrangement identified in human solid tumours. As a consequence, ERG_and ETV1 oncogenes are regulated by the androgen-responsive promoter of TMPRSS2, possibly providing a selective mechanism for early transformation. The occurrence of TMPRSS2:ERG or TMPRSS2:ETV1 translocations appears to be mutually exclusive, suggesting a specific mechanism required for tumour initiation or, more likely, maintenance. The TMPRSS2:ERG rearrangement, the commonest, is rather complex because both genes are located on the same chromosome and have the same transcriptional orientation. The translocation can generate ≤8 different types of mRNA, by alternative splicing and variant breakpoints. 19 One of these, known as TMPRSS2 ATG (with native translocation initiation codons in frame with the ERG protein), seems to be associated with aggressive disease. 19
Over the last decade, PCa has also been investigated for epigenetic changes—inheritable gene expression alterations without alterations in DNA sequence—which are proving to be of relevance. Studies on DNA methylation status have shown hypermethylation of CpG islands in the promoter of several tumour suppressor genes—e.g. Rb, p16INK4A, MLH1, MSH2, GSTP1 and APC1—which are thereby transcriptionally silenced.20 Most promising among many potential oncogenetic candidates is GSTP1, a member of a family of enzymes playing an important part in cell detoxification. Methylation of GSTP1 is detected in several cancer types, including breast and hepatocellular carcinomas, but only in PCa does it have a prevalence of >90% (reviewed in21). This alteration has also been detected with high frequency in proliferative inflammatory atrophy (PIA). PIA is proposed to be a common proliferative response to environmental damage and, because it may be seen in proximity to high-grade prostatic intraepithelial neoplasia (HGPIN) and expresses molecular signals of early neoplastic transformation (e.g. GSTP1 hypermethylation) it has been suggested that PIA is an early precursor of PCa (reviewed in 21).
Recently, GSTP1 methylation analysis has been detected in cell-free circulating DNA, which has turned out to be a promising seric biomarker of PCa detection and possibly biochemical recurrence.22
Metabolic alterations in tumour initiation and in androgen-independent disease
Pioneering efforts on the characterization of tumour metabolism have revealed that cancer cells rely on anaerobic pathways to convert glucose to ATP, even in abundant oxygen.23 This phenomenon, known as the Warburg effect, occurs despite the fact that the anaerobic pathway is less efficient for energy supply than aerobic respiration.
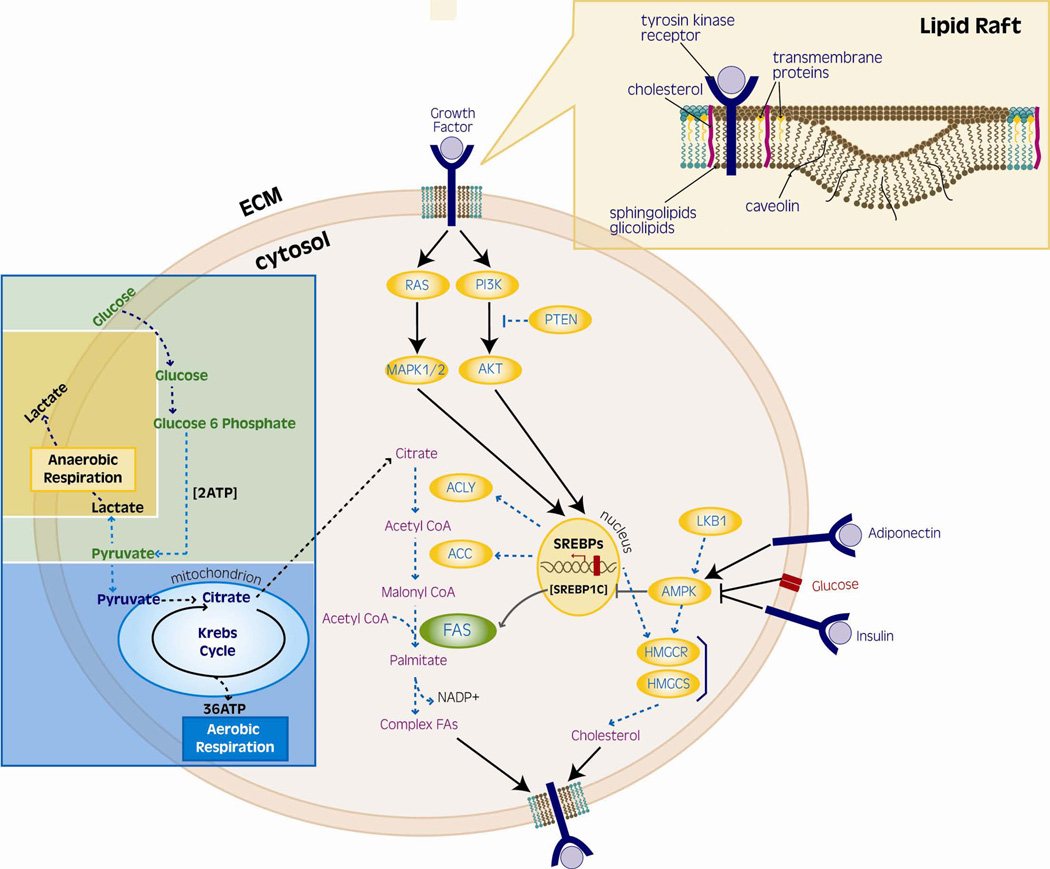
Warburg claimed that the first phase of cancer genesis is irreversible injury to the respiratory chain, followed by a long struggle of the injured cells to survive, achieving this through fermentation (glycolysis with lactate as the final product).23 If on the one hand glycolysis is less efficient than aerobic respiration, on the other hand it produces ATP at a rate 100-times faster than mitochondrial oxidation. Tumour cells therefore efficiently extract glucose from the host and process it by adaptive metabolic activities. The high intake of glucose provides, by pyruvate synthesis, energy and a readily available source of acetyl CoA for mitochondrial synthesis of citrate, necessary for fatty acid/cholesterol synthesis (Figure 1).24 Under normal conditions, most fatty acids are derived from intake in food, and their endogenous synthesis is usually minimal while, in tumour cells, almost all fatty acids are produced via de novo synthesis, despite high levels of ambient fatty acids.25 Thus it is speculated that the increased metabolism of glucose contributes to the tumorigenic process by promoting de novo_synthesis of fatty acids,26 even if the mechanism by which fatty acids might promote prostate cancer growth and survival is poorly understood. Consistent with this hypothesis, numerous studies show that most lipogenic enzymes, such as fatty acid synthase (FASN) and acetyl-CoA carboxylase, are overexpressed in a wide variety of tumour types. The overexpression of FASN in the prostate occurs during the earliest stages of neoplastic transformation (PIN lesions) and in nearly all invasive prostate carcinomas.21, 25 Altered metabolism is not a cause of malignancies, although tumour cells may depend on altered metabolic pathways (see below).
Figure 1. Lipogenesis from glucose metabolism and lipid rafts genesis.
The pathway involving the conversion of glucose to fatty acids (FAs) begins with citrate metabolism. In health, citrate is fully oxidized into the Krebs cycle to generate ATP. In tumour cells, citrate is preferentially transported to the cytoplasm, where it is converted into acetyl-CoA, which constitutes the building block of FAs. Complex FAs, in turn, tend to partition into detergent-resistant membrane microdomains called lipid rafts, which have a role in cell membranes genesis and signalling molecules regulation.Key molecule in this metabolic switch-essential for tumour cells survival-is FASN, the enzyme that catalyzes the first step of de novo synthesis of FAs.
Next, we review the evidence supporting a crucial role of fatty acid-related metabolism in the pathogenesis and progression of prostate malignancy, pointing out that the understanding of the alterations in intermediary metabolism in prostate cells may offer new opportunities in the prevention, diagnosis and treatment of PCa.
FASN as a metabolic oncogene in PCa
FASN, a 270 kD cytosolic complex enzyme that functions as a homodimer, catalyzes the synthesis of palmitate by the condensation of malonyl-CoA and acetyl-CoA. FASN plays an important part in energy homeostasis by converting excess carbon intake into fatty acids for storage. For the reason mentioned above, FASN is expressed at low-to-undetectable levels in normal adult human tissues, except in the liver, adipose tissue, and breast of lactating women. FASN is overexpressed in many human cancers, including PCa.27 We have previously shown that FASN expression progressively increases throughout the natural history of a substantial proportion (two-thirds) of PCas, beginning with PIN, and reaching the highest values in androgen-independent PCas metastatic to bone. FASN-overexpressing PCas have a distinctive gene expression signature.28
Human PCas have genomic amplification of FASN (reviewed in26), suggesting that FASN confers a selective growth advantage to tumour cells. The biochemical and metabolic basis for this overexpression and its consequences are not well understood, but it is known that prostate tumours expressing high FASN levels are associated with poor prognosis (rev in29).
Treatment of human PCa cell lines with cerulenin, a natural irreversible inhibitor of FASN, reduces cell proliferation and, more significantly, induces cell death,26, 30 while systemic treatment of nude mice bearing PCa xenografts with the more stable fatty acid synthase inhibitor C75 (α-methylene-γ-butyrolactone) significantly reduces the size of PCa masses that overexpress FASN.30 A recent study has shown that FASN expression and activity is higher in TRAMP mouse models compared to non-transgenic littermates, and increases with age, tumour progression, and in metastatic lesions. In these models, FASN activity inhibited by cerulenin and C-75 results in apoptosis.31
Polyphenol epigallocatecin gallate (EGCG), in part responsible for the cancer-preventive effects of green tea, is also a potent inhibitor of fatty acid synthase.26
The negligible activity of FASN in normal tissues and its seemingly essential role in tumours, begs the question ‘why FASN activity is essential for tumour cell survival?’ Several hypotheses can be entertained.
FASN has been shown to maintain membrane biogenesis for the endoplasmatic reticulum (ER), which is specialized in the synthesis of secretory and membrane proteins, as well as of phospholipids. A recent study showed that FASN inhibitors induce ER stress in tumour cells.32 ER stress is a coordinated cellular response to alterations of ER homeostasis that results in the activation of an adaptive survival response. The same study showed that FASN inhibitors cooperate with the ER stress-inducer thapsigargin to cause cell death. It has thus been proposed that FASN overexpression in tumour cells may be necessary to preserve ER function.
Detergent-resistant membrane microdomains may be altered by increased lipid synthesis, thus affecting signal transduction. Most newly synthesized lipids are phospholipids enriched in saturated and monounsaturated fatty acyl chains. These phospholipids, together with cholesterol, tend to partition into detergent-resistant membrane microdomains called lipid rafts (Figure 1)33 Raft microdomains are also rich in proteins and serve as membrane platforms for signal transduction in various cellular pathways, including the protein kinase C (PKC), mitogen-activated protein kinase (MAPK), and PI3K pathways. Lipid rafts mobilize or silence the membranous second messengers of these pathways.34 The first evidence linking lipid rafts to PCa comes from the identification of the raft-associated protein caveolin1 (Cav-1) as a marker for aggressive PCa.35 In a recent study aimed at investigating Cav-1 in the onset and progression of PCa, Cav-1 null mice were interbred with TRAMP mice. Inactivation of Cav-1 resulted in significant reductions in prostate tumour size and occurrence of metastases.36 This effect appears to be mediated, at least in part, by FASN, which is downstream of Cav-1.37 Signal transduction through rafts in cancer cells may be also be caveolin-independent. Zhuang et al demonstrated that in caveolin-negative human PCa cells, LNCaP the EGFR-mediated activation of the PI3K/Akt signaling pathway, is downregulated after treatment with the raft-disrupting agent filipin, and is totally recovered by repletion of the membrane with cholesterol. Treatment with filipin alone resulted in cell apoptosis to a similar extent as the PI3K inhibitor, suggesting that the raft microdomains are prominent mediators of survival in PCa cells.38 Given the abundance of complex fatty acids in liquid-ordered lipid microdomains, FASN-driven increase in the proportion of membrane phospholipids may result in a modified organization of signalling proteins on the membrane, and thus in a deregulation of several processes (e.g. cell growth and survival).
PCa cells overexpressing FASN may rely on palmitate metabolism to maintain their malignant phenotype. Many of the effects of FASN inhibition such as apoptosis, reactive oxygen species (ROS) production and in vivo growth inhibition, have been shown to be at least partially rescued by palmitate.39 Palmitate, the principal product of FASN enzyme, is a signal for cellular membrane localization and activation for many proteins. Palmitoylation of oncogenic proteins like Ras and Wnt is an essential posttranslational modification critical for cancer progression.40 In prostate cells overexpressing FASN, global protein palmitoylation profiles often reveal unique patterns compared to isogenic cells without FASN overexpression (Migita, Loda et al, unpublished), suggesting an important mechanism through which high levels of FASN, or possibly even exogenous fatty acids (such as those derived from the diet) can influence signalling in PCa cells.
Tumour cells consistently rely on anaerobic pathways: this limits their respiratory chain and, in turn, their oxidizing power. Cancer cells may therefore favour synthesis and chain elongation of fatty acids because they supply oxidizing power for key oxidative steps, to improve the cellular redox balance despite hypoxic conditions.41This would accomplish a reduction in lactic acidosis and the synthesis of fatty acids to be utilized as structural lipids or as an energy source.
It is unclear which of the mechanisms mentioned above, by no means mutually exclusive, is principally responsible for the oncogenic properties of FASN.
FASN regulation
the sterol regulatory element binding proteins (SREBPs) are key players in the transcriptional regulation of lipogenic enzymes. FASN_transcription is modulated by the androgen-dependent SREBP1c in the androgen-dependent and ligand-independent (castration-resistant) state. The putative role of FASN in the progression of PCa toward androgen independence is unknown. In the CWR22 model of PCa, FASN protein expression, tumour size and proliferation rate were decreased after castration and returned to normal with androgen replenishment.42 Tumours in castrated mice, which relapsed after a long latency, displayed high levels of FASN protein. These data suggest an androgen-independent, but AR-dependent mechanism of FASN induction and are akin to the human situation.43 Tumour growth arrest by FASN inhibitors has been also achieved in some androgen-independent prostatic cancer xenografts.39 Thus, FASN, which is required for survival, represents an attractive drug target in androgen-dependent and - independent disease.
A substantial number of PCa patients exhibit discordance between FASN mRNA and protein levels, suggesting post-transcriptional regulatory mechanisms.43 FASN protein levels are modulated via deubiquitination by the isopeptidase USP2a. USP2a is a pre-proteosomal, androgen-regulated deubiquitinating enzyme overexpressed in ~40% of PCa44 that plays a key part in PCa cell survival through stabilization of FASN protein.45 RNA interference of USP2a results in apoptosis of PCa cells, which is rescued by FASN overexpression, suggesting that FASN downregulation can also be achieved by targeting USP2a in PCa.
FASN and the metabolic syndrome (MS)
MS is a pathological condition characterized primarily by insulin resistance, and is associated with a high risk for cardiovascular disease and death. The overabundance of circulating fatty acids is the major contributor to the development of insulin resistance in diabetic patients.46 In large epidemiologic studies, obesity and the MS have been related to risk of advanced or lethal PCa.47 Among men diagnosed with PCa, weight gain before and obesity at the time of cancer diagnosis are associated with an increased probability of PSA failure after prostatectomy.
Lack of AMPK (5'AMP-activated protein kinase) activity is associated with the MS.46 AMPK acts as an energy sensor or ‘fuel gauge’ with the ultimate aim of enhancing ATP generation in low fuel (high AMP/ATP) conditions and plays a crucial part in systemic energy balance by integrating nutritional and hormonal signals in peripheral tissues and also in the hypothalamus.26 AMPK modulates FASN expression (by regulating the transcriptional factor SREBP1c) and FASN activity (by phosphorylating and inhibiting acetyl-CoA carboxylase (ACC), which catalyzes the formation of malonyl CoA, the rate-limiting step in fatty acid synthesis, Figure 1).26 AMPK stimulation is capable of inhibiting FASN activity in vitro despite constitutive FASN expression in PCa cells.
Therefore, one could speculate that prolonged inactivation of AMPK may contribute to abnormal cellular metabolism and tumour cell transformation/survival through an increase in the availability of circulating fatty acids or the deregulated activity of FASN itself, thus linking predisposing dietary factors and intrinsic alterations of metabolic enzymes, such as FASN. Further evidence is necessary to substantiate the hypothesis that chronic inactivation of AMPK results in unchecked activity of FASN.
FASN: structure–function relationship
The FDA-approved drug orlistat can induce apoptosis in FAS-overexpressing prostate tumours by inhibiting the thioesterase domain of FAS.39The structure of the thioesterase domain of FAS has been resolved.48 The structure of FAS26, USP249 and AMPK50 have also been defined. This will allow for the design of more specific drugs that will affect metabolic pathways crucial to the survival of PCa cells.
Conclusions
In this review, the authors suggested that interfering with lipid metabolism has detrimental effects on prostate tumour cell survival. Studies are under way to determine the precise mechanisms responsible for tumour initiation and maintenance by altered metabolic pathways.
From a broader perspective, the authors would like to underscore the necessity of more thorough investigations of the metabolic alterations in cancer, and speculate that combining metabolic profiling with genome-wide analyses will drive new understanding in PCa biology and subsequently treatment.
Practice points
Novel molecular biomarkers have the potential to improve risk stratification among patients with prostate cancer (PCa) and allow for better decision-making about treatment.
Candidate high-risk genes that are potentially associated with hereditary PCa are RNAse L and MSR1; many sporadic PCas, characterized by heterogeneous patterns of oncogene activation, show the commonest chromosomal rearrangement yet identified in human solid tumours, resulting in the fusion of TMPRSS2 (21q22.2) with ERG_(21q22.3) or ETV1 (7p21.2).
Upregulation of fatty acid synthase (FASN), the key metabolic enzyme that is responsible for the terminal
catalytic step in de novo synthesis of fatty acids (FAs), represents a common characteristic in human malignancies.
FASN seems to play a crucial part in PCa by conferring growth and survival advantages to cancer cells, maintaining membrane biogenesis and membranous signalling proteins regulation, and providing protein palmitoylation, which is a post-translational modification critical in cancer progression.
The metabolic syndrome (MS), which is a pathological condition characterized by primarily insulin resistance and associated with the overabundance of circulating fatty acids, has been related to risk of advanced PCa. Prolonged inactivation of AMPK in patients with PCa may contribute to abnormal cellular metabolism and tumour cell transformation/survival by promoting the unchecked activity of FASN.
Tumour exacerbated lipogenesis might represent a potential marker in tumour diagnosis (because it emerges early in carcinogenesis) and a target in treatment of advanced carcinomas.
Acknowledgments
We thank members of Loda laboratory for fruitful discussions, particularly Emanuele Palesandolo, Carmen Priolo, Stephen Finn and Giorgia Zadra; Sonal Jhaveri-Schneider for critical review of the manuscript; Marleen Marino and Jane Hayward for graphic assistance.
This work was supported by grants from the NIH (Specialized Programs of Research Excellence 5P50CA90381, PO1 CA089021) and the Prostate Cancer Foundation (Massimo Loda).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elisa Benedettini, Dana-Farber Cancer Institute, Massachusetts, USA..
Paul Nguyen, Dana-Farber Cancer Institute and Harvard Medical School Massachusetts, USA..
Massimo Loda, Dana-Farber Cancer Institute and Harvard Medical School Massachusetts, USA. He is a consultant for Novartis Pharmaceuticals, Incorporated, and is a member of the Center for Cancer Genome Discovery, Dana Farber Cancer Institute..
REFERENCES
- 1.Walczak JR, Carducci MA. Prostate cancer: a practical approach to current management of recurrent disease. Mayo Clin Proc. 2007;82:243–249. doi: 10.4065/82.2.243. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007 doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 4.Loeb S, Catalona WJ. Prostate-specific antigen in clinical practice. Cancer Lett. 2007;249:30–39. doi: 10.1016/j.canlet.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med. 2004;350:2239–2246. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 9.Bostwick DG, Shan A, Qian J, et al. Independent origin of multiple foci of prostatic intraepithelial neoplasia: comparison with matched foci of prostate carcinoma. Cancer. 1998;83:1995–2002. doi: 10.1002/(sici)1097-0142(19981101)83:9<1995::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.DeMarzo AM, Nelson WG, Isaacs WB, Epstein JI. Pathological and molecular aspects of prostate cancer. Lancet. 2003;361:955–964. doi: 10.1016/S0140-6736(03)12779-1. [DOI] [PubMed] [Google Scholar]
- 11.Freedman ML, Haiman CA, Patterson N, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Nat Acad Sci USA. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amundadottir LT, Sulem P, Gudmundsson J, et al. A common variant associated with prostate cancer in European and African populations. Nat Genet. 2006;38:652–658. doi: 10.1038/ng1808. [DOI] [PubMed] [Google Scholar]
- 13.Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12:19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- 14.Bradford TJ, Tomlins SA, Wang X, Chinnaiyan AM. Molecular markers of prostate cancer. Urol Oncol. 2006;24:538–551. doi: 10.1016/j.urolonc.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Yu YP, Landsittel D, Jing L, et al. Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol. 2004;22:2790–2799. doi: 10.1200/JCO.2004.05.158. [DOI] [PubMed] [Google Scholar]
- 16.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 17.Majumder PK, Yeh JJ, George DJ, et al. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: the MPAKT model. Proc Nat Acad Sci USA. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Cai Y, Ren C, Ittmann M. Expression of variant TMPRSS2/ERG fusion messenger RNAs is associated with aggressive prostate cancer. Cancer Res. 2006;66:8347–8351. doi: 10.1158/0008-5472.CAN-06-1966. [DOI] [PubMed] [Google Scholar]
- 20.Li LC, Carroll PR, Dahiya R. Epigenetic changes in prostate cancer: implication for diagnosis and treatment. J Natl Cancer Inst. 2005;97:103–115. doi: 10.1093/jnci/dji010. [DOI] [PubMed] [Google Scholar]
- 21.De Marzo AM, DeWeese TL, Platz EA, et al. Pathological and molecular mechanisms of prostate carcinogenesis: implications for diagnosis, detection, prevention, and treatment. J Cell Biochem. 2004;91:459–477. doi: 10.1002/jcb.10747. [DOI] [PubMed] [Google Scholar]
- 22.Henrique R, Jeronimo C. Molecular detection of prostate cancer: a role for GSTP1 hypermethylation. Eur Urol. 2004;46:660–669. doi: 10.1016/j.eururo.2004.06.014. discussion p669. [DOI] [PubMed] [Google Scholar]
- 23.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 24.Costello LC, Franklin RB. 'Why do tumour cells glycolyse?': from glycolysis through citrate to lipogenesis. Mol Cell Biochem. 2005;280:1–8. doi: 10.1007/s11010-005-8841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 26.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 27.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Nat Acad Sci USA. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Molecul Can Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 29.Baron A, Migita T, Tang D, Loda M. Fatty acid synthase: a metabolic oncogene in prostate cancer? J Cell Biochem. 2004;91:47–53. doi: 10.1002/jcb.10708. [DOI] [PubMed] [Google Scholar]
- 30.Pizer ES, Pflug BR, Bova GS, Han WF, Udan MS, Nelson JB. Increased fatty acid synthase as a therapeutic target in androgen- independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 31.Pflug BR, Pecher SM, Brink AW, Nelson JB, Foster BA. Increased fatty acid synthase expression and activity during progression of prostate cancer in the TRAMP model. Prostate. 2003;57:245–254. doi: 10.1002/pros.10297. [DOI] [PubMed] [Google Scholar]
- 32.Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- 33.Swinnen JV, Van Veldhoven PP, Timmermans L, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 34.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 35.Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999;59:5719–5723. [PubMed] [Google Scholar]
- 36.Williams TM, Hassan GS, Li J, et al. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in tramp mice. J Biol Chem. 2005;280:25134–25145. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- 37.Di Vizio D, Sotgia F, Williams TM, et al. Caveolin-1 is required for the upregulation of fatty acid synthase (FASN), a tumor promoter, during prostate cancer progression. Cancer Biol Ther. 2007;6:1263–1268. doi: 10.4161/cbt.6.8.4447. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- 39.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 40.Kato K, Der CJ, Buss JE. Prenoids and palmitate: lipids that control the biological activity of Ras proteins. Semin Cancer Biol. 1992;3:179–188. [PubMed] [Google Scholar]
- 41.Hochachka PW, Rupert JL, Goldenberg L, Gleave M, Kozlowski P. Going malignant: the hypoxia-cancer connection in the prostate. Bioessays. 2002;24:749–757. doi: 10.1002/bies.10131. [DOI] [PubMed] [Google Scholar]
- 42.Myers RB, Oelschlager DK, Weiss HL, Frost AR, Grizzle WE. Fatty acid synthase: an early molecular marker of progression of prostatic adenocarcinoma to androgen independence. J Urol. 2001;165:1027–1032. [PubMed] [Google Scholar]
- 43.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 44.Priolo C, Tang D, Brahamandan M, et al. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 2006;66:8625–8632. doi: 10.1158/0008-5472.CAN-06-1374. [DOI] [PubMed] [Google Scholar]
- 45.Graner E, Tang D, Rossi S, et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/s1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- 46.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. doi: 10.1038/nrd1344. [DOI] [PubMed] [Google Scholar]
- 47.Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13:1646–1650. [PubMed] [Google Scholar]
- 48.Chakravarty B, Gu Z, Chirala SS, Wakil SJ, Quiocho FA. Human fatty acid synthase: structure and substrate selectivity of the thioesterase domain. P Proc Nat Acad Sci USA. 2004;101:15567–15572. doi: 10.1073/pnas.0406901101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Renatus M, Parrado SG, D'Arcy A, et al. Structural basis of ubiquitin recognition by the deubiquitinating protease USP2. Structure. 2006;14:1293–1302. doi: 10.1016/j.str.2006.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Townley R, Shapiro L. Crystal structures of the adenylate sensor from fission yeast AMP-activated protein kinase. Science. 2007 doi: 10.1126/science.1137503. [DOI] [PubMed] [Google Scholar]