Summary
In contrast to the relatively uniform pathology and the unyielding dismal outcome associated with infiltrating ductal adenocarcinoma of the pancreas, cystic lesions have a broad spectrum of gross and microscopic pathologies, and a range of clinical outcomes. The common cystic lesions of the pancreas are reviewed with emphasis on practical tips for distinguishing between the main entities.
Introduction
With the increasing use and resolution of abdominal imaging, ever increasing numbers of cystic lesions of the pancreas are being discovered.1,2 For example, Pitt et al reviewed all computed tomography (CT) and magnetic resonance imaging (MRI) scans performed at the Medical College of Wisconsin on patients without pancreatitis between January 1995 through December 2002, and found that 1.2% of the 24 039 patients had pancreatic cysts.1 Similarly, Laffan et al recently reported that 2.6% of 2832 consecutive patients who underwent a contrast enhanced multidetector CT (MDCT) scanning of the abdomen and pelvis at the Johns Hopkins Hospital for a nonpancreatic indication had an asymptomatic pancreatic cyst.2 Clearly, a significant number of individuals have asymptomatic pancreatic cysts.
The most common symptomatic cystic lesion of the pancreas is the non-neoplastic pseudocyst.3 The common neoplastic cystic lesions include serous cystic neoplasm, mucinous cystic neoplasm, intraductal papillary mucinous neoplasm and solid-pseudopapillary neoplasm.4 In addition, usually solid lesions, such as pancreatic endocrine neoplasms, may undergo cystic degeneration. The most appropriate treatment and likely prognosis for patients with a cystic lesion of the pancreas differ greatly depending on the type of cyst. For example, pseudocysts are benign and often managed medically or simply drained, while mucinous cystic neoplasms can harbour an associated invasive carcinoma and are best treated by complete surgical resection.
General approach
As illustrated in Table 1, an integration of six clinical and pathological features can be used to distinguish between the various cystic neoplasms of the pancreas:4
The patient’s sex can help suggest a diagnosis. Mucinous cystic neoplasms and solid-pseudopapillary neoplasms are significantly more common in women than in men.4
The location of the cyst within the pancreas can help prioritize the differential diagnosis. Mucinous cystic neoplasms are more common in the tail of the gland than in the head, while the opposite is true for intraductal papillary mucinous neoplasms.4–9
The relationship of the cysts that comprise a cystic neoplasm to the larger pancreatic ducts helps distinguish mucinous cystic neoplasms from intraductal papillary mucinous neoplasms. Intraductal papillary mucinous neoplasms, by definition, involve the larger pancreatic ducts, while mucinous cystic neoplasms do not.4 The relationship between pancreatic ducts and cysts can often be determined preoperatively with currently available imaging technologies such as endoscopic ultrasound, endoscopic retrograde pancreatography and CT.4
The contents of the cysts can help distinguish between the various cystic neoplasms. Mucinous cystic neoplasms and intraductal papillary mucinous neoplasms contain copious amounts of thick mucin, while serous cystic neoplasms, as the name suggests, produce a watery almost clear fluid, and solid-pseudopapillary neoplasms contain haemorrhagic necrotic material.4 Cyst fluid can be obtained preoperatively by final needle aspiration.10
The type of epithelium. Mucinous cystic neoplasms and intraductal papillary neoplasms are lined by columnar mucin-producing epithelium, serous cystic neoplasms by cuboidal glycogen-rich cells, and solid-pseudopapillary neoplasms are composed of uniform poorly cohesive cells.4
The nature of the stroma. Mucinous cystic neoplasms have a distinctive ‘ovarian-type’ stroma, while the stroma in the other neoplasms is composed predominantly of collagen and scattered fibroblasts.4–6,11
The last two features, the nature of the epithelium and stroma, are best appreciated on biopsy or surgical resection, but the epithelium can be sampled by fine needle aspiration biopsy as well.10
Table 1.
Features of cystic neoplasms of the pancreas
| Mucinous cystic neoplasm | Intraductal papillary mucinous neoplasm | Solid-pseudopapillary neoplasm | Serous cystic neoplasm | |
|---|---|---|---|---|
| Sex ratio (F:M) | 20:1 | 1:1.5 | 10:1 | 7:3 |
| Head/tail | Tail | Head | Tail = head | Tail = head |
| Connectivity to duct | None | Always | None | None |
| Cyst contents | Mucinous | Mucinous | Necrotic/haemorrhagic | Serous |
| Epithelium | Mucinous | Mucinous | Noncohesive | Serous |
| Stroma | Ovarian | None | None | None |
The following sections will consider each of the most common cystic neoplasms in greater detail.
Serous cystic neoplasm
The serous cystadenoma is a benign neoplasm composed of uniform cuboidal epithelial glycogen-rich cells.4,12 The cells line numerous small cysts containing clear straw-coloured fluid.4,12 Synonyms include microcystic serous cystadenoma, glycogen-rich cystadenoma and microcystic adenoma.
Serous cystadenomas are slightly more common in women (female:male ratio 7:3) and the mean age at diagnosis is 65 years.4,12–14 Most serous cystic neoplasms are sporadic, but they can also arise in association with the von Hippel-Lindau (VHL) syndrome.15 Serous cystadenomas usually present clinically as large abdominal masses, and common symptoms include abdominal pain, early satiety, weight loss, dyspepsia, and nausea and vomiting.4,12–14 CT will reveal a well-demarcated mass composed of innumerable small cysts. The lesions often have a central stellate calcified scar, and when the pancreatic duct can be visualized, it is usually seen to drape around the mass.
Grossly, serous cystadenomas can involve any portion of the pancreas. Most are solitary and large (mean 6.0 cm). They are well-demarcated and composed of innumerable small (< 2 mm) thin-walled cysts filled with clear straw-coloured (serous) fluid (Fig. 1).4,12–14 As noted in the description of the radiographic appearance of these neoplasms, they often contain a calcified central stellate scar. Two less common variations on the gross appearance of serous cystadenomas deserve note. Serous neoplasms can rarely be solid (the solid variant) or macrocystic (the macrocystic variant).4 Synonyms for the macrocystic serous cystadenoma include oligocystic serous cystadenomas, serous oligocystic adenoma and ill-demarcated adenoma. Solid serous cystadenomas mimic well-differentiated endocrine neoplasms, and macrocystic serous cystadenomas mimic mucinous cystic neoplasms.
Figure 1.
Cross-section of a serous cystadenoma. Note the central scar and the innumerable small cysts.
Microscopically the cysts of serous cystadenomas are lined by clear cuboidal cells with uniform round nuclei (Fig. 2).4,12–14 These cells can focally form microscopic papillae that project into the cysts, a finding that has no clinical significance. Stains for glycogen, such as the periodic acid-Schiff stain, can be used to highlight abundant intracytoplasmic glycogen.
Figure 2.
Serous cystadenoma. The cysts are lined by cuboidal cells with clear cytoplasm.
The cells immunohistochemically label with antibodies to pancytokeratin (AE1/AE3), CAM5.2, epithelial membrane antigen (EMA), and for cytokeratins 7, 8, 18, and 19.4,12–14 Loss of heterozygosity on chromosome 3p25, the location of the VHL gene, is one of the most frequently identified molecular alterations.16
The vast majority of serous cystic neoplasms are entirely benign and patients are cured if the lesion is resected. Very rare examples of locally invasive, and even metastatic, serous cystic neoplasms have been reported.17 Serous cystic neoplasms that have metastasized are designated ‘serous cystadenocarcinoma’. Recently, Galanis et al suggested that the identification of locally aggressive disease in a resected neoplasm may indicate an increased likelihood of recurrence or metachronous metastasis.13
Mucinous cystic neoplasm
By definition, the mucinous cystic neoplasm has both a mucin-producing epithelium and an ovarian-type of stroma.4,11 The neoplastic epithelial cells line larger cysts (1–3 cm) containing mucous. These cysts do not communicate with the larger pancreatic ducts.
Mucinous cystic neoplasms arise in women much more commonly than in men (female:male ratio 20:1), and the mean age at diagnosis is 45 years.4–6,11 Symptoms are similar to those associated with serous cystic neoplasms and include abdominal pain, early satiety, weight loss, dyspepsia, and nausea and vomiting. Abdominal imaging, such as CT, demonstrates a mass composed of 1–3-cm cysts with thick walls.
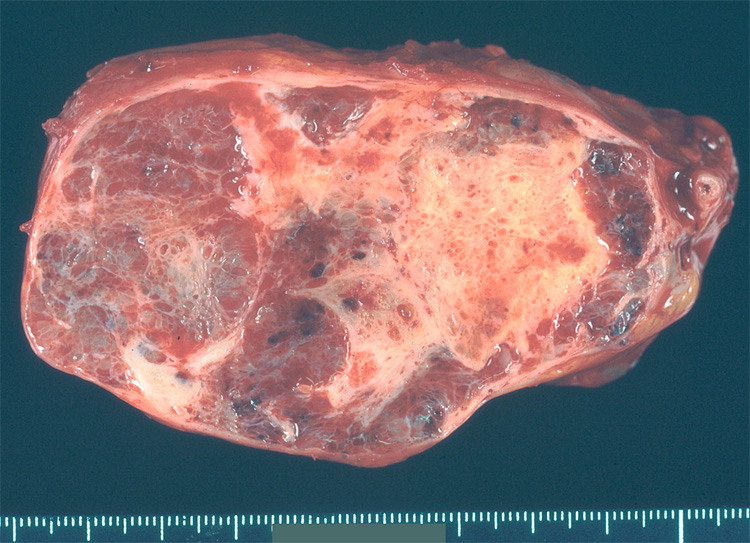
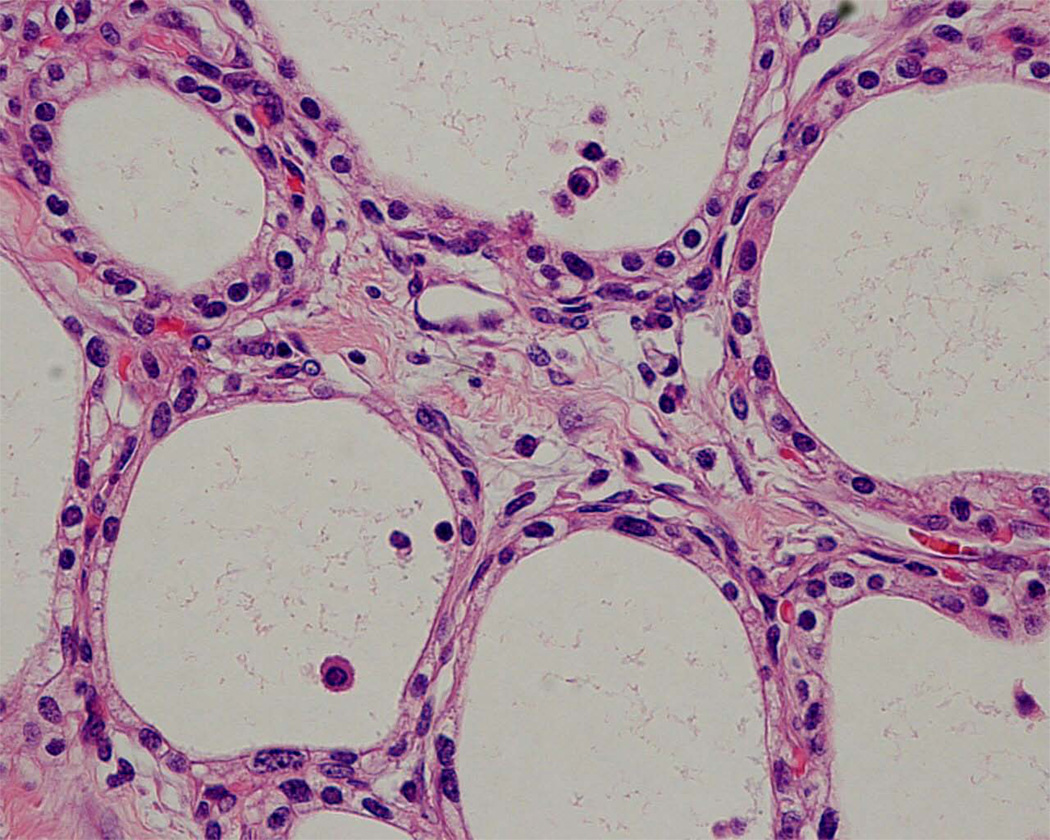
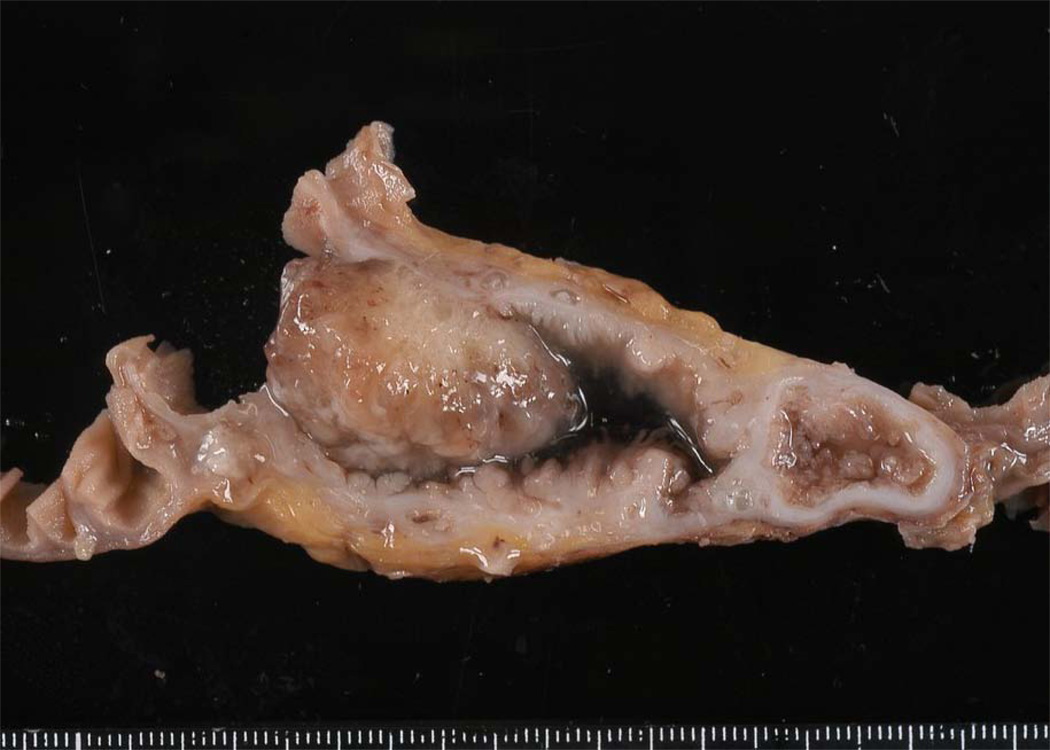
Mucinous cystic neoplasms involve the tail of the pancreas more frequently than they do the head.4–6,11 Grossly, these neoplasms are usually solitary and well-demarcated. On cross-section the 1–3-cm cysts usually contain tenacious mucin, but some can contain degraded blood (Fig. 3). The walls of the cysts are thick (1–3 mm). Microscopically, by definition, the cysts are lined by columnar mucin-producing epithelial cells, and the stroma in the septa of the cysts resembles ovarian stroma with crowded spindle cells with thin cytoplasm (Fig. 4).4–6,11 Ovarian stroma is seen even in mucinous cystic neoplasms that arise in men. The epithelium can show varying degrees of dysplasia, and noninvasive mucinous cystic neoplasms can be divided into three grades: mucinous cystic neoplasm with low-grade dysplasia, mucinous cystic neoplasm with moderate dysplasia, and mucinous cystic neoplasm with high-grade dysplasia based on the degree of architectural and cytological atypia.4 A third of mucinous cystic neoplasms have an associated invasive carcinoma and, not surprisingly, these are designated mucinous cystic neoplasm with an associated invasive carcinoma.
Figure 3.
Cross-section of a mucinous cystic neoplasm. The cysts are large and have thick walls. As is common, this example arose in the tail of the pancreas.
Figure 4.
Mucinous cystic neoplasm. The cysts are lined by columnar mucin-producing epithelial cells. Note the dense ‘ovarian-type’ stroma.
The neoplastic cells label with antibodies to pan-cytokeratin, cytokeratins 7, 8, 18 and 19, CAM5.2 and carcinoembryonic antigen (CEA), while the stromal cells label with antibodies to vimentin, inhibin, progesterone receptors and oestrogen receptors.4,11 Activating point mutations in the KRAS2 oncogene and inactivation of the TP53 tumour suppressor gene have been reported in mucinous cystic neoplasms, particularly those with high-grade dysplasia.
The prognosis for patients with a surgically resected noninvasive mucinous cystic neoplasm is excellent. The vast majority of these patients are cured.4–6,11 Most examples of metastasizing ‘noninvasive mucinous cystic neoplasms’ reported in the literature were likely to be mucinous cystic neoplasms with an associated invasive carcinoma in which the invasive component was simply not sampled. Clearly extensive, if not complete, histological sampling of mucinous cystic neoplasms is advisable.
The best prognosticator for a patient with a mucinous cystic neoplasm is the presence or absence of an associated invasive carcinoma.4–6,11 If an invasive carcinoma is present, then the size of the invasive component, depth of invasion, resectabilty and stage all become useful prognosticators. The 5-year survival rate for patients with an invasive carcinoma arising in association with mucinous cystic neoplasm is 50%.
Intraducatal papillary mucinous neoplasm
The intraductal papillary mucinous neoplasm, as the name so aptly describes, is a mucin-producing, usually papillary, epithelial neoplasm that arises in one of the larger pancreatic ducts.4,7,8 As is true for mucinous cystic neoplasms, about one-third of these are associated with an invasive carcinoma.
In contrast to mucinous cystic neoplasms, intraductal papillary mucinous neoplasms are more common in men than in women.4,7–9 The mean age at diagnosis is 65 years. Presenting symptoms typically include abdominal and back pain, anorexia, weight loss and recurrent episodes of pancreatitis.4,7–9 Abdominal imaging will reveal a cystic mass that communicates with one of the larger pancreatic ducts. The main pancreatic duct is often dilated. Features on imaging that suggest a malignancy include size > 3 cm, a mural nodule and dilatation of the main pancreatic duct.18–20 Mucin oozing from the ampulla of Vater is almost diagnostic of an intraductal papillary mucinous neoplasm on endoscopy.
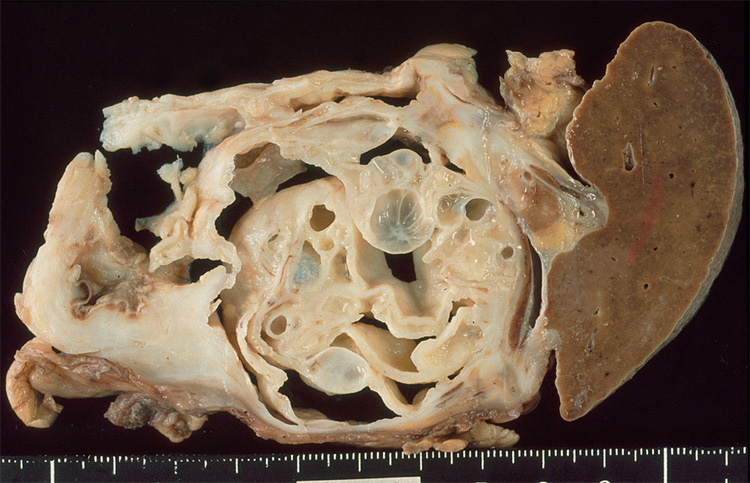
In contrast to mucinous cystic neoplasms, intraductal papillary mucinous neoplasms involve the head more frequently than the tail of the gland, and intraductal papillary mucinous neoplasms are often multifocal.4,21 Grossly, on sectioning the pancreatic duct or one of its branches will be distended with thick mucin. Papillary projections into the lumen can be observed (Fig. 5). Those that involve the main pancreatic duct are designated ‘main-duct type’, while those that predominantly involve a branch duct are designated ‘branch-duct type’.
Figure 5.
Cross-section of an intraductal papillary mucinous neoplasm. Note the long papillae projecting into the lumen.
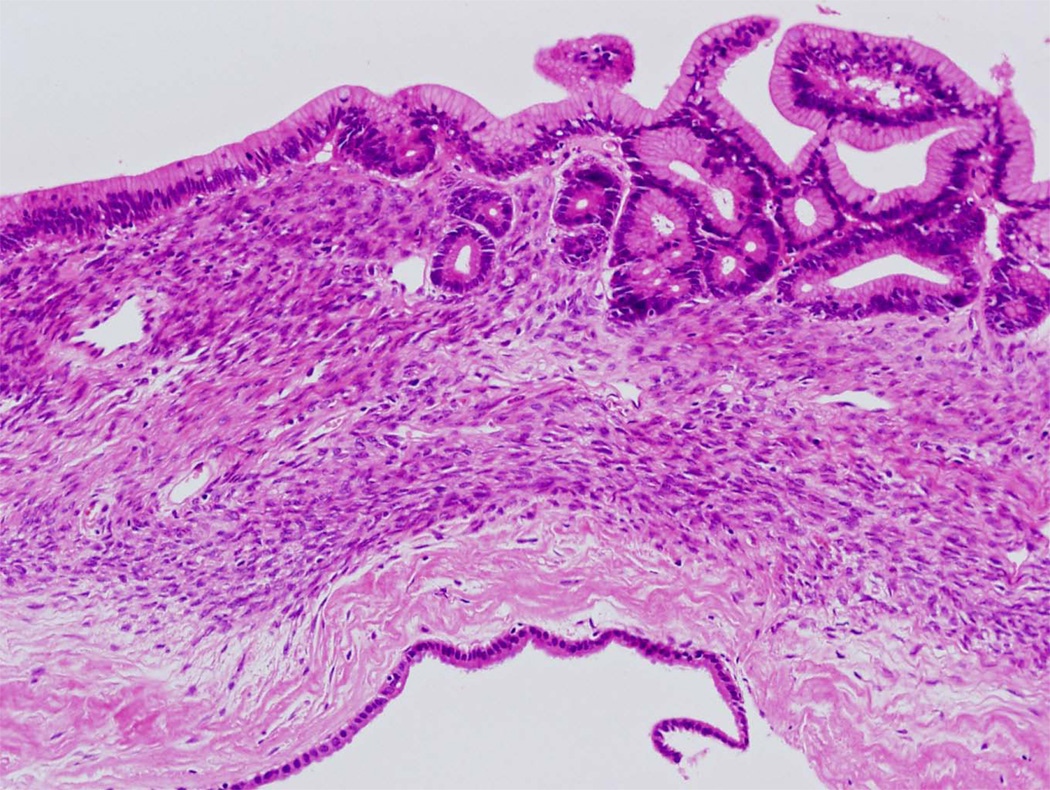
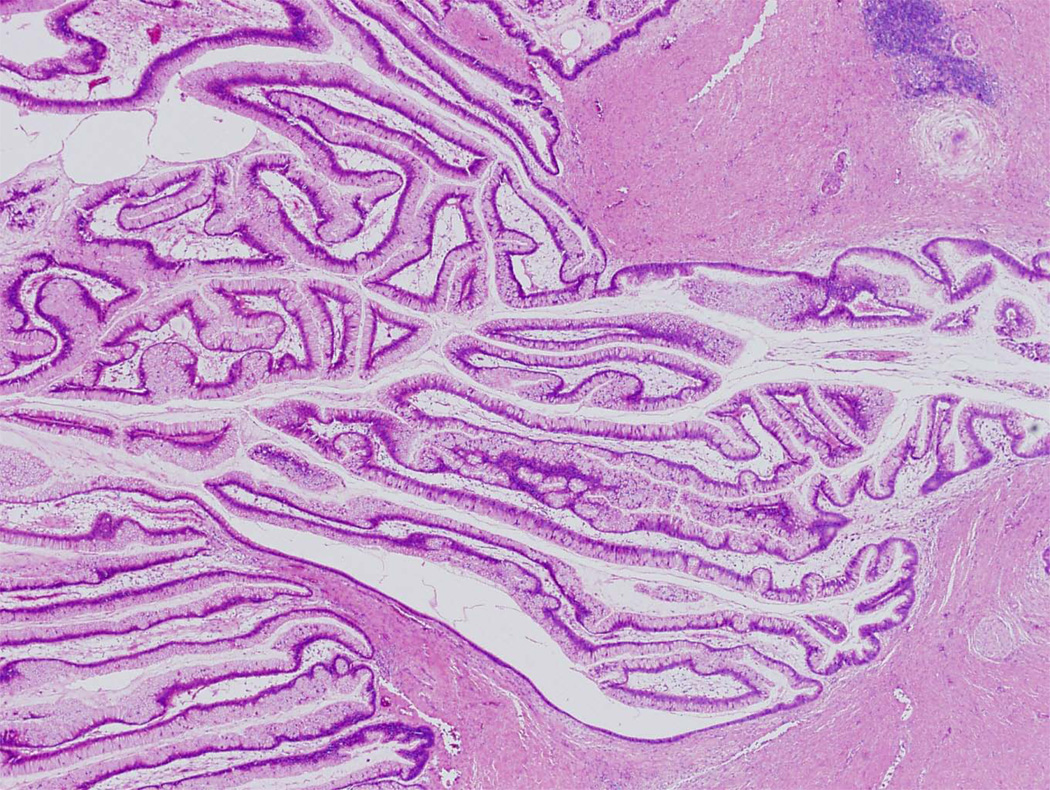
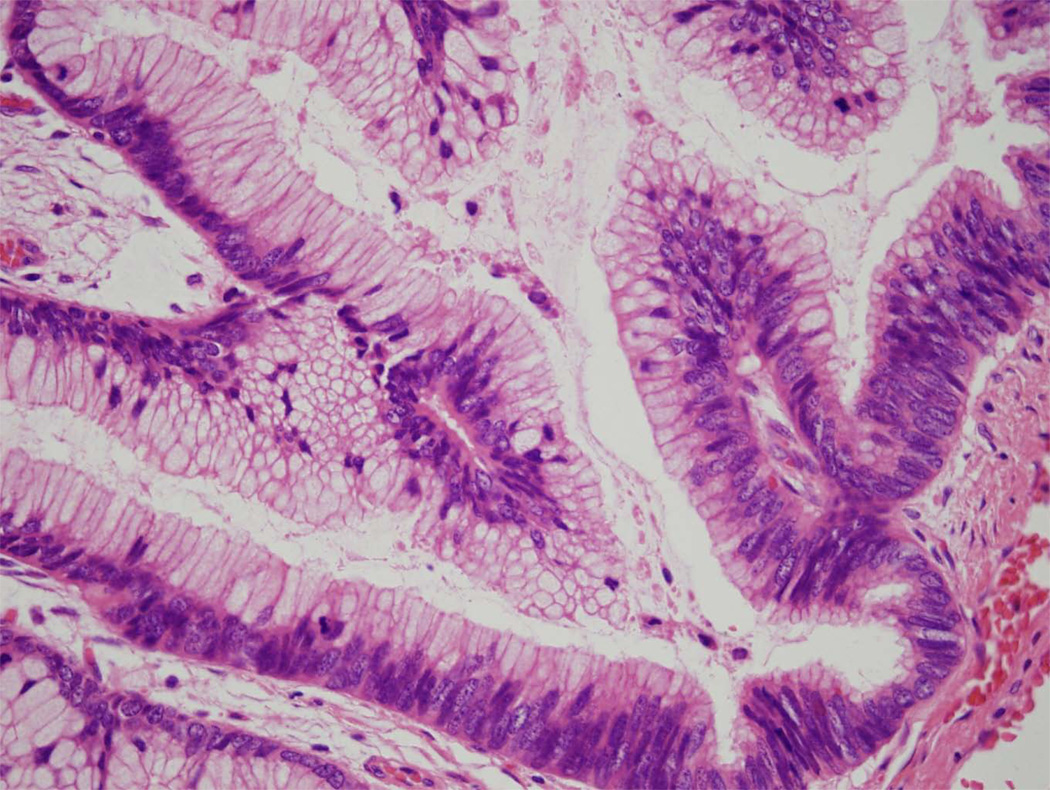
Microscopic examination typically reveals long thin intraductal papillae lined by mucin-producing columnar epithelial cells (Fig. 6).4,7–9 In contrast to mucinous cystic neoplasms, the stroma is composed of loose, relatively acellular, connective tissue. As is true for mucinous cystic neoplasms, the epithelium of intraductal papillary mucinous neoplasms can show varying degrees of dysplasia, and noninvasive intraductal papillary mucinous neoplasms can be divided into three grades based on the degree of architectural and cytological atypia. The three grades are intraductal papillary mucinous neoplasm with low-grade dysplasia, intraductal papillary mucinous neoplasm with moderate dysplasia, and intraductal papillary mucinous neoplasm with high-grade dysplasia (Fig. 7).4 A third of intraductal papillary mucinous neoplasms have an associated invasive carcinoma and, not surprisingly, these are designated intraductal papillary mucinous neoplasm with an associated invasive carcinoma. These invasive carcinomas are usually either colloid carcinomas or ductal carcinomas.4,22,23
Figure 6.
Intraductal papillary mucinous neoplasm. Long finger-like papillae project into the duct. The papillae are lined by tall columnar mucin-producing cells.
Figure 7.
Intraductal papillary mucinous neoplasm with high-grade dysplasia. Note the pleomorphism and the numerous mitotic figures.
Immunolabelling can be used to demonstrate the expression of cytokeratin, and intraductal papillary mucinous neoplasms label with antibodies to pancytokeratin (AE1/AE3), CAM5.2 and cytokeratins 7, 8, 18 and 19, and variably cytokeratin 20.4,7,24,25 They express CEA and those with ‘intestinal’ phenotype are strongly MUC2 positive.4,22 Intraductal papillary mucinous neoplasms with ‘pancreatobiliary’ histology may express MUC1 instead of MUC2.22 Activating point mutations in the KRAS2 oncogene and inactivation of the TP53 tumour suppressor gene have been reported in these neoplasms. The STK11/LKB1 gene is biallelically inactivated in a minority of cases.26 As is true for mucinous cystic neoplasms, the number of genetic alterations in intraductal papillary mucinous neoplasms typically increases with increasing degrees of dysplasia.
The single most important prognostic factor is the presence or absence of an associated invasive carcinoma.4,9,21 Unlike mucinous cystic neoplasms, however, patients with a surgically resected noninvasive papillary mucinous neoplasm still have to be carefully followed because of the real risk of multifocal disease.21 If an invasive carcinoma is present, then the size of the invasive component, resectabilty and stage are all useful prognosticators. The 5-year survival rate for patients with an invasive carcinoma arising in association with an intraductal papillary mucinous neoplasm is 40%.
Solid-pseudopapillary neoplasm
Solid-pseudopapillary neoplasms are difficult to define because they do not have a recognizable direction of differentiation. Instead, these distinctive neoplasms are defined by their gross and morphological appearance as epithelial neoplasms composed of discohesive uniform cells that surround delicate blood vessels and form solid masses with frequent cystic degeneration and intracystic haemorrhage.4,27
Almost all solid-pseudopapillary neoplasms occur in women (female:male ratio 10:1), and the mean age at diagnosis is ~25 years.4,27 Patients usually present nonspecifically with abdominal pain, early satiety, weight loss, dyspepsia, and nausea and vomiting. Abdominal imaging will reveal a solid mass, a cystic mass or, more commonly, a solid and cystic mass.
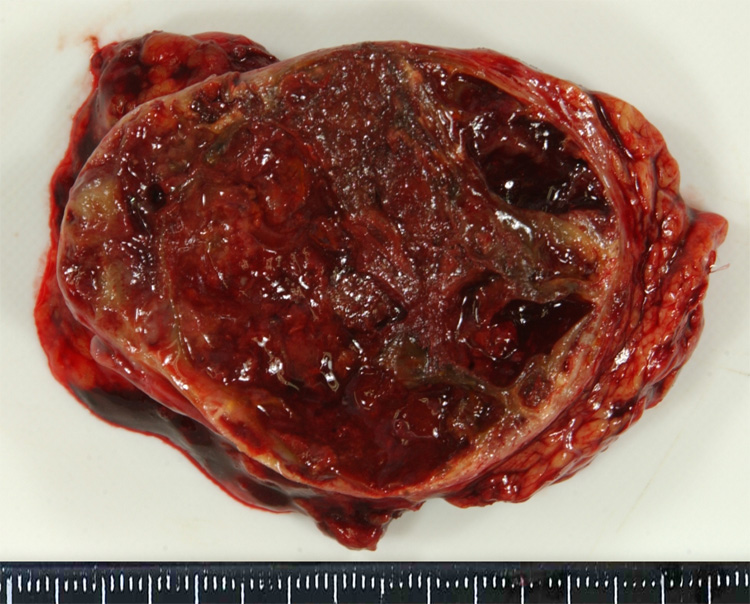
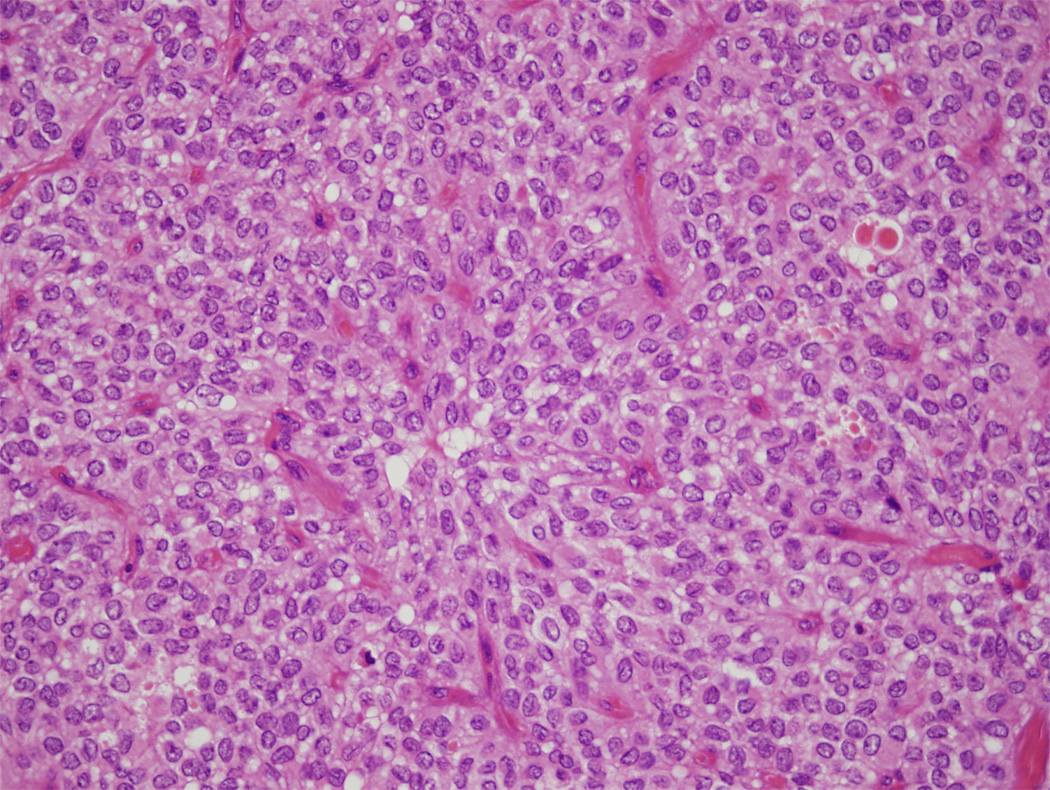
Solid-pseudopapillary neoplasms can arise anywhere in the gland and they have a distinctive gross appearance. They typically form well-demarcated masses with solid areas and irregular foci containing friable necrotic material and haemorrhagic debris (Fig. 8).4,27
Figure 8.
Cross-section of a solid-pseudopapillary neoplasm. This example is extensively haemorrhagic.
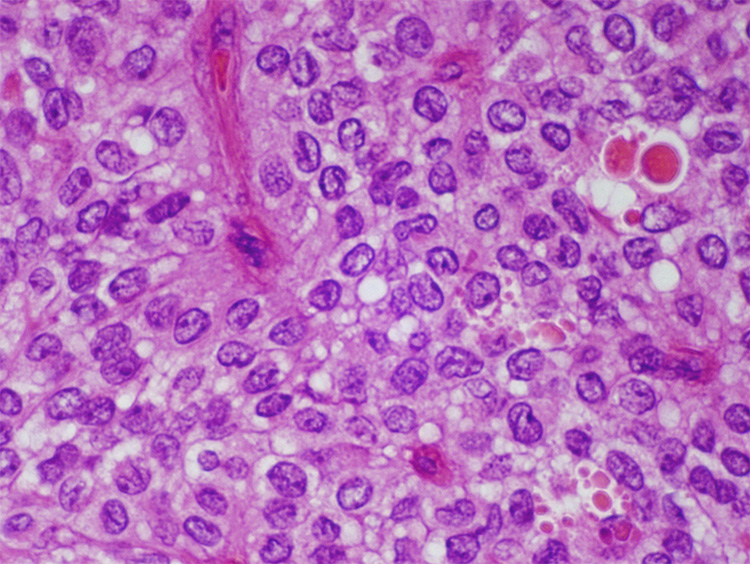
Microscopically they are composed of loosely cohesive uniform cells that cling to delicate capillaries (Fig 9 and Fig 10).4,27 The nuclei are uniform and oval, and typically have nuclear grooves. Eosinophilic hyaline globules, cholesterol clefts and multinucleated giant cells are also seen. Immunolabelling will reveal that the neoplastic cells express vimentin, α-1 antitrypsin, CD10, neuron-specific enolase and CD56.4,27 The expression of synaptophysin and cytokeratin (AE1/AE3, CAM5.2) is variable. Almost all harbour mutations in the β-catenin gene and, as a result, immunolabelling with antibodies to the β-catenin protein will show an abnormal nuclear pattern of labelling.28
Figure 9.
Solid-pseudopapillary neoplasm. Note the branching delicate vessels lined by uniform poorly-cohesive cells.
Figure 10.
Solid-pseudopapillary neoplasm with uniform loosely cohesive cells and hyaline globules.
Solid-pseudopapillary neoplasms are considered low-grade malignancies, and the prognosis for most patients is excellent. Only 10–15% metastasize and even metastases can be surgically resected with good outcome.4,27
Other cystic lesions
In addition to the common cystic neoplasms discussed above, it should be noted that usually solid neoplasms, such as well-differentiated pancreatic endocrine neoplasms, can undergo cystic degeneration and present as a cystic mass. In addition, not all cystic lesions in the pancreas are neoplasms. Pseudocysts, paraduodenal wall cysts (‘groove pancreatitis’) and retention cysts all need to be considered in the differential diagnosis of a cyst in or adjacent to the pancreas.3
Conclusion
Cystic lesions of the pancreas are becoming more and more important. As the resolution of abdominal imaging improves, we are finding that as many as 3% of the population has a pancreatic cyst.1,2 Some of these cysts will be precursors to invasive pancreatic cancer, and these represent an opportunity to cure pancreatic neoplasia. On the other hand, many will be harmless non-neoplastic cysts, and many will be such early neoplasms that surgical intervention is not justified. The challenge of the coming decade will be to develop new techniques that can be used to distinguish between the many cysts that can involve the pancreas.
Practice points
Ovarian stroma and lack of connectivity to the major duct system distinguish mucinous cystic neoplasms from intraductal papillary mucinous neoplasms.
Whenever possible, mucinous cystic neoplasms should be completely resected.
A noninvasive diagnosis should not be established for a mucinous cystic neoplasm unless the lesion has been entirely examined histologically.
In contrast to mucinous cystic neoplasms, intraductal papillary mucinous neoplasms can be multifocal. Careful clinical follow-up is therefore warranted for patients with an intraductal papillary mucinous neoplasm.
Immunolabelling for β-catenin can help establish the diagnosis of a solid-pseudopapillary neoplasm.
Cystic degeneration of an otherwise solid neoplasm should be considered in the differential diagnosis of a cystic mass in the pancreas.
Research directions
New approaches to detect asymptomatic cystic neoplasms of the pancreas.
New techniques to distinguish preoperatively among the many cysts that can involve the pancreas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ralph H. Hruban, Department of Pathology and Department of Oncology, The Sol Goldman Pancreatic Cancer Research Center, The Johns Hopkins Medical Institutions, Baltimore, MD, USA.
Noriyoshi Fukushima, Department of Pathology, The University of Tokyo, Tokyo, Japan.
REFERENCES
- 1.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–657. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffan TA, Horton KM, Klein AP, Fishman EK, Johnson PT, Hruban RH. Prevelance of unsuspected pancreatic cysts on MDCT. doi: 10.2214/AJR.07.3340. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klöppel G. Pseudocysts and other non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 2000;17:7–15. [PubMed] [Google Scholar]
- 4.Hruban RH, Pitman MB, Klimstra DS. Atlas of Tumor Pathology. Washington, DC: American Registry of Pathology and Armed Forces Institute of Pathology; 2007. Tumors of the pancreas. [Google Scholar]
- 5.Wilentz RE, Albores-Saavedra J, Hruban RH. Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol. 2000;17:31–42. [PubMed] [Google Scholar]
- 6.Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Longnecker DS, Adler G, Hruban RH, Klöppel G. Intraductal papillary-mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 237–240. [Google Scholar]
- 8.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 9.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centeno BA, Pitman MB. Fine Needle Aspiration Biopsy of the Pancreas. Oxford: Butterworth Heinemann; 1999. [Google Scholar]
- 11.Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. [Google Scholar]
- 12.Capella C, Solcia E, Klöppel G, Hruban RH. Serous cystic neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 231–233. [Google Scholar]
- 13.Galanis C, Zamani A, Cameron JL, et al. Resected srous cystic neoplasms of the pancreas: A review of 158 patients with recommendations for treatment. J Gastrointest Surg. 2007;11:820–826. doi: 10.1007/s11605-007-0157-4. [DOI] [PubMed] [Google Scholar]
- 14.Compton CC. Serous cystic tumors of the pancreas. Semin Diagn Pathol. 2000;17:43–55. [PubMed] [Google Scholar]
- 15.Neumann HP, Dinkel E, Brambs H, et al. Pancreatic lesions in the von Hippel-Lindau syndrome. Gastroenterology. 1991;101:465–471. doi: 10.1016/0016-5085(91)90026-h. [DOI] [PubMed] [Google Scholar]
- 16.Vortmeyer AO, Lubensky IA, Fogt F, Linehan WM, Khettry U, Zhuang Z. Allelic deletion and mutation of the von Hippel-Lindau (VHL) tumor suppressor gene in pancreatic microcystic adenomas. Am J Pathol. 1997;151:951–956. [PMC free article] [PubMed] [Google Scholar]
- 17.Eriguchi N, Aoyagi S, Nakayama T, et al. Serous cystadenocarcinoma of the pancreas with liver metastases. J Hepatobiliary Pancreat Surg. 1998;5:467–470. doi: 10.1007/s005340050075. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Chari S, Adsay NV, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanno S, Nakano Y, Nishikawa T, et al. Natural history of branch duct intraductal papillary-mucinous neoplasms of the pancreas without mural nodules: Long-term follow-up results. Gut. 2008;57:339–343. doi: 10.1136/gut.2007.129684. [DOI] [PubMed] [Google Scholar]
- 21.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 22.Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- 23.Seidel G, Zahurak M, Iacobuzio-Donahue CA, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Paal E, Thompson LD, Przygodzki RM, Bratthauer GL, Heffess CS. A clinicopathologic and immunohistochemical study of 22 intraductal papillary mucinous neoplasms of the pancreas, with a review of the literature. Mod Pathol. 1999;12:518–528. [PubMed] [Google Scholar]
- 25.Terada T, Ohta T, Kitamura Y, Ashida K, Matsunaga Y. Cell proliferative activity in intraductal papillary-mucinous neoplasms and invasive ductal adenocarcinomas of the pancreas: an immunohistochemical study. Arch Pathol Lab Med. 1998;122:42–46. [PubMed] [Google Scholar]
- 26.Su GH, Hruban RH, Bova GS, et al. Germline and somatic mutations of the STK11/LKB1 Peutz-Jeghers gene in pancreatic and biliary cancers. Am J Pathol. 1999;154:1835–1840. doi: 10.1016/S0002-9440(10)65440-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klöppel G, Lüttges J, Klimstra DS, Hruban RH, Kern SE, Adler G. Solid-pseudopapillary neoplasm. In: Hamilton SR, Aaltonen LA, editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 246–248. [Google Scholar]
- 28.Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Abraham SC, Klimstra DS, Wilentz RE, et al. Solid-pseudopapillary tumors of the pancreas are genetically distinct from pancreatic ductal adenocarcinomas and almost always harbor beta-catenin mutations. Am J Pathol. 2002;160:1361–1369. doi: 10.1016/s0002-9440(10)62563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adsay NV, Merati K, Andea A, et al. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087–1095. doi: 10.1097/01.MP.0000028647.98725.8B. [DOI] [PubMed] [Google Scholar]
- Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- Zamboni G, Scarpa A, Bogina G, et al. Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol. 1999;23:410–422. doi: 10.1097/00000478-199904000-00005. [DOI] [PubMed] [Google Scholar]