Abstract
BACKGROUND AND OBJECTIVE
Affect and how it is regulated plays a role in pain perception, maintenance of pain, and its resolution. This randomized, controlled trial evaluated an innovative affective self-awareness (ASA) intervention, which was designed to reduce pain and improve functioning in individuals with fibromyalgia.
PARTICIPANTS AND METHODS
Forty-five women with fibromyalgia were randomized to a manualized ASA intervention (n = 24) or wait-list control (n = 21). The intervention began with a one-time physician consultation, followed by 3 weekly, 2-h group sessions based upon a mind-body model of pain. Sessions focused on structured written emotional disclosure and emotional awareness exercises. Outcomes in both conditions were measured by a blinded assessor at baseline, post-intervention, and 6-month follow-up.
MEASURES
The primary outcome was pain severity (Brief Pain Inventory); secondary outcomes included tender-point threshold and physical function (SF-36 Physical Component Summary). Intent-to-treat analyses compared groups on outcomes using analysis of covariance and on the proportion of patients achieving ≥30% and ≥50% pain reduction at 6 months.
RESULTS
Adjusting for baseline scores, the intervention group had significantly lower pain severity (p < 0.001), higher self-reported physical function (p < 0.001), and higher tender-point threshold (p = 0.02) at 6 months compared to the control group. From baseline to 6 months, 45.8% of the ASA intervention group had ≥30% reduction in pain severity, compared to none of the controls (p < 0.001).
CONCLUSIONS
The affective self-awareness intervention improved pain, tenderness, and self-reported physical function for at least 6 months in women with fibromyalgia compared to wait-list control. This study suggests the value of interventions targeting emotional processes in fibromyalgia, although further studies should evaluate the efficacy of this intervention relative to active controls.
KEY WORDS: fibromyalgia, psychophysiological, mind-body, clinical trial, randomized, psychological, meditation, therapeutic writing
INTRODUCTION
Fibromyalgia is characterized by chronic widespread pain (for at least 3 months in four body quadrants) and excessive tenderness in at least 11 of 18 tender points1. Common co-morbid conditions include fatigue, headaches, irritable bowel syndrome, and temporo-mandibular joint disorder2,3. Fibromyalgia affects 2–4% of the population, particularly women4, and despite neurological underpinnings5–7, many clinicians view fibromyalgia in the spectrum of medically unexplained syndromes8.
The suffering of this population is substantial, there is little spontaneous improvement in symptoms over time9,10, and effective treatment approaches are needed11. Despite new pharmaceutical options, lack of therapeutic response occurs in at least half of patients12,13. Non-pharmacological treatments, such as exercise, behavioral activation, and cognitive-behavioral therapy, enhance physical function and mood, but have limited effects on pain14–17.
The limited efficacy of current treatments for fibromyalgia may stem, in part, from the minimal emphasis placed upon psychological stress regulation of affect. Individuals with fibromyalgia report elevated lifetime experiences of victimization, including childhood trauma (e.g., physical or sexual abuse) and adult stressors (e.g., marital discord, work conflict)18–20. Such histories likely contribute to the elevated anxiety and depressive disorders found in these individuals21–23. These retrospective data are supported by prospective findings that workers exposed to workplace bullying had a four-fold increased rate of developing fibromyalgia24. Negative effects of stressors can be maintained by avoiding or inhibiting emotions25. Studies suggest that individuals with fibromyalgia are more likely than controls to have deficits in emotional awareness, difficulty distinguishing positive from negative emotions, and reluctance to verbally express feelings, particularly anger26–30. These emotional limitations are linked to increased pain28,30.
We developed a treatment approach for pain disorders of central pain augmentation7, including fibromyalgia, which focuses on the relevance of emotional factors in the onset and exacerbation of symptoms. This model was influenced by one popularized in the lay press31,32 that emphasizes the importance of an internal locus of control over one’s health33. This approach is also supported by recent theories on emotion and pain, including the roles of fear avoidance34 and acceptance of pain experiences35. Finally, there is evidence demonstrating the beneficial effects of writing about stressful experiences and mindfulness meditation in fibromyalgia36–40.
We report here on a randomized, wait-list controlled trial demonstrating post-treatment and 6-month follow-up effectiveness of an Affective Self-Awareness (ASA) intervention, which places primary importance on the awareness and expression of emotions underlying the initiation and exacerbation of fibromyalgia symptoms.
METHODS
Participants
Participants were recruited through flyers sent to physicians, local advertisements, and presentations at fibromyalgia support group meetings. Interested individuals underwent a screening interview and 2-h evaluation performed by a physical medicine and rehabilitation specialist (M.C.H.) that included a thorough medical history and physical, along with baseline tender point assessment to confirm the diagnosis.
Inclusion criteria were: female; aged 18 or older; and fulfilling the 1990 criteria for fibromyalgia1. To increase generalizability, we had few exclusion criteria: serious co-morbid medical conditions that could confound the influence of fibromyalgia in the next 6 months (e.g., cancer, heart disease); current, serious psychiatric disorders involving psychotic symptoms, recent suicide risk or substance abuse, as determined by a structured interview based on DSM-IV criteria41; and changes in pain medication within 1 month prior to enrollment, to maintain stability in medical care. We did not exclude patients in psychiatric treatment or who had other psychiatric disorders (e.g., anxiety, post-traumatic stress disorder, depression), autoimmune disorders, or other disorders of central sensitization (e.g., irritable bowel syndrome, migraine headaches). All participants signed IRB-approved informed consents and were reimbursed $50 for completing the assessments; the intervention was free-of-charge.
Randomization and Assessor Blinding
A computer-generated randomization scheme allocated cases. Sealed envelopes containing assignment to the intervention or wait-list (WL) control were handed to qualifying subjects following the baseline assessment, and the assessor remained blinded to group assignments throughout the 6-month study period.
Study Interventions
The ASA intervention followed a manualized protocol. The physician (H.S.) first conducted a 90-min individual consultation to investigate participants’ medical and psychosocial history and identify linkages between life stressors, emotional responses, and onset and exacerbation of symptoms of fibromyalgia and associated disorders42. The pain experience of each participant was validated, and a model for understanding pain as a “mind-body syndrome” was offered: affectively charged experiences amplify pain processing in the central nervous system, contributing to the development and maintenance of fibromyalgia symptoms. Participants were asked to read The Mindbody Prescription by Sarno as a standardized text supporting the intervention31.
Following the consultation, groups of 8 to 12 patients attended three 2-h small-group sessions, held at 1-week intervals, conducted by the physician (H.S.). The curriculum adhered to the treatment manual and consisted of four components: education regarding a psychophysiological model of chronic pain, written emotional disclosure about stress, affective awareness techniques, and re-engagement in previously avoided activities. The education component included research and case studies documenting the central role of biopsychosocial processes in fibromyalgia and associated conditions. Written emotional disclosure was performed for 30 min daily as homework and consisted of writing about stress and emotions in free-writing prose, unsent letters, and imagined dialogues. Affective awareness techniques consisted of daily CD-guided exercises that encouraged mindfulness towards one's breath, body, and emotions; non-judgmental awareness of these emotions; and affirmations of self-acceptance and self-healing. Re-engagement in activity consisted of increased physical and leisurely activity and to not allow pain to dissuade them from engaging in important relational experiences and other activities. After the final session, the physician contacted each participant for a 15–20-min phone call to address any remaining concerns and encourage further practice. Participants randomized to the control group were free to engage in any interventions on their own, as recommended by their providers, and were invited to participate in the ASA intervention following a 6-month waiting period.
Outcome Measures
All measures, except tender-point threshold, were assessed at baseline, post-intervention (6 weeks post-randomization for controls), and 6 months post-randomization. The primary outcome measure was pain severity, measured by the Brief Pain Inventory (BPI43) Pain Severity scale, which yields four pain intensity ratings: current pain, worst, least, and average pain over the previous week, each rated on a 0–10 scale (0 = “no pain” and 10 = “pain as bad as you can imagine”). The BPI is well validated in chronic pain populations44.
Secondary pain outcomes included: The number of painful body regions (BPI Painful Body Regions, range 0–29); interference of pain on daily tasks (BPI Pain Interference, range 0–10); tender-point threshold (explained below); health-related physical and mental function (SF-3645, Physical Component Summary and Mental Component Summary (mean = 50, SD = 10; higher scores indicating better functioning); fatigue (Multidimensional Fatigue Inventory46, “General Fatigue” scale, range 4–20); sleep disturbance (Medical Outcomes Study Sleep Scale47, “Sleep Problems Index 2”, range 0–100); beliefs about pain control (Beliefs about Pain Control Questionnaire48, “Powerful Others” scale, range 4–24), which is a measure of locus of control. These measures have been widely used in studies of fibromyalgia or related conditions and have good reliability and validity.
Tender-point threshold was assessed at baseline and 6 months post-randomization using a modified dolorimeter (Chatillon, model LG-010), containing a 1-cm diameter rubber stopper over the bottom shaft of the force gauge. At each of the 18 tender points1, increasing pressure was applied at a rate of 1 kg/s, and the participant was instructed to say “pain” as soon as the sensation changed from an experience of pressure to definitive pain. Tender-point threshold was calculated as the average pain threshold (kg) over all 18 tender points, with a higher threshold indicating decreased pain sensitivity49,50. Mean tender point threshold has been used as a secondary outcome measure in several fibromyalgia clinical trials51–53.
Sample Size Calculation and Statistical Analysis
Although previous pilot data were not available, we expected a modest (1.5 points on a 10-point scale) reduction in BPI Pain Severity score attributable to the intervention, relative to control. Given previously reported SDs of 1.8 on this scale in fibromyalgia54, the sample size needed to detect an effect of this size, with a two-tailed alpha of 0.05 and power of 0.80, was 18 per group. Accounting for an expected 20% drop-out rate, a total sample size of 45 was necessary. All variables were tested for normal distribution prior to analyses. To determine the success of randomization, baseline values were compared between groups using two-tailed independent samples t-tests. For outcomes, we used intent-to-treat analyses with last observation carried forward for those few patients who withdrew, thereby analyzing all randomized participants. Outcome analyses used analysis of covariance (ANCOVA) with the baseline score serving as the covariate. Also, the primary outcome was examined for “responder” status, that is, whether a patient experienced 30% or 50% reductions in pain severity from baseline to 6-month follow-up, and whether the patient scored less than 3 out of 10 on the BPI Pain Severity scale at 6-month follow-up. These analyses used two-sided Fisher’s (chi-square) exact tests. All tests used alpha of 0.05.
Within-group effect sizes of the ASA intervention on the primary outcome were calculated as a change score (post-treatment or follow-up minus baseline) divided by the ASA group’s baseline SD. Between-groups effect sizes were calculated as the difference between group change scores, divided by the baseline SD for the entire sample.
RESULTS
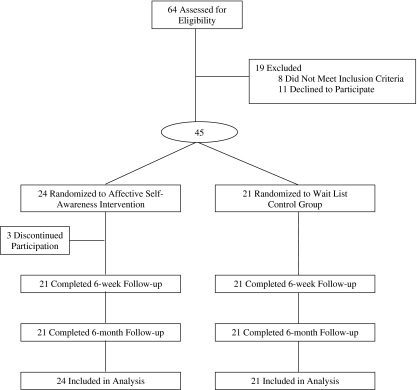
Figure 1 shows participant flow through the study; of the eight women who did not meet the study criteria, seven did not have fibromyalgia, and one had a comorbid medical condition.
Figure 1.
Subject flow diagram.
Forty-five participants were randomized to the ASA group (n = 24) or control group (n = 21). Three intervention participants withdrew after the initial consultation session because of scheduling difficulties. There were no other drop-outs in either condition throughout the remainder of the study.
Of the 45 randomized participants, the ASA and control groups did not differ (all p > 0.22) in demographics or medical history; the sample averaged 50.1 years of age (SD = 10.0, range: 25–66) and 12.7 years since fibromyalgia pain onset (SD = 11.6, range: 1–45); 40% were receiving disability, 55.5% were college graduates, 44.4% had co-morbid affective disorder, 42.2% had cluster B or C personality disorder, and 29% had current or past treatment with duloxetine or pregabalin. Table 1 shows baseline data for each group on the outcome measures. By chance, the ASA group had greater baseline pain severity, fatigue, health-related physical disability, and sleep problems than the WL group, further supporting our decision to covary baseline values in the analyses.
Table 1.
Baseline, Post-treatment, and 6-month Post-Randomization Scores for Primary and Secondary Outcome Measures, by Group
| Characteristics | ASA mean (SD) | WL mean (SD) | t/Fb | p-value |
|---|---|---|---|---|
| Primary outcome | ||||
| BPI-pain severity (range 0–10) | ||||
| Baseline | 6.18 (1.58) | 5.04 (1.18) | 2.7 | 0.009 |
| Post treatment | 4.43 (2.69) | 5.01 (1.80) | 5.1 | 0.03 |
| 6-month post randomization | 4.38 (2.16) | 5.43 (1.31) | 17.0 | <0.001 |
| Secondary outcomes | ||||
| BPI-pain interference (0–10) | ||||
| Baseline | 6.45 (1.62) | 5.37 (2.52) | 1.7 | 0.09 |
| Post treatment | 4.24 (3.09) | 4.68 (2.75) | 2.8 | 0.10 |
| 6-month post randomization | 4.29 (2.56) | 5.51 (2.54) | 7.4 | 0.009 |
| BPI–painful body regions (0–29) | ||||
| Baseline | 19.6 (5.71) | 17.6 (6.22) | 1.1 | 0.27 |
| Post treatment | 10.3 (7.50) | 16.9 (7.98) | 15.1 | <0.001 |
| 6-month post randomization | 11.2 (5.81) | 17.1 (7.42) | 12.5 | 0.001 |
| Tender-point threshold, kg a | ||||
| Baseline | 2.43 (0.65) | 2.81 (0.66) | 2.0 | 0.06 |
| 6-month post randomization | 3.04 (0.86) | 2.66 (0.78) | 6.0 | 0.02 |
| MFI–general fatigue (4–20) | ||||
| Baseline | 17.5 (2.48) | 15.7 (3.13) | 2.2 | 0.03 |
| Post treatment | 14.3 (4.14) | 15.8 (2.62) | 6.9 | 0.01 |
| 6-month post randomization | 16.2 (3.17) | 16.1 (3.73) | 0.4 | 0.54 |
| SF-36 PCS (US mean 50, SD 10)a | ||||
| Baseline | 27.0 (6.68) | 33.5 (7.94) | 2.9 | 0.005 |
| Post treatment | 39.3 (10.0) | 32.8 (8.74) | 24.3 | <0.001 |
| 6-month post randomization | 36.4 (9.58) | 33.9 (8.41) | 15.2 | <0.001 |
| SF-36 MCS (US mean 50, SD 10)a | ||||
| Baseline | 38.7 (11.3) | 41.3 (12.1) | 0.7 | 0.47 |
| Post treatment | 41.6 (14.6) | 40.9 (11.3) | 0.6 | 0.43 |
| 6-month post randomization | 39.4 (14.2) | 43.3 (11.4) | 0.5 | 0.49 |
| MOS Sleep Scale–SPI2 (0–100) | ||||
| Baseline | 48.4 (10.5) | 41.8 (10.8) | 2.1 | 0.045 |
| Post treatment | 42.5 (20.8) | 47.9 (18.8) | 3.1 | 0.08 |
| 6-month post randomization | 51.6 (18.3) | 49.2 (19.5) | 0.2 | 0.67 |
| BPCQ–powerful others (4–24) | ||||
| Baseline | 9.42 (2.52) | 11.1 (3.55) | 1.9 | 0.072 |
| Post treatment | 7.38 (3.49) | 11.1 (4.06) | 6.9 | 0.01 |
| 6-month post randomization | 7.25 (2.82) | 11.6 (3.33) | 17.6 | <0.001 |
For the WL group, 6-week post-randomization scores are used as “post-treatment” scores
aHigher scores indicate better outcome. For all other measures, higher scores indicate worse outcome
bBaseline group differences were tested with a t-test on 43 df. Post-treatment and 6-month post-randomization group differences were tested with an ANCOVA, covarying baseline score, yielding an F(1,42) statistic
Abbreviations: ASA, Affective Self-Awareness workshop group; WL, wait-list control group; ES, effect size; BPI, Brief Pain Inventory; MFI, Multidimensional Fatigue Inventory; SF-36, Medical Outcomes Study 36-Item Short Form Health Survey; PCS, Physical Component Summary; MCS, Mental Component Summary; BPCQ, Beliefs about Pain Control Questionnaire; MOS, Medical Outcomes Study; SPI2, Sleep Problems Index 2
Effectiveness of the ASA Intervention
Table 1 presents group outcomes. Controlling for baseline pain level, the ASA group showed significantly lower pain severity at both post-treatment and follow-up than did the WL group. The within-group treatment effect size was 1.11 SD post-treatment and 1.14 SD at 6 months. The between-groups effect size was 1.14 SD post treatment and 1.46 SD at 6 months, favoring the ASA group.
At 6 months, 45.8% of treatment participants had at least 30% pain reduction, and 20.8% had at least 50% pain reduction, which were significantly greater than the 0% of controls on either index (Χ2 = 12.74, p < 0.001, and Χ2 = 4.92, p = 0.03, respectively). Also, a greater proportion of ASA group participants (25%) had less than 3 out of 10 pain severity by 6 months, compared to none of the WL group (Χ2 = 6.06, p = 0.02).
Regarding secondary outcomes, the ASA group had lower 6-month scores for pain interference and lower post-treatment and 6-month scores for painful body regions. ASA participants had higher post-treatment and 6-month scores for health-related physical function and higher 6-month tender-point thresholds. The ASA group had less fatigue at post-treatment than the control group, but this difference was not observed at 6 months. No significant group differences were noted in mental function or sleep disturbance. Finally, ASA participants had lower post-treatment and 6-month scores for the belief that one’s pain relief depends on “powerful others,” such as physicians.
Ancillary analyses addressed the possibility that group differences in outcomes were due to initial baseline differences, that is, that a few ASA group patients with elevated baseline scores subsequently regressed to the mean. We repeated ANCOVAs after removing patients whose baseline scores were beyond 1.5 inter-quartile range (IQR) above the 75th percentile for pain sensitivity or fatigue, or 1.5 IQR below the 25th percentile for physical function. All significant group differences from the original analyses retained significance and directionality in this sub-analysis. Thus, baseline differences did not account for the positive effect of the ASA intervention.
DISCUSSION
This is the first randomized, controlled study to demonstrate the benefits of a primarily affectively oriented group intervention for fibromyalgia. We found that a relatively short-duration but intensive intervention (i.e., one individual session and three group sessions over 4 weeks) yielded substantial benefits within 6 weeks, and these benefits were maintained at the 6-month endpoint. It is noteworthy that long-term benefits were observed not only in subjective pain, but also in pressure pain threshold and physical functioning. Improvements in both pain and physical function at 6 months or beyond, using intent-to-treat analyses, have thus far been shown in only a handful of interventions for fibromyalgia55–59.
To date, among non-pharmacological treatments for fibromyalgia, cognitive-behavioral therapy (CBT) has the strongest level of evidence supporting its use14. ASA shares some aspects of CBT, particularly a shift in one’s perception that health is controlled by external factors, such as physicians, to internal control. However, CBT emphasizes modifying maladaptive thinking and behavioral responses to pain, and typically either avoids patients’ negative emotional experiences, or attempts to reduce negative emotions as directly as possible. In contrast, ASA emphasizes the value of approaching or confronting one’s stressful experiences, developing awareness of one’s emotions and motivations, and encouraging verbal rather than somatic expression27,28,60.
Two prior fibromyalgia studies using written emotional disclosure asked patients to write about stressful experiences for three or four sessions, and improved pain and function were observed after 3 or 4 months, relative to controls36,37. The between-group effect sizes on pain in these earlier studies, however, were only 0.22 and 0.49—substantially smaller than the effects noted in the current study (1.46 at 6 months). It is possible that the additional techniques—such as a clear statement of the key role played by emotions, more intensive and varied written emotional disclosure, inclusion of mindfulness exercises, and a group format—substantially increased the effect compared with expressive writing or mindfulness meditation alone36,37,40.
There are a few noteworthy limitations of this study. First, the control group did not receive any active or placebo intervention, and therefore did not serve as a control for provider attention, peer-group interaction, personal time devoted to recovery, and other non-specific treatment effects. We did not monitor the interventions received by the control group. Second, perhaps due to our rather small sample size, randomization did not evenly distribute patients on some baseline measures. Covariance analyses and removal of baseline outliers, however, did not change the significance of any of our results, and the ASA group had ending values on significant outcomes that were superior to the controls, suggesting that the improvement in the ASA group extended beyond a regression to the mean.
Third, as in any clinical trial, a biased sample may have chosen to participate in this study. However, this sample of women with fibromyalgia was comparable to those reported in highly cited studies with respect to pain13, physical function and fatigue55, and comorbid affective disorder56, and more than one-third of our participants had already tried pharmacological agents approved for the treatment of fibromyalgia in the US (e.g., pregabalin or duloxetine). Thus, the marked improvement seen in this study is unlikely to be explained by sampling bias leading to an easier-to-treat sample population. Nonetheless, it is likely that individuals who agreed to participate were at least open to the possibility that affective factors could be playing a role in their illness.
Despite our theoretical model, we do not know the mechanisms responsible for the benefits of the ASA intervention, because we did not assess treatment processes, such as changes in emotional awareness or expression. And despite limited sharing of personal information, aspects of the provider-patient relationship and the dynamics of group interactions may play significant roles in patient improvement. As mentioned above, there were no drop-outs from the intervention groups. In addition, attendance was nearly perfect, and the very few patients who missed a single session were contacted by the group leader with the homework assignments. Participants were asked to devote at least an hour per day to course homework and self-care activities, which may have significant benefit, as may behavioral and physical activation17,61.
To address these limitations, we are currently planning a larger study that will not only assess the efficacy of this type of intervention in comparison to an active control group, but will allow for assessment of mediating and moderating variables to help determine mechanisms of action and subgroups of patients that respond best to this intervention.
In conclusion, an affective self-awareness intervention resulted in a sustained reduction in pain and improvement in physical functioning in a sample of women with fibromyalgia compared to wait-listed controls. A notable advantage of the ASA intervention used in this study is the relatively low amount of provider time needed to treat each individual and the relatively short duration protocol. Furthermore, this intervention does not require expensive equipment or pharmaceuticals and may prove to be a preferred adjunctive intervention for fibromyalgia in the primary care setting. Finally, although some practitioners suspect that patients with fibromyalgia are unwilling to consider a psychologically oriented, self-management approach, our experience found substantial interest in this intervention among patients, and attrition was very low. Individuals with fibromyalgia in this study appeared to accept the central messages of the intervention: that the experience of pain in fibromyalgia is real, that fibromyalgia pain is processed in the central nervous system, that unresolved emotional experiences can initiate and perpetuate physical symptoms, and that the mind-body link can be tapped to empower individuals with fibromyalgia to more effectively diminish pain and associated symptoms.
Acknowledgments
Our research was supported in part by the Scott F. Nadler, DO, Research Grant (Physiatric Association of Spine, Sports, and Occupational Rehabilitation); and by grant numbers U020912 (Michigan Institute of Clinical and Health Research); T32-HD007422, K12HD001097 (NICHD/NIH); AR049059, (NIAMS/NIH); and DAMD 17-00-2-0018 (Department of Defense).
These data were presented at the American Academy of Physical Medicine and Rehabilitation; November 22, 2008; San Diego, CA, and the North American Research Council for Complementary and Integrative Medicine, May 14, 2009, Minneapolis, MN.
Conflict of interest statement There is one potential conflict of interest to report. Dr. Schubiner developed the program used in this study. He conducts this program at Providence Hospital and on the Internet. It is unlikely that this was a significant factor in this study since Dr. Hsu, who performed all of the baseline and outcome assessments and all data analyses, remained a blinded assessor throughout the study.
Footnotes
Supported in part by the Scott F. Nadler, DO, Research Grant (Physiatric Association of Spine, Sports, and Occupational Rehabilitation) and by grant numbers U020912 (Michigan Institute of Clinical and Health Research); T32-HD007422, K12HD001097 (NICHD/NIH); AR049059 (NIAMS/NIH); and DAMD 17-00-2-0018 (Department of Defense).
Clinicaltrials.gov identifier NCT00437411
References
- 1.Wolfe F, Smythe HA, Yunus MB, et al. The American College of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 2.Aaron LA, Buchwald D. Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Pract Res Clin Rheumatol. 2003;17:563–74. doi: 10.1016/S1521-6942(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Bradley LA, Clauw DJ, Glass JM, Goldenberg DL. Evaluating and diagnosing fibromyalgia and comorbid psychiatric disorders. J Clin Psychiatry. 2008;69:e28. doi: 10.4088/JCP.1008e28. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe F, Ross K, Anderson J, Russell IJ. Aspects of fibromyalgia in the general population: sex, pain threshold, and fibromyalgia symptoms. J Rheumatol. 1995;22:151–6. [PubMed] [Google Scholar]
- 5.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10:777–91. doi: 10.1016/j.jpain.2009.01.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–22. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36:339–56. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Jackson JL, Kroenke K. Prevalence, impact, and prognosis of multisomatoform disorder in primary care: a 5-year follow-up study. Psychosom Med. 2008;70:430–4. doi: 10.1097/PSY.0b013e31816aa0ee. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner E, Finckh A, Cedraschi C, Vischer TL. A 6 year prospective study of a cohort of patients with fibromyalgia. Ann Rheum Dis. 2002;61:644–5. doi: 10.1136/ard.61.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe F, Anderson J, Harkness D, et al. Health status and disease severity in fibromyalgia: results of a six-center longitudinal study. Arthritis Rheum. 1997;40:1571–9. doi: 10.1002/art.1780400905. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JL, Kroenke K. Managing somatization: medically unexplained should not mean medically ignored. J Gen Intern Med. 2006;21:797–9. doi: 10.1111/j.1525-1497.2006.00514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crofford LJ, Mease PJ, Simpson SL, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain. 2008;136:419–31. doi: 10.1016/j.pain.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 13.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136:432–44. doi: 10.1016/j.pain.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. JAMA. 2004;292:2388–95. doi: 10.1001/jama.292.19.2388. [DOI] [PubMed] [Google Scholar]
- 15.Williams DA, Cary MA, Groner KH, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. J Rheumatology. 2002;29:1280–6. [PubMed] [Google Scholar]
- 16.White KP, Nielson WR. Cognitive behavioral treatment of fibromyalgia syndrome: a followup assessment. J Rheumatol. 1995;22:717–21. [PubMed] [Google Scholar]
- 17.Hassett AL, Bevirtz RN. Nonpharmacologic treatment for fibromyalgia: patient education, cognitive-behavioral therapy, relaxation techniques, and complementary and alternative medicine. Rheum Dis Clin N Am. 2009;35:393–407. doi: 10.1016/j.rdc.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Houdenhove B, Luyten P. Stress, depression and fibromyalgia. Acta Neurol Belg. 2006;106:149–56. [PubMed] [Google Scholar]
- 19.Houdenhove B, Egle U, Luyten P. The role of life stress in fibromyalgia. Curr Rheumatol Rep. 2005;7:365–70. doi: 10.1007/s11926-005-0021-z. [DOI] [PubMed] [Google Scholar]
- 20.Walker EA, Keegan D, Gardner G, Sullivan M, Bernstein D, Katon WJ. Psychosocial factors in fibromyalgia compared with rheumatoid arthritis: II. Sexual, physical, and emotional abuse and neglect. Psychosom Med. 1997;59:572–7. doi: 10.1097/00006842-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Arnold LM, Hudson JI, Keck PE, Auchenbach MB, Javaras KN, Hess EV. Comorbidity of fibromyalgia and psychiatric disorders. J Clin Psychiatry. 2006;67:219–25. doi: 10.4088/JCP.v67n0807. [DOI] [PubMed] [Google Scholar]
- 22.Sherman JJ, Turk DC, Okifuji A. Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. Clin J Pain. 2000;16:127–34. doi: 10.1097/00002508-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Raphael KG, Janal MN, Nayak S. Comorbidity of fibromyalgia and posttraumatic stress disorder symptoms in a community sample of women. Pain Med. 2004;5:33–41. doi: 10.1111/j.1526-4637.2004.04003.x. [DOI] [PubMed] [Google Scholar]
- 24.Kivimäki M, Leino-Arjas P, Virtanen M, et al. Work stress and incidence of newly diagnosed fibromyalgia: prospective cohort study. J Psychosom Res. 2004;57:417–22. doi: 10.1016/j.jpsychores.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz MJ. Stress-response syndromes: a review of posttraumatic and adjustment disorders. Hosp Community Psychiatry. 1986;37:241–9. doi: 10.1176/ps.37.3.241. [DOI] [PubMed] [Google Scholar]
- 26.Dailey PA, Bishop GD, Russell IJ, Fletcher EM. Psychological stress and the fibrositis/fibromyalgia syndrome. J Rheumatol. 1990;17:1380–5. [PubMed] [Google Scholar]
- 27.Brosschot JF, Aarsse HR. Restricted emotional processing and somatic attribution in fibromyalgia. Int J Psychiatry Med. 2001;31:127–46. doi: 10.2190/K7AU-9UX9-W8BW-TETL. [DOI] [PubMed] [Google Scholar]
- 28.Middendorp H, Lumley MA, Jacobs JWG, Doornen LJP, Bijlsma JWJ, Geenen R. Emotions and emotional approach and avoidance strategies in fibromyalgia. J Psychosom Res. 2008;64:159–67. doi: 10.1016/j.jpsychores.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Sayar K, Gulec H, Topbas M. Alexithymia and anger in patients with fibromyalgia. Clin Rheumatol. 2004;23:441–8. doi: 10.1007/s10067-004-0918-3. [DOI] [PubMed] [Google Scholar]
- 30.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 31.Sarno JE. The Mindbody Prescription: Healing the Body, Healing the Pain. New York: Warner Books; 1998. [Google Scholar]
- 32.Selfridge N, Peterson F. Freedom from Fibromyalgia. New York: Three Rivers Press; 2001. [Google Scholar]
- 33.Torres X, Collado A, Arias A, et al. Pain locus of control predicts return to work among Spanish fibromyalgia patients after completion of a multidisciplinary pain program. Gen Hosp Psychiatry. 2009;31:137–45. doi: 10.1016/j.genhosppsych.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Vlaeyen JW, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 35.McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107:159–66. doi: 10.1016/j.pain.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Gillis ME, Lumley MA, Mosley-Williams A, Leisen JC, Roehrs T. The health effects of at-home written emotional disclosure in fibromyalgia: a randomized trial. Ann Behav Med. 2006;32:135–46. doi: 10.1207/s15324796abm3202_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broderick JE, Junghaenel DU, Schwartz JE. Written emotional expression produces health benefits in fibromyalgia patients. Psychosom Med. 2005;67:326–34. doi: 10.1097/01.psy.0000156933.04566.bd. [DOI] [PubMed] [Google Scholar]
- 38.Smyth J, Helm R. Focused expressive writing as self-help for stress and trauma JCLP/In Session: Psychotherapy in Practice. 2003;59:227–35. [DOI] [PubMed]
- 39.Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Soc Sci Med. 1988;26:327–32. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- 40.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness training as an intervention for fibromyalgia: evidence of post-intervention and 3-year follow-up benefits in well-being. Psychother Psychosom. 2007;76:226–33. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 41.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 42.Schubiner H, Betzold M. Unlearn Your Pain. Pleasant Ridge, MI: Mind Body Publishing; 2010. [Google Scholar]
- 43.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 44.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Ware JE, Kosinski M, Dewey J. How to Score Version Two of the SF-36r Health Survey. Lincoln, RI: QualityMetric, Inc; 2000. [Google Scholar]
- 46.Smets EM, Garssen B, Bonke B, DeHaes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–25. doi: 10.1016/0022-3999(94)00125-O. [DOI] [PubMed] [Google Scholar]
- 47.Stewart AL, Ware JEJ, editors. Measuring Functioning and Well-being: The Medical Outcomes Study Approach. Durham NC: Duke University Press; 1992. [Google Scholar]
- 48.Skevington SM. A standardized scale to measure beliefs about controlling pain. (BPCQ): a preliminary study. Psychol Health. 1990;4:221–32. doi: 10.1080/08870449008400392. [DOI] [Google Scholar]
- 49.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–13. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 50.Fischer AA. Pressure threshold meter: its use for quantification of tender spots. Arch Phys Med Rehabil. 1986;67:836–8. [PubMed] [Google Scholar]
- 51.Arnold LM, Pritchett YL, D'Souza DN, Kajdasz DK, Iyengar S, Wernicke JF. Duloxetine for the treatment of fibromyalgia in women: pooled results from two randomized, placebo-controlled clinical trials. J Womens Health (Larchmt). 2007;16:1145–56. doi: 10.1089/jwh.2006.0213. [DOI] [PubMed] [Google Scholar]
- 52.Arnold LM, Goldenberg DL, Stanford SB, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–44. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 53.Arnold LM, Lu Y, Crofford LJ, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50:2974–84. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 54.Ang D, Kesavalu R, Lydon JR, Lane KA, Bigatti S. Exercise-based motivational interviewing for female patients with fibromyalgia: a case series. Clin Rheumatol. 2007;26:1843–9. doi: 10.1007/s10067-007-0587-0. [DOI] [PubMed] [Google Scholar]
- 55.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30:1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Lemstra M, Olszynski WP. The effectiveness of multidisciplinary rehabilitation in the treatment of fibromyalgia: a randomized controlled trial. Clin J Pain. 2005;21:166–74. doi: 10.1097/00002508-200503000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Thieme K, Gromnica-Ihle E, Flor H. Operant behavioral treatment of fibromyalgia: a controlled study. Arthritis Rheum. 2003;49:314–20. doi: 10.1002/art.11124. [DOI] [PubMed] [Google Scholar]
- 58.Mannerkorpi K, Nyberg B, Ahlmén M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. J Rheumatol. 2000;27:2473–81. [PubMed] [Google Scholar]
- 59.Evcik D, Kizilay B, Gökçen E. The effects of balneotherapy on fibromyalgia patients. Rheumatol Int. 2002;22:56–9. doi: 10.1007/s00296-002-0189-8. [DOI] [PubMed] [Google Scholar]
- 60.Burns JW, Quartana P, Gilliam W, et al. Effects of anger suppression on pain severity and pain behaviors among chronic pain patients: evaluation of an ironic process model. Health Psychol. 2008;27:645–52. doi: 10.1037/a0013044. [DOI] [PubMed] [Google Scholar]
- 61.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]