Abstract
BACKGROUND
Racial differences in mortality among veterans with diabetes are less well characterized than those in the general population.
OBJECTIVE
To examine racial differences in all-cause mortality in a large sample of veterans with diabetes.
DESIGN
A retrospective cohort.
PARTICIPANTS
Participants comprised 8,812 veterans with type 2 diabetes.
MEASUREMENTS
The main outcome measure was time to death. The main predictor was race/ethnicity. Other risk factors (or covariates) included age, gender, marital status, employment, glycosylated hemoglobin (HgbA1c), and several ICD-9 coded physical and mental health comorbidities.
RESULTS
Average follow-up was 4.5 years; 64% of veterans were non-Hispanic whites (NHW), 97% male, and 84% at least 50 years old. The overall mortality rate was 15% and was significantly lower for non-Hispanic blacks (NHB). Baseline HgbA1c values also differed for NHW (mean = 7.05) and NHB (mean = 7.65) (p < 0.001). In sequentially-built models NHB race was associated with a lower risk of mortality with HR ranging 0.80-0.92. After adjusting for all significant covariates, the risk of mortality remained lower for NHB (HR = 0.84, 95% CI: 0.75, 0.94). Increased mortality risk was associated with age, not being employed or retired, poor glycemic control, cancer, Coronary Heart Disease (CHD), and anxiety disorder; while a lower risk was associated with being female and ever being married.
CONCLUSIONS
The risk of death among NHB veterans with diabetes remained significantly lower than that of NHW after controlling for important confounding variables. Future studies in the VA need to examine detailed contributions of patient, provider and system-level factors on racial differences in mortality in adults with diabetes, especially if the findings of this study are replicated at other sites or using national VA data.
KEY WORDS: race, all-cause mortality, diabetes, veterans
INTRODUCTION
Diabetes is a major contributor to disability and is the sixth leading cause of death1. Cardiovascular disease (CVD) is the leading cause of death in the United States and the predominant cause of death among individuals with diabetes accounting for nearly 65% of all deaths2,3. While overall age-adjusted mortality rates have declined 25% since 1980, diabetes prevalence has increased with age and over time4,5. Several population-based studies show trends in age-adjusted mortality by race. Overall, blacks have a 30% higher mortality rate than whites and have twice the rate of diabetes-related deaths as whites1,5.
Regarding disease management, epidemiologic evidence shows worse glycemic control6 among blacks. Higher rates of chronic hyperglycemia have been associated with an increased risk of CVD mortality in persons with diabetes7. While there is an established evidence base for differences in mortality among blacks and whites, racial disparities in mortality among a high-risk population of veterans with diabetes lacks strong convincing data. The results of several large-scale studies have used administrative data to support the finding of lower mortality among elderly black veterans8; not among those younger than 65 years of age9,10. A major limitation of administrative datasets in reporting disease states is its heavy dependence on accurate coding for record-keeping and/or payment purposes11 rather than accurate patient-level data, thus providing a picture for disease burden but lacking in other important confounders. Therefore, relevant clinical variables (such as disease duration and severity, laboratory markers, etc.) are often excluded but are imperative in determining the contribution of these factors on clinical outcomes and mortality. The findings from these studies need to be substantiated by trials that take into account clinically relevant factors such as disease severity, disease management, and co-morbid conditions12.
In the era of reducing and eliminating health disparities and improving health outcomes it is crucial that studies are based on data with greater predictive ability12, especially the impact of race on the mortality outcome of a potentially devastating disease such as diabetes. This study uses a 9-year time period of data from veterans with type 2 diabetes and a semi-parametric Cox model to study the association between the risk of mortality and race after adjusting for several covariates.
METHODS
Study Design
Veterans with type 2 diabetes (hereafter referred to as diabetes) were identified based on having at least two ICD-9 codes for diabetes (250.xx) in either outpatient or inpatient files and having two or more visits each year since diagnosis based on a previously validated algorithm. We used these criteria to assemble a retrospective cohort of 8,812 veterans. We included those having two or more HgbA1c measurements in the interval of April 1997 to May 2006 at a southeastern VA facility. Socio-demographic and clinical variables were collected from medical records. Date of death was obtained from administrative files.
Subjects
A dataset was created from this cohort of veterans with diabetes using multiple patient and administrative files from the Veterans Health Administration (VHA) Decision Support System (DSS) files linked by Social Security Number (SSN). SSN were stripped after creating the database to maintain protection of personal information. The VHA DSS is a national automated management information system based on commercial software to integrate data from clinical and financial systems for both inpatient and outpatient care. The following DSS datasets were merged in developing the diabetes dataset: 1) Discharge files; 2) Outpatient files; 3) Laboratory files—laboratory results datasets for specific lists of tests separated into inpatient and outpatient files; 4) Pharmacy files—prescription, unit dose, and intravenous pharmacy detail for inpatient and outpatient files; 5) Treating specialty — specialty directing treatment on admission and specialty on discharge; and 6) Cost—costs by Diagnostic Related Groups (DRGs), readmissions within a certain numbers of days, average patient costs, and cost details for selected clinic stops. The datasets were merged and cleaned and then used as the final dataset for analysis.
Outcome Measures
The main outcome measure was time to death, which was defined in two different ways: first, time to death was defined as time to death in quarters (i.e., 3-month periods) between date of entry into the study and date of death (or date last seen or May 2006) and second, time to death was defined as the number of years from age at time of entry into the study to age at time of death (or day last seen or May 2006). We report analysis based on age as time scale13. Time to death is considered in two different ways—calendar time and age14,15 and results were compared. This comparison helps to investigate sensitivity of the results to the choice of time scale. We reported results based on age since both methods lead to similar results.
Covariates
Race/ethnicity was based on self-report. The analysis was limited to NHW and NHB to allow for easier comparison with prior studies. Hispanics were excluded since the sample size was too small to make meaningful inferences. Socio-demographic and clinical variables were compared across racial groups using pooled t-tests for continuous variables and chi-square tests for categorical variables. Other risk factors (or covariates) included age, gender, marital status, employment status, glycemic control, and ICD-9 coded co-morbidities (coronary heart disease–CHD, depression, hypertension, stroke, cancer, major depressive disorder-MDD, generalized anxiety disorder-GAD, bipolar disorder, post-traumatic stress disorder-PTSD, psychotic disorder, substance abuse disorder). Age was categorized into four groups (<50, 50-64, 65-74, and 75+ years). Gender was treated as a categorical variable. Marital status was classified as never married, married, or separated/widowed/divorced. Employment was classified as employed, not employed, or retired. Glycemic control was coded as a 1 when the glycosylated hemoglobin (HgbA1c) was ≥8.0% and 0 when HgbA1c <8.0%. Important co-morbidity variables with high prevalence were defined based on enhanced ICD-9 codes using validated algorithms16. Cancer was defined with codes 140-208; psychotic disorder—codes 295.1, 295.2, 295.3, 295.4, 295.6, and 295.7; bipolar disorder—codes 296.0, 296.4, 296.5, 296.6, and 296.7; MDD—codes 296.2 and 296.3; GAD—code 300.2; substance abuse disorder—codes 303.9, 304, and 305.x; PTSD—code 309.81; hypertension—codes 401-405; CHD—codes 410-414; and stroke—codes 430-438.
Statistical Analysis
In preliminary analyses crude associations were examined between mortality and all measured covariates in our study population of NHB and NHW patients with diabetes using chi-square tests for categorical variables and t-tests for continuous variables. Cox regression methods were used to model the association between race and time to death. Time to death was defined as the number of years from age at time of entry to the cohort to age at time of death or censoring (i.e., day last seen or May 2006). Death was coded as 1 and 0 if censored. For the Cox model, appropriateness of the assumption of proportionality was determined by examining log{-log(time)} plots and by testing the coefficients of the interactions of time with the respective covariate in multivariate analyses. Initially, four models were created to provide hazard ratios (HR) for mortality risk, all adjusted by race and then sequentially by a specific set of covariates. The first model was adjusted for race only; the second model was adjusted for race and age. The third model was adjusted with race and the set of socio-demographic variables; and the fourth model was adjusted for race and the set of comorbid conditions. The final Cox model was adjusted for all statistically significant covariates (race, socio-demographics, and comorbidities). Inference on these parameters is based on the sandwich variance estimator17, which accounts for the possible bias in the variance of the estimated parameters due to unaccounted random effects. The Kaplan–Meier method was used to plot the survival function. Residual analysis was used to assess goodness-of-fit of each of the models. All data analyses were conducted using SAS 9.1.318.
RESULTS
Table 1 shows socio-demographic and clinical characteristics of the study cohort by racial/ethnic group. The mean length of follow up for the cohort was 4.5 years. NHW comprised 64% of the sample; 96% were male. The average age at entry was 61.5 years (sd 11.3) and showed a statistically significant difference (p < 0.001) between races with NHB being younger than NHW (63 and 58 years, respectively). The overall mortality rate was 15% with a statistically significant difference between races. Of the 5,666 NHW veterans, 16% died and 14% of the 3,146 NHB veterans died. Baseline HgbA1c values were also significantly different by race [NHW mean 7.05 (sd 1.65); NHB 7.65 (2.18); p < 0.001] with an overall mean of 7.27 (sd 1.88). At baseline, the distribution of the four most common co-morbid conditions was 31% with hypertension, 18% with substance abuse disorder, 17% with CHD, and 10% with MDD. When comparing these co-morbid conditions and socio-demographics by race; all were significant (p < 0.05) except for stroke and bipolar disorder.
Table 1.
Socio-Demographic and Clinical Characteristics
| All | Non-Hispanic White | Non-Hispanic Black | p-value | |
|---|---|---|---|---|
| N (%) | 8812 (100) | 5666 (64) | 3146 (36) | <0.001 |
| Survivor Status | ||||
| Dead | 1348 (15) | 902 (16) | 446 (14) | |
| Alive | 7464 (85) | 4764 (84) | 2700 (86) | 0.029 |
| Length of Follow-up | 4.5 (2.5) | 4.3 (2.5) | 4.9 (2.6) | <0.001 |
| Age at Entry, years (mean ± sd) | 61.5 ± 11.3 | 63.1 ± 10.6 | 58.5 ± 12.0 | <0.001 |
| Age Category | ||||
| <50 years | 1371 (16) | 556 (10) | 815 (26) | |
| 50-64 years | 3649 (41) | 2405 (42) | 1244 (40) | |
| 65-74 years | 2602 (30) | 1841 (33) | 761 (24) | |
| 75+ years | 1190 (13) | 864 (15) | 326 (10) | <0.001 |
| Gender | ||||
| Male | 8589 (97) | 5546 (98) | 3043 (97) | |
| Female | 223 (3) | 120 (2) | 103 (3) | 0.001 |
| Baseline HgbA1c (mean ± sd) | 7.27 ± 1.88 | 7.05 ± 1.65 | 7.65 ± 2.18 | <0.001 |
| Average Glycemic Control (HgbA1c) | ||||
| Good, <8.0% | 5742 (65) | 3912 (69) | 1812 (58) | |
| Poor, ≥8.0% | 3088 (35) | 1754 (31) | 1334 (42) | <0.001 |
| Marital Status | ||||
| Never married | 562 (6) | 259 (4) | 303 (10) | |
| Married | 5701 (65) | 3831 (68) | 1870 (59) | |
| Separated, divorced or widowed | 2549 (29) | 1576 (28) | 973 (31) | <0.001 |
| Employment Status | ||||
| Active military duty or employed | 1801 (20) | 1093 (19) | 708 (23) | |
| Not employed | 4361 (50) | 2733 (48) | 1628 (52) | |
| Retired | 2650 (30) | 1840 (33) | 810 (25) | <0.001 |
| Hypertension | 2711 (31) | 1650 (29) | 1061 (34) | <0.001 |
| Coronary Heart Disease | 1515 (17) | 1133 (20) | 382 (12) | <0.001 |
| Cancer | 530 (6) | 294 (5) | 236 (8) | <0.001 |
| Stroke | 343 (4) | 238 (4) | 105 (3) | 0.050 |
| Bipolar Disorder | 229 (4) | 144 (3) | 85 (3) | 0.069 |
| Generalized Anxiety Disorder | 276 (3) | 212 (2) | 64 (1) | 0.001 |
| Major Depressive Disorder | 883 (10) | 531 (9) | 352 (11) | 0.015 |
| Posttraumatic Stress Disorder | 556 (6) | 280 (5) | 276 (9) | <0.001 |
| Psychotic | 258 (3) | 98 (2) | 160 (5) | <0.001 |
| Substance Abuse | 1618 (18) | 908 (16) | 710 (23) | <0.001 |
P-value < 0.05 is statistically significant
Intermediate regression models were sequentially created to examine the impact of specific sets of covariates on the association between risk of mortality and race among veterans with diabetes. Table 2 demonstrates the HR and confidence intervals for four such sequentially-built models. The first model adjusted only for race showed an association with 20% lower risk of death among NHB compared to NHW. In the second model which is adjusted for age, race was similarly significantly associated. Moreover, a nearly threefold higher mortality risk among younger elders, and greater than fivefold increased risk among older elders, all compared to those <50 years old. This association with higher mortality risk with increasing age remained significant in the next model adjusted for race and demographics. NHB race was significantly associated with lower mortality risk, HR 0.88 (079, 0.99) than NHW (p = 0.033). Other variables showing a statistically significant association with lower mortality risk was female gender and being married, while being unemployed or retired was associated with a higher risk of death. In the fourth model adjusting for glycemic control and co-morbidities NHB race was associated with a HR of 0.81 (0.72, 0.91; p < 0.001).
Table 2.
Sequentially-Built Models for Cox Regression*
| Hazard Ratio | 95% Confidence | Limits | p-value | |
|---|---|---|---|---|
| Model 1: Race Only | ||||
| Non-Hispanic Black, NHB (Ref: Non-Hispanic White, NHW) | 0.80 | 0.71 | 0.90 | <0.001 |
| Model 2: Race and Age Category | ||||
| NHB (Ref: NHW) | 0.92 | 0.82 | 1.03 | 0.165 |
| Model 3: Race and Socio-Demographics | ||||
| NHB (Ref: NHW) | 0.88 | 0.79 | 0.99 | 0.033 |
| Model 4: Race and All Covariates | ||||
| NHB (Ref: NHW) | 0.81 | 0.72 | 0.91 | <0.001 |
*Model 1 = unadjusted. Model 2 = adjusted for age category (<50 years, 50-64 years, 65-74 years and 75+ years). Model 3 = adjusted for socio-demographics (age category, gender, marital status, and employment status). Model 4 = adjusted for all covariates (socio-demographics; HgbA1c, a measure of glycemic control; and ICD-9 coded co-morbidities such as coronary heart disease, hypertension, stroke, cancer, bipolar disorder, generalized anxiety disorder, major depressive disorder, posttraumatic stress disorder, psychotic disorder and substance abuse)
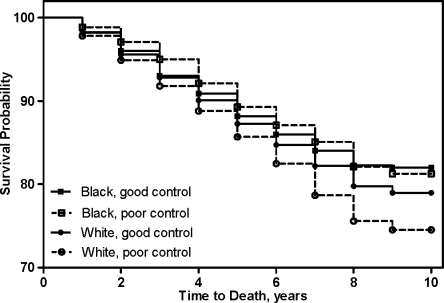
Table 3 shows the parameter estimates for all of the significant covariates in the final Cox model, which includes race, age, gender, employment, marital status, cancer, CHD, AND PTSD. The interaction of race with age (p > 0.10) and with HgbA1c (p = 0.13) were tested and none were significant. When comparing the mortality rates by race after adjusting for all significant covariates, risk of death was significantly lower in NHB race (HR = 0.84, 95% CI 0.75, 0.94) compared to NHW (p = 0.003). Poor glycemic control (HgbA1c >8.0%) was associated with a 29% higher risk of death (also see Fig. 1). Moreover, a lower risk of death was associated with being female and being married. Among comorbid conditions, the risk of mortality was more strongly associated with cancer, (HR 1.79, 95% CI 1.51, 2.12) followed by hypertension, (HR 1.25, 95% CI 1.07, 1.45) and CHD, (HR 1.18, 95% CI 1.02, 1.36) while a PTSD diagnosis was associated with a 20% lower risk of mortality. None of the other comorbidities were significant.
Table 3.
Final Cox Regression Model for Risk of Mortality
| Hazard Ratio | 95% Confidence | Limits | p-value | |
|---|---|---|---|---|
| NHB (Ref: NHW) | 0.84 | 0.75 | 0.94 | 0.003 |
| Age Category (Ref: <50 years) | ||||
| 50-64 years | 1.35 | 1.08 | 1.70 | 0.009 |
| 65-74 years | 2.29 | 1.82 | 2.88 | <0.001 |
| 75+ years | 4.06 | 3.20 | 5.15 | <0.001 |
| Female (Ref: Male) | 0.53 | 0.31 | 0.89 | 0.017 |
| Not Employed | 0.76 | 0.60 | 0.96 | 0.020 |
| Retired | 0.72 | 0.57 | 0.90 | 0.004 |
| Divorced | 2.70 | 2.14 | 3.42 | <0.001 |
| Never Married | 1.99 | 1.55 | 2.55 | <0.001 |
| HgbA1c (Ref: Good Control <8.0%) | 1.29 | 1.15 | 1.44 | <0.001 |
| Hypertension | 1.25 | 1.07 | 1.45 | 0.004 |
| Coronary Heart Disease | 1.18 | 1.02 | 1.36 | 0.025 |
| Cancer | 1.79 | 1.51 | 2.12 | <0.001 |
| Posttraumatic Stress Disorder | 0.83 | 0.64 | 1.08 | 0.171 |
P-value < 0.05 is statistically significant
Figure 1.
Survival curve for time to death by racial group and glycemic control.
DISCUSSION
A significant difference in mortality rates was observed in NHB veterans with diabetes compared to NHW, despite a higher average glycemic level among NHB (7.6) than NHW (7.0). NHB veterans with diabetes also had a mortality benefit with 9% to 25% lower risk after adjusting for all measured socio-demographics (including employment status) or co-morbidities, respectively. This analysis was unique in considering other important confounders including disease severity (by baseline HgbA1c) and glycemic control (HgbA1c over time). A lower risk of death was also associated with being female and ever being married. Not surprisingly, increasing age and having cancer or CHD presented significantly greater associations with risk of death.
The relationship between glycemic control and mortality has been well-established2,7,19. Population-based studies showed that NHB have higher glycemia or worse diabetes control compared to NHW6,20. A recent meta-analysis confirms our finding that NHB have a higher mean HgbA1c than NHW6. Another meta-analysis of studies examining HgbA1c and mortality among people with diabetes showed each 1-point percentage increase in HgbA1c confers a 16% higher risk of CHD-related death7 and a 25% higher risk of total mortality19. Furthermore, a large clinical trial in a non-veteran sample, the Multiple Risk Factor Intervention Trial (MRFIT)21, examined this race–mortality relationship and found a RR of 0.71 (95% CI: 0.53-0.95) among middle-aged NHB compared to NHW for CHD-related mortality after adjusting for socio-demographic characteristics and CVD risk factors. No significant difference was seen in other underlying causes of death (CVD, stroke, renal disease, diabetes, cancer, other causes, or all) between NHB and NHW21. Our findings in a relatively older veteran sample are consistent with the findings of this non-veteran study.
A “reverse disparity” in the race–mortality relationship, where NHB elderly (>65 years old) have a survival benefit over elderly NHW, has been demonstrated in studies examining all-cause or specific cause-related deaths in individuals with diabetes8–10,21–23. The sample in this study represents an older (generally elderly) cohort of individuals with type 2 diabetes. Although standardized mortality rates are typically higher among NHB than NHW and increasingly older populations5, findings from prospective21,22 and retrospective8–10,23 studies consistently show a higher risk and rate of death among elderly White men compared to elderly Black men. Both inpatient and post-discharge (30-day and 6-month) mortality rates remained consistently lower among black veterans (RR 0.77-0.86) compared to their white counterparts8. Another study of racial differences in mortality among veterans examined 30-day mortality for common conditions including acute myocardial infarction, pneumonia, congestive heart failure, hip fracture, stroke, and gastrointestinal bleeding10. A significantly lower risk-adjusted, 30-day mortality was demonstrated among those black veterans who were older than 65 years of age (OR ranging 0.70 for CHF to 0.90 for pneumonia); otherwise, no mortality difference was seen by race for younger veterans. This mortality advantage persisted for each measurement point even after adjusting for patient and hospital characteristics8,10. This study differs from these two important studies in that we examined the race–mortality relationship in a longitudinal sample, we included veterans cared for in both inpatient and outpatient settings and focused on diabetes-related mortality, we included a more homogeneous sample with less regional variability and we used clinical variables to better assess diabetes severity. Thus, our findings strongly support the conclusion of a survival benefit among older NHB veterans in an equal-access VA healthcare system.
Although these results offers important information regarding the association between race/ethnicity and mortality derived from a large sample of veterans followed in a longitudinal study, the reason for lower mortality among NHB compared to NHW is not readily apparent. We can offer the following explanations for the mortality difference by race based on data from our study and evidence from prior studies: (1) NHW are more likely to be dual users of the VA and commercial health systems, which has been associated with more fragmented care, therefore an increased attributable risk of death (15.6%) compared to those who are solely VA users24,25; (2) NHB who survive to their elderly years (65 and older) may be healthier than those who die before 65 years of age (survivor bias26,27); (3) in this region with a high obesity prevalence NHB veterans may have lower mortality rates than NHW across similar levels of obesity as measured by BMI28; (4) worse disease severity (poorer glycemic control) among NHB veterans with diabetes may increase the likelihood of providers intensifying their treatment and monitoring (possibly reflected by significantly longer follow-up among NHB in this study); (5) more NHW veterans had tighter glycemic control than NHB (A1c 7.0 versus 7.6, respectively) which may be associated with poorer outcomes29, and (6) NHB veterans more readily access medical care, have greater personal resources and social supports30, and are offered more social resources that have increased their capacity to perform better self-care or self-management behaviors translating into better mortality outcomes.
It should be realized that these explanations on racial disparity in mortality among those veterans with diabetes have not taken into account several potentially confounding factors. In this analysis we were not able to include specific CVD risk factors such as previous CVD event, smoking31, hyperlipidemia or use of anti-hyperlipidemic medications, the extent of blood pressure control32 or adherence to diabetes, lipid, and blood pressure lowering medications. Furthermore, socio-demographic, clinical, and behavioral variables that have evidence of an impact on diabetes management such as education31 and/or income, residence, routine medical care or health services utilization, diabetes history (onset and duration), BMI or obesity (weight and height data generally inaccurate), and use of insulin were not examined in the analysis. These results also have limited generalizability to female veterans, to younger veterans and to non-NHB ethnic minorities. In addition, excluding some veterans with missing race data could have biased our findings. While we believe the unreported race information is missing at random, we did a sensitivity analysis and found that there were no significant differences by age, gender, insulin use at baseline, or mean HgbA1c except for some comorbidities and marital status. But, maximum likelihood methods are valid under an ignorable missing data mechanism;33 hence these would not impact the validity of our analysis. Finally, including medication adherence (MPR) reduced our sample size from 8,812 to 7,482 possibly biasing the results by the missing data. Again, the unreported MPR information is likely missing at random; however, we also did a sensitivity analysis by imputing the missing values with either of the two possible values since maximum likelihood methods are valid under the missing at random mechanism. An OR of 0.83 (0.73, 0.93) resulted after imputing missing MPR by 0 and OR 0.84 (0.74, 0.94) after imputing by 1 which are not different from reported results when we ignore the missing values 0.84 (0.74, 0.95).
CONCLUSIONS
The finding of lower mortality risk in a highly disadvantaged population, within a healthcare system that allows for equitable access to care, points to the potential moderating effect of access to care on risk of death. It also points to a need for better understanding of how different racial/ethnic groups engage the system and use services within the VA. It will be important to understand whether the observed differences are a function of increased access to care for NHB veterans, less fragmented care for NHB veterans, differential utilization of available resources for diabetes self-management in the VA, more aggressive management, or whether due to non-clinical variables such as social support. Future studies in the VA need to examine detailed contributions of patient, provider and system-level factors on racial/ethnic differences in mortality in adults with diabetes, especially if the findings of this study are replicated at other sites or using national VA data.
Acknowledgment
This study represents work supported by the use of resources at the VA HSR&D Funded Center for Disease Prevention and Health Interventions for Diverse Populations (REA 08-261) in Charleston, SC.
Conflict of Interest None disclosed.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2008.
- 2.Khaw KT, Wareham N, Bingham S, Luben R, Welch A, Day N. Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann Intern Med. 2004;141(6):413–20. doi: 10.7326/0003-4819-141-6-200409210-00006. [DOI] [PubMed] [Google Scholar]
- 3.Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med. 2004;140(11):945–50. doi: 10.7326/0003-4819-140-11-200406010-00035. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Gu Q, Cheng YJ, Narayan KM, Cowie CC. Mortality trends in men and women with diabetes, 1971 to 2000. Ann Intern Med. 2007;147(3):149–55. doi: 10.7326/0003-4819-147-3-200708070-00167. [DOI] [PubMed] [Google Scholar]
- 5.Heron MP, Hoyert DL, Murphy SL, Xu JQ, Kochanek KD, Tejada-Vera B. Deaths: Final data for 2006. National vital statistics reports; vol 57 no 14. Hyattsville: National Center for Health Statistics; 2009. [PubMed] [Google Scholar]
- 6.Kirk JK, D'Agostino RB, Jr, Bell RA, et al. Disparities in HgbA1c levels between African-American and non-Hispanic white adults with diabetes: a meta-analysis. Diab Care. 2006;29(9):2130–6. doi: 10.2337/dc05-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med. 2004;141(6):421–31. doi: 10.7326/0003-4819-141-6-200409210-00007. [DOI] [PubMed] [Google Scholar]
- 8.Jha AK, Shlipak MG, et al. Racial differences in mortality among men hospitalized in the Veterans Affairs health care system. JAMA. 2001;285(3):297–303. doi: 10.1001/jama.285.3.297. [DOI] [PubMed] [Google Scholar]
- 9.Polsky D, Lave J, Klusaritz H, et al. Is lower 30-day mortality posthospital admission among blacks unique to the Veterans Affairs health care system? Med Care. 2007;45(11):1083–9. doi: 10.1097/MLR.0b013e3180ca960e. [DOI] [PubMed] [Google Scholar]
- 10.Volpp KG, Stone R, Lave JR, et al. Is thirty-day hospital mortality really lower for black veterans compared with white veterans? Health Serv Res. 2007;42(4):1613–31. doi: 10.1111/j.1475-6773.2006.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billings J. Using Administrative Data To Monitor Access, Identify Disparities, and Assess Performance of the Safety Net. Tools for Monitoring the Health Care Safety Net. . Rockville, MD: Agency for Healthcare Research and Quality; September 2003: http://www.ahrq.gov/data/safetynet/billings.htm. Accessed May 4, 2010.
- 12.Mark DH. Race and the limits of administrative data. JAMA. 2001;285(3):337–8. doi: 10.1001/jama.285.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145(1):72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. Hoboken: John Wiley & Sons Inc.; 2002. [Google Scholar]
- 15.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott Williams and Wilkins; 1998. [Google Scholar]
- 16.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 17.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distribution. J Am Stat Assoc. 1989;84:1065–73. doi: 10.2307/2290084. [DOI] [Google Scholar]
- 18.SAS 9.1.3 (Cary, NC).
- 19.Shalev V, Chodick G, Bialik M, Green MS, Heymann AD. In a population-based cohort of diabetes patients, men and women had similar risks for all-cause mortality. J Clin Epidemiol. 2007;60(1):86–93. doi: 10.1016/j.jclinepi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Ford ES, Li C, Little RR, Mokdad AH. Trends in A1C concentrations among U.S. adults with diagnosed diabetes from 1999 to 2004. Diab Care. 2008;31(1):102–4. doi: 10.2337/dc07-0565. [DOI] [PubMed] [Google Scholar]
- 21.Vaccaro O, Stamler J, Neaton JD. Sixteen-year coronary mortality in black and white men with diabetes screened for the Multiple Risk Factor Intervention Trial (MRFIT) Int J Epidemiol. 1998;27(4):636–41. doi: 10.1093/ije/27.4.636. [DOI] [PubMed] [Google Scholar]
- 22.Valdez R, Narayan KM, Geiss LS, Engelgau MM. Impact of diabetes mellitus on mortality associated with pneumonia and influenza among non-Hispanic black and white US adults. Am J Public Health. 1999;89(11):1715–21. doi: 10.2105/AJPH.89.11.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polsky D, Jha AK, Lave J, et al. Short- and long-term mortality after an acute illness for elderly whites and blacks. Health Serv Res. 2008;43(4):1388–402. doi: 10.1111/j.1475-6773.2008.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolinsky FD, Miller TR, An H, Brezinski PR, Vaughn TE, Rosenthal GE. Dual use of Medicare and the Veterans Health Administration: are there adverse health outcomes? BMC Health Serv Res. 2006;6:131. doi: 10.1186/1472-6963-6-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmer D, Sambamoorthi U, Shen Y, et al. Opting out of an integrated healthcare system: dual-system use is associated with poorer glycemic control in veterans with diabetes. Prim Care Diabetes. 2008;2(2):73–80. doi: 10.1016/j.pcd.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Johnson N. The racial crossover in comorbidity, disability, and mortality. Demography. 2000;37(3):267–83. doi: 10.2307/2648041. [DOI] [PubMed] [Google Scholar]
- 27.Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–10. doi: 10.1681/ASN.2007070747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341(15):1097–110. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 29.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 30.Hermos JA. Health outcomes for black and white patients in the Veterans Affairs health care system. JAMA. 2001;285(14):1837. doi: 10.1001/jama.285.14.1837. [DOI] [PubMed] [Google Scholar]
- 31.Miech RA, Kim J, McConnell C, Hamman RF. A growing disparity in diabetes-related mortality U.S. trends, 1989–2005. Am J Prev Med. 2009;36(2):126–32. doi: 10.1016/j.amepre.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heisler M, Faul JD, Hayward RA, Langa KM, Blaum C, Weir D. Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the health and retirement study. Arch Intern Med. 2007;167(17):1853–60. doi: 10.1001/archinte.167.17.1853. [DOI] [PubMed] [Google Scholar]
- 33.Little RJA, Rubin DB. Statistical analysis with missing data. 2. Hoboken: Wiley; 2002. [Google Scholar]