Abstract
BACKGROUND
Medications for the prevention and treatment of cardiovascular disease save lives but adherence is often inadequate. The optimal role for physicians in improving adherence remains unclear.
OBJECTIVE
Using existing evidence, we set the goal of evaluating the physician’s role in improving medication adherence.
DESIGN
We conducted systematic searches of English-language peer-reviewed publications in MEDLINE and EMBASE from 1966 through 12/31/2008.
SUBJECTS AND INTERVENTIONS
We selected randomized controlled trials of interventions to improve adherence to medications used for preventing or treating cardiovascular disease or diabetes.
MAIN MEASURES
Articles were classified as either (1) physician “active”—a physician participated in designing or implementing the intervention; (2) physician “passive”—physicians treating intervention group patients received patient adherence information while physicians treating controls did not; or (3) physicians noninvolved. We also identified studies in which healthcare professionals helped deliver the intervention. We did a meta-analysis of the studies involving healthcare professionals to determine aggregate Cohen’s D effect sizes (ES).
KEY RESULTS
We identified 6,550 articles; 168 were reviewed in full, 82 met inclusion criteria. The majority of all studies (88.9%) showed improved adherence. Physician noninvolved studies were more likely (35.0% of studies) to show a medium or large effect on adherence compared to physician-involved studies (31.3%). Among interventions requiring a healthcare professional, physician-noninvolved interventions were more effective (ES 0.47; 95% CI 0.38–0.56) than physician-involved interventions (ES 0.25; 95% CI 0.21–0.29; p < 0.001). Among physician-involved interventions, physician-passive interventions were marginally more effective (ES 0.29; 95% CI 0.22–0.36) than physician-active interventions (ES 0.23; 95% CI 0.17–0.28; p = 0.2).
CONCLUSIONS
Adherence interventions utilizing non-physician healthcare professionals are effective in improving cardiovascular medication adherence, but further study is needed to identify the optimal role for physicians.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-010-1387-9) contains supplementary material, which is available to authorized users.
KEY WORDS: medication adherence, pharmaceutical care, doctor–patient relationships, preventive care, systematic reviews
BACKGROUND
Non-adherence to essential, chronic medications is common1,2, with profound consequences. Medication non-adherence has been shown to be a critical source of morbidity3 and mortality4, with annual costs in the U.S. estimated in excess of $100 billion5. As a result, increasing attention is being directed at developing interventions to improve adherence to chronic therapy. Rigorous analyses of these interventions can be used to better understand characteristics of adherence interventions that predict success, to evaluate their comparative effectiveness and costs, and to develop best-practices to encourage appropriate medication-taking.
While several systematic reviews of adherence interventions indicate that multi-factorial strategies are more effective than simple ones6,7, there is little data to compare the effectiveness of specific characteristics of adherence interventions. Specifically, little is known about who is best able to deliver adherence interventions, and what role should be assumed by the physician. Considering the high cost of physician time as compared to some other health professionals, a better understanding of the effectiveness of interventions provided by physicians can help guide intervention development.
We conducted a systematic review of interventions to improve adherence to medications for cardiovascular disease and diabetes, a cardiovascular disease equivalent8. We explored the effectiveness of interventions that utilized physicians and compared them to interventions that relied on other healthcare professionals or no professionals at all. Using existing evidence, our goal was to evaluate the physician’s role in improving medication adherence.
METHODS
We performed a systematic search of articles published in peer-reviewed health-care related journals between 1966 and December 31, 2008 using MEDLINE and EMBASE with the help of a professional librarian. We limited our search to randomized controlled trials.
We used search terms related to the type of study (randomized controlled trial), adherence (i.e. “compliance” OR “adherence” OR “medication adherence” or “treatment compliance”), prescription drugs (i.e. “drug,” OR “medication” OR “antihypertensive” OR “antihyperlipidemic” OR “hypoglycemic agents”) and cardiovascular disease and diabetes (myocardial infarction, coronary heart disease, heart failure; hypertension; hyperlipidemia; and diabetes.) Articles with at least 1 search term in all three of the main categories (study type AND adherence AND either drug OR disease) met criteria for the title/abstract review.
Search terms and parameters were adjusted for both databases while maintaining a common overall architecture. Search results from MEDLINE and EMBASE were combined and screened for duplicate entries.
Study Selection
Studies were included if they reported the results of randomized controlled trials studying interventions to improve adherence to medications used for the prevention or treatment of cardiovascular disease or diabetes. Studies were limited to adult subjects only (age ≥18) with adherence measured in the outpatient setting (i.e. take place exclusively in the outpatient setting or bridge the inpatient/outpatient transition with data gathered on outpatient adherence). Studies were excluded if they were written in a language other than English. We assigned each to 1 of 5 categories: Remind/Reinforce, Education, Behavioral, Simplify Regimen, and Complex/Combination. Complex/Combination interventions were those that (1) did not clearly fall into one of the previous four categories because they combined two or more of these categories or (2) those requiring the ongoing involvement of a health professional (nurse, pharmacist, or physician) in a manner that could not be clearly classified into one of the above four categories. For this review, we excluded those interventions characterized solely by regimen simplification.
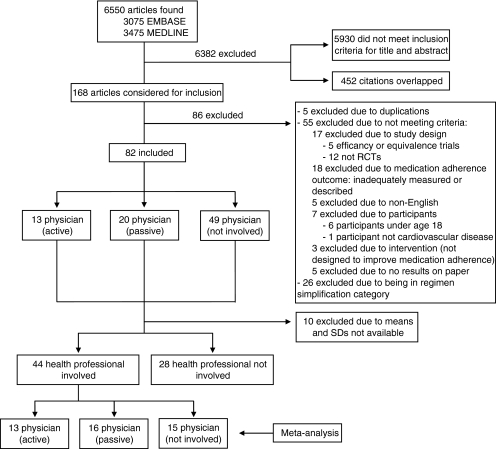
The remaining 82 articles (see Fig. 1) were classified as either (1) physician “active”—a physician participated in designing and/or implementing the intervention; (2) physician “passive”—physicians treating the intervention group potentially received different medication adherence information about their patients than physicians treating the control group; or (3) physicians uninvolved in the intervention.
Figure 1.
Article selection.
To compare the effectiveness of health care professionals as purveyors of the intervention and to limit the heterogeneity of studies included, we divided the 82 studies into those involving a health professional (nurse, pharmacist, or physician) and those that did not and performed a meta-analysis on the 44 studies involving healthcare professionals.
Data Extraction
Data were extracted by one investigator (SLC) and checked by a second (WS) with disagreements resolved by consensus. We assessed a number of variables related to the organization and outcome of studies including: the study design, setting, characteristics of population studied, the number of participants, the mean age (or age range) of participants, characteristics of intervention, level of physician involvement in intervention, methods used to measure medication adherence, clinical outcomes, medication adherence outcomes, and listed source of funding.
For the meta-analysis we identified those randomized controlled trials where means and standard deviations for medication adherence outcomes were presented. We combined these studies using Cohen’s D statistics which can be calculated for outcomes that are either binary (e.g. survey responses or predefined adherence cutoffs) or continuous (e.g. proportion of days covered)9,10. The effect sizes compare the difference in effect between the study groups divided by the standard deviation of this difference11. When standard deviations were not reported we derived them from the p-value or t statistic. We performed a meta-regression using study estimates of the Cohen's D. The method involved maximum likelihood estimation of the treatment difference from a weighted least-squares regression predicting the Cohen's D from physician involvement category. Weights were specified as the precision of the estimated Cohen's Ds.12
We considered an effect size of less than 0.2 to be very small, an effect size of 0.2 to 0.5 to be small, an effect size of 0.5 to 0.8 to be medium, and an effect size of greater than 0.8 to be large. This measure is independent of the measurement used, the sample size, and the standard deviation of the outcome measure. It therefore allowed us to aggregate different end points across studies to obtain effect sizes with 95% CIs for each category of physician involvement (i.e. physician-active; physician-passive; physician-non-involved.) In the meta-analysis, we derived combined summary estimates for physician-active, physician-passive, and physician-non-involved groups. We also derived a combined summary estimate for all physician-involved vs. all physician non-involved interventions.
RESULTS
Our search retrieved a total of 6550 articles, of which 168 were reviewed in full and 82 articles met inclusion criteria (see Online Tables 113–25, 226–45, 346–68, and 469–94).
There were few adherence improvement trials published before 1990 that involved physicians; physician-involved adherence interventions were reported with increased frequency only in the past ten years. Years of publication for all studies ranged from 1976 through 2008, with 50% of all studies published in or after 2003.
The majority (56.1%) of all interventions were aimed at subjects with hypertension. Other patient populations studied included those with diabetes (8.5% of all interventions); coronary artery disease (9.8%); congestive heart failure (11.0%); or dyslipidemia (7.3%). Additionally, 7.3% of the studies evaluated patients with a mix of cardiovascular and noncardiovascular diseases.
PHYSICIAN-ACTIVE INTERVENTIONS
Interventions characterized as physician-active were described in 13 articles. The mean age of patients included in these studies ranged from 53 to 68 years, for those studies in which a mean age was available.
Physician-active interventions were generally characterized by moderate time investment on the part of the physician and could be grouped into four categories. These articles described (1) physicians receiving additional education (physicians wrote hypertension guidelines, took intensive classes on hypertension management, or were mailed information reminding them of appropriate treatment regimen); (2) physicians recruited as agents of intervention via cues (all intervention physicians sent prompts urging medication regimen assessment); (3) physicians varying their own exposure to patients (changing frequency of follow up visits or ceding primary care of patients to a pharmacist); or (4) physicians directly involving themselves in delivery of intervention (physicians giving educational lectures or distributing pamphlets, or physicians reviewing patient data and approving various strategies for improving adherence).
Articles in the first three categories consistently demonstrated very small or small effects on adherence. For those articles in which physicians directly involved themselves in delivery of an intervention, results were mixed and difficult to interpret. The intervention studied by Logan et al. had a nonsignificant negative impact on adherence, worsening adherence slightly22. In this study, the physician’s role was to approve compliance-improving strategies after reviewing blood pressure and compliance information gathered by an occupational health nurse. Hunt et al. also found a negative impact on adherence20. In this study, the role of the physician was to approve and co-sign pharmacist recommendations, and also to discuss treatment plans for hypertension as part of a collaborative pharmacist protocol. Four studies in this category showed medium to large improvement on adherence (Gonzalez-Fernandez21, Kelly19, Yilmaz25, Antonicelli24), but two of these were of short duration (2–8 weeks); it is possible that their effect might have been attenuated if patients were followed for longer duration. In addition, one of these studies (Kelly19) examined the impact of a directly observed test dose of sublingual nitroglycerin administered in the physician’s office. The adherence outcome assessed in this study was use of the medication at least once before the second office visit. It is likely that this intervention and adherence outcome measure are not easily generalizable to most chronic cardiovascular medications.
PHYSICIAN-PASSIVE INTERVENTIONS
Interventions characterized as physician-passive were described in 20 articles. For those studies in which a mean age was available, mean ages ranged between 46 and 76 years.
Articles in which the physician was passively involved required minimal time commitments from the physician. In cases where no adherence problems were identified, such interventions were often designed to minimize or completely avoid using the physician’s time. These interventions fell into four categories describing (1) pharmacist-initiated physician contact (i.e., in order to communicate clinical or adherence data, the pharmacist contacted the physician by phone, issued a report, entered data into medical records, sent recommendations or referred the patient to the physician); (2) nurse-initiated physician contact (i.e. for the same reasons and in the same ways, nurses initiated contact); (3) patient-initiated contact (i.e., patient prompted to speak to a physician based on patient self monitoring and algorithm); or (4) data sent directly to the physician, to be communicated subsequently to patients.
Almost all the articles describing nurse-initiated contact, patient-initiated contact or data sent directly to the physician demonstrated a very small or small impact on adherence, with only one exception. Johnson et al. examined the impact of monthly home visits, self-monitoring of blood pressure, or both on patient compliance and found a large effect (Cohen’s D 1.51, CI 0.96, 2.05)45.
Articles describing pharmacist-initiated contact were the most common (14 articles, n = 4536) and displayed a range of effect sizes. They were the only group of articles displaying a negative impact on adherence (n = 109) and were also the only group to include a significant number of interventions (n = 829) with medium to large effects on adherence.
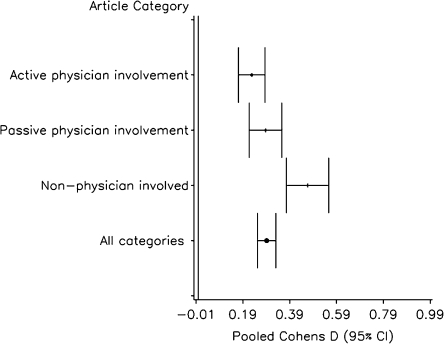
In our meta-analysis limited to those interventions requiring a healthcare professional (Fig. 2), physician-passive interventions were marginally more effective (pooled Cohen’s D = 0.29, CI 0.22, 0.36) than physician-active interventions (pooled Cohen’s D = 0.23, CI 0.17, 0.28). This difference was not statistically significant (p = 0.2).
Figure 2.
Meta-analysis of studies involving a health professional.
PHYSICIAN-NONINVOLVED INTERVENTIONS
Interventions characterized as physician-noninvolved were described in 49 articles. For those studies in which a mean age was available, mean ages fell between 49 and 85 years.
Articles in which physicians were not involved were grouped into four categories. These articles described (1) educational interventions aimed at patients; (2) reminders or reinforcement of adherence aimed at patients; (3) behavioral interventions aimed at patients; (4) complex/combination interventions, requiring involvement of a healthcare professional or combining two or more of the above categories.
The most successful category of intervention was the complex/combination category, which had a majority of interventions (n = 9284) yielding medium to large effects (pooled Cohen’s D = 0.49, CI 0.41, 0.57); compared to pooled behavioral interventions, Cohen’s D = 0.13, CI 0.01, 0.26); pooled educational interventions, Cohen’s D = 0.10, 0.04, 0.16); and pooled remind/reinforce interventions, Cohen’s D = 0.18, CI 0.13, 0.23).
Two studies in the complex/combination category had small negative effect sizes. Tsuyuki et al.86, combined patient education, adherence aids, phone follow-up and a monthly newsletter; Vivian et al.87 provided monthly pharmacist counseling.
Five interventions were characterized as primarily educational. The majority (4) of these had a very small or small effect, while one educational intervention (Morisky et al.66), a randomized factorial intervention providing hypertension education and training to both patients and family members, yielded a large effect.
Sixteen interventions used reminder or reinforcement techniques. The majority of these showed very small or small effects while three yielded large effects. McKenney et al.53 tested the effect of timepiece pill bottle caps along with pocket cards for recording blood pressure while Skaer et al.57,58 examined the effect of mailed refill reminders, unit of use packaging or the combination on patients with hypertension57 and in diabetic patients58.
The majority (88.9%) of all studies for which Cohen’s D values could be calculated showed improved adherence (Cohen’s D >0). Physician-noninvolved studies showed a higher rate of a medium or large effect (35.0% of studies) on adherence compared to physician-involved studies (31.3%). Similarly, only 7.5% of physician-noninvolved studies displayed a negative effect or lack of any effect compared to 15.6% of physician-involved studies. Physician-passive studies were slightly more likely to have a medium or large effect (31.6%) compared to physician-active studies (30.8%) and similarly likely (15.8% vs. 15.4%) to negatively impact adherence.
In our meta-analysis limited to interventions requiring involvement of a healthcare professional (Fig. 2), physician-non-involved interventions were substantially more effective (pooled Cohen’s D = 0.47, CI 0.38–0.56) than physician-involved interventions (combined physician-involved pooled Cohen’s D = 0.25, CI 0.21–0.29; p < 0.001).
DISCUSSION
Our findings demonstrate that existing physician-based adherence interventions have been less effective than strategies relying on other healthcare professionals. Healthcare professionals with specialized skills in pharmaceutical counseling or with expertise in behavioral interventions may be better equipped to address medication adherence challenges, and may be able to do so in more cost-effective ways.
Although small, the positive effect of physician-involved interventions must not be overlooked. While primary responsibility for time-intensive adherence interventions may not be ideally suited for physicians, it is still essential that we identify the most effective components of the physician adherence intervention. The strength in a physician-involved adherence intervention may lie, for example, in a patient’s perception of the physician’s expertise or in the trust built through long-term relationships. Referral networks that link physicians to other adherence experts could take advantage of the strengths of the physician-involved adherence intervention while limiting demands placed on a physician’s time.
Our review identified a number of areas in which well-designed studies were lacking. Few studies provided physicians with quantitative real-time adherence information, though several did describe interventions in which pharmacists or nurses provided the physician with ongoing medication information. Future interventions providing real-time quantitative adherence data might improve doctor–patient communication about medication adherence, though we need to consider whether the physician is the healthcare professional best-equipped to invest large amounts of time in such communication. In addition, few studies provided information on the cost of interventions. Future trials should provide estimates of the cost of their intervention so that comparisons can be made on this basis.
Our review was limited to published randomized controlled trials. Best practices have not yet been developed for adherence interventions, and future studies may better identify effective roles for physicians to improve patient behavior. The trials included in this evaluation were heterogeneous. While Cohen’s D effect sizes provide a means for standardizing, the clinical significance of these effect sizes may be hard to interpret. It is possible that there was publication bias in the studies we included in this review; however, we do not expect that publication bias should disproportionately affect any of the categories we used to differentiate interventions. It is possible that the chosen measure of adherence may have impacted the outcomes. However, if self-report yields a higher estimate of adherence compared to more objective measures16, the presence of a higher number of self-reported adherence outcomes in the physician-involved studies would over-estimate the effectiveness of this group, and our findings would be conservative. Several studies identified in our review followed subjects for short periods of time (less than 6 months). Given the long-term commitment necessary in the case of most cardiovascular medications, these studies may not be as readily generalizable.
As we continue to strive for evidence-based approaches to improve adherence to essential medications, we must also consider who is best able to deliver interventions that are both effective and cost-effective. Our results suggest that non-physician healthcare professionals such as pharmacists and nurses can play an important role in improving adherence. Existing studies have not provided a compelling evidence-base to support large investments in interventions that are delivered by physicians. Further study is needed to better understand the role of the physician in improving patient adherence in a cost-effective manner and how physicians should collaborate best with other healthcare professionals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Physician-Active Adherence Interventions (DOC 55 kb)
Physician-Passive Adherence Interventions (DOC 67 kb)
Physician-Noninvolved Adherence Interventions: Reminder/Reinforcement, Behavioral and Educational (DOC 72 kb)
Physician-Noninvolved Adherence Interventions: Complex/Combination Interventions (DOC 71 kb)
Acknowledgements and Conflicts of Interest Disclosure
This work was supported by a research grant from CVS Caremark. All data analysis and evaluation took place at Brigham and Women’s Hospital. CVS Caremark did not play a role in the design and conduct of the study, the collection, management, analysis, or interpretation of the data, the preparation, review or approval of the manuscript. Josh Liberman and Troy Brennan, both co-authors, are employees of CVS Caremark. Dr. Shrank is supported by a career development award from the National Heart, Lung and Blood Institute (HL-090505). Dr. Brookhart is supported by a career development award from the National Institute of Health (AG-027400). Dr. Cutrona has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
At the 32nd annual national meeting of the Society of General Internal Medicine on May 14, 2009, Dr. Cutrona presented findings from this paper as a nominee for Milton W. Hamolsky Award.
This work was supported by a research grant from CVS Caremark. Josh Liberman and Troy Brennan, both co-authors, are employees of CVS Caremark. All data analysis and evaluation took place at Brigham and Women’s Hospital. Dr. Shrank is supported by a career development award from the National Heart, Lung and Blood Institute (HL-090505). Dr. Brookhart is supported by a career development award from the National Institute of Health (AG-027400).
References
- 1.Adherence to long-term therapies: evidence for action. Geneva, Switzerland: WHO; 2003. [Google Scholar]
- 2.Avorn J, Monette J, Lacour A, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–62. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 3.Stuart BC, Simoni-Wastila L, Zhao L, Lloyd JT, JA D. Increased persistency in medication use by U.S. Medicare beneficiaries with diabetes is associated with lower hospitalization rates and cost savings. Diab Care. 2009;32(4):647–9. doi: 10.2337/dc08-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297(2):177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–50. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008(2):CD000011. [DOI] [PubMed]
- 8.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed]
- 9.Cohen J. Statistical power analysis for the behavioral sciences. New York: Lawrence Erlbaum associates; 1988. [Google Scholar]
- 10.Dunlap W, Cortina J, Vaslow J, Burke M. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Meth. 1996;1:170–7. doi: 10.1037/1082-989X.1.2.170. [DOI] [Google Scholar]
- 11.Hedges L, Olkin I. Statistical methods for meta-analysis. San Diego: Academic Press Inc; 1985. [Google Scholar]
- 12.Whitehead A. Meta-analysis of controlled clinical trials, series: statistics. In: Senn S, Barnett V, eds. Practice: Human and Biological Sciences. Published Online: 7 Feb 2003. Accessed at http://www3.interscience.wiley.com/cgi-bin/bookhome/102529859 on March 17, 2010.
- 13.Avanzini F, Corsetti A, Maglione T, et al. Simple, shared guidelines raise the quality of antihypertensive treatment in routine care. Am Heart J. 2002;144(4):726–32. doi: 10.1067/mhj.2002.125327. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi NN, Hatcher J, Chaturvedi N, Jafar TH. Effect of general practitioner education on adherence to antihypertensive drugs: cluster randomised controlled trial. Br Med J. 2007;335(7628):1030–3. doi: 10.1136/bmj.39360.617986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DH, Kramer JM, Perrin N, et al. A randomized trial of direct-to-patient communication to enhance adherence to beta-blocker therapy following myocardial infarction. Arch Intern Med. 2008;168(5):477–83. doi: 10.1001/archinternmed.2007.132. [DOI] [PubMed] [Google Scholar]
- 16.Rosen MI, Rigsby MO, Salahi JT, Ryan CE, Cramer JA. Electronic monitoring and counseling to improve medication adherence. Behav Res Ther. 2004;42(4):409–22. doi: 10.1016/S0005-7967(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 17.Birtwhistle RV, Godwin MS, Delva MD, et al. Randomised equivalence trial comparing three and six months of follow up of patients with hypertension by family practitioners. Br Med J. 2004;328(7433):204–206B. doi: 10.1136/bmj.37967.374063.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawkins DW, Fiedler FP, Douglas HL, Eschbach RC. Evaluation of a clinical pharmacist in caring for hypertensive and diabetic patients. Am J Hosp Pharm. 1979;36(10):1321–5. [PubMed] [Google Scholar]
- 19.Kelly JM. Sublingual nitroglycerin: improving patient compliance with a demonstration dose. J Am Board Fam Pract. 1988;1(4):251–4. [PubMed] [Google Scholar]
- 20.Hunt JS, Siemienczuk J, Pape G, et al. A Randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008. [DOI] [PMC free article] [PubMed]
- 21.Gonzalez-Fernandez RA, Rivera M, Torres D, Quiles J, Jackson A. Usefulness of a systemic hypertension in-hospital educational-program. Am J Cardiol. 1990;65(20):1384–6. doi: 10.1016/0002-9149(90)91332-Z. [DOI] [PubMed] [Google Scholar]
- 22.Logan AG, Milne BJ, Flanagan PT, Haynes RB. Clinical effectiveness and cost-effectiveness of monitoring blood-pressure of hypertensive employees at work. Hypertension. 1983;5(6):828–36. doi: 10.1161/01.hyp.5.6.828. [DOI] [PubMed] [Google Scholar]
- 23.Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions—Statin choice randomized trial. Arch Intern Med. 2007;167(10):1076–82. doi: 10.1001/archinte.167.10.1076. [DOI] [PubMed] [Google Scholar]
- 24.Antonicelli R, Testarmata P, Spazzafumo L, et al. Impact of telemonitoring at home on the management of elderly patients with congestive heart failure. J Telemed Telecare. 2008;14(6):300–5. doi: 10.1258/jtt.2008.071213. [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz MB, Pinar M, Naharci I, et al. Being well-informed about statin is associated with continuous adherence and reaching targets. Cardiovasc Drugs Ther. 2005;19(6):437–40. doi: 10.1007/s10557-005-5202-5. [DOI] [PubMed] [Google Scholar]
- 26.Blenkinsopp A, Phelan M, Bourne J, Dakhil N. Extended adherence support by community pharmacists for patients with hypertension: a randomised controlled trial. Int J Pharm Pract. 2000;8(3):165–75. [Google Scholar]
- 27.Bouvy ML, Heerdink ER, Urquhart J, Grobbee DE, Hoe AW, Leufkens HGM. Effect of a pharmacist-led intervention on diuretic compliance in heart failure patients: a randomized controlled study. J Card Fail. 2003;9(5):404–11. doi: 10.1054/S1071-9164(03)00130-1. [DOI] [PubMed] [Google Scholar]
- 28.Grant RW, Devita NG, Singer DE, Meigs JB. Improving adherence and reducing medication discrepancies in patients with diabetes. Ann Pharmacother. 2003;37(7–8):962–9. doi: 10.1345/aph.1C452. [DOI] [PubMed] [Google Scholar]
- 29.Jaffray M, Bond C, Watson M, et al. The MEDMAN study: a randomized controlled trial of community pharmacy-led medicines management for patients with coronary heart disease. Fam Pract. 2007;24(2):189–200. doi: 10.1093/fampra/cml075. [DOI] [PubMed] [Google Scholar]
- 30.Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy. 2000;20(11):1384–9. doi: 10.1592/phco.20.17.1384.34891. [DOI] [PubMed] [Google Scholar]
- 31.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure—a randomized trial. Ann Intern Med. 2007;146(10):714–25. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 32.Odegard PS, Goo A, Hummel J, Williams KL, Gray SL. Caring for poorly controlled diabetes mellitus: a randomized pharmacist intervention. Ann Pharmacother. 2005;39(3):433–40. doi: 10.1345/aph.1E438. [DOI] [PubMed] [Google Scholar]
- 33.Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. Br J Clin Pharmacol. 2005;60(2):183–93. doi: 10.1111/j.1365-2125.2005.02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon DK, Portner TS, Bass GE, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. J Am Pharm Assoc (Wash) 1998;38(5):574–85. doi: 10.1016/s1086-5802(16)30371-0. [DOI] [PubMed] [Google Scholar]
- 35.Sookaneknun P, Richards RM, Sanguansermsri J, Teerasut C. Pharmacist involvement in primary care improves hypertensive patient clinical outcomes. Ann Pharmacother. 2004;38(12):2023–8. doi: 10.1345/aph.1D605. [DOI] [PubMed] [Google Scholar]
- 36.Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. Am J Health-Syst Pharm. 2003;60(11):1123–9. doi: 10.1093/ajhp/60.11.1123. [DOI] [PubMed] [Google Scholar]
- 37.Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: Interventions and outcomes. Pharmacotherapy. 1999;19(7):860–9. doi: 10.1592/phco.19.10.860.31565. [DOI] [PubMed] [Google Scholar]
- 38.Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting. J Am Pharm Assoc (Wash) 1996;NS36(7):443–51. doi: 10.1016/s1086-5802(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 39.Edworthy SM, Baptie B, Galvin D, et al. Effects of an enhanced secondary prevention program for patients with heart disease: a prospective randomized trial. Can J Cardiol. 2007;23(13):1066–72. doi: 10.1016/s0828-282x(07)70875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Logan AG, Milne BJ, Achber C, Campbell WP, Haynes RB. Work-site treatment of hypertension by specially trained nurses—controlled trial. Lancet. 1979;2(8153):1175–8. doi: 10.1016/S0140-6736(79)92397-3. [DOI] [PubMed] [Google Scholar]
- 41.Rudd P, Miller NH, Kaufman J, et al. Nurse management for hypertension—a systems approach. Am J Hypertens. 2004;17(10):921–7. doi: 10.1016/j.amjhyper.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 42.Piette JD, Weinberger M, McPhee SJ, Mah CA, Kraemer FB, Crapo LM. Do automated calls with nurse follow-up improve self-care and glycemic control among vulnerable patients with diabetes? Am J Med. 2000;108(1):20–7. doi: 10.1016/S0002-9343(99)00298-3. [DOI] [PubMed] [Google Scholar]
- 43.Zarnke KB, Feagan BG, Mahon JL, Feldman RD. A randomized study comparing a patient directed hypertension management strategy with usual office-based care. Am J Hypertens. 1997;10(1):58–67. doi: 10.1016/S0895-7061(96)00305-6. [DOI] [PubMed] [Google Scholar]
- 44.Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension—impact on medication adherence and blood pressure control. Am J Hypertens. 1996;9(4):285–92. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 45.Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J. 1978;119(9):1034–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Barrios V, Escobar C, Navarro A, Calderon A, Ruilope LM. Antihypertensive effectiveness of lercanidipine administered using an electronic pillbox compared with usual care in a cohort of mild-to-moderately hypertensive patients: The ELECTRA study. Therapy. 2007;4(4):433–40. doi: 10.2217/14750708.4.4.433. [DOI] [Google Scholar]
- 47.Becker LA, Glanz K, Sobel E, Mossey J, Zinn SL, Knott KA. A randomized trial of special packaging of antihypertensive medications. J Fam Pract. 1986;22(4):357–61. [PubMed] [Google Scholar]
- 48.Fulmer TT, Feldman PH, Kim TS, et al. An intervention study to enhance medication compliance in community-dwelling elderly individuals. J Gerontol Nurs. 1999;25(8):6–14. doi: 10.3928/0098-9134-19990801-04. [DOI] [PubMed] [Google Scholar]
- 49.Guthrie RM. The effects of postal and telephone reminders on compliance with pravastatin therapy in a national registry: results of the first myocardial infarction risk reduction program. Clin Ther. 2001;23(6):970–80. doi: 10.1016/S0149-2918(01)80084-9. [DOI] [PubMed] [Google Scholar]
- 50.Hagstrom B, Mattsson B, Rost IM, Gunnarsson RK. What happened to the prescriptions? a single, short, standardized telephone call may increase compliance. Fam Pract. 2004;21(1):46–50. doi: 10.1093/fampra/cmh110. [DOI] [PubMed] [Google Scholar]
- 51.Johnson SS, Driskell MM, Johnson JL, et al. Transtheoretical model intervention for adherence to lipid-lowering drugs. Dis Manag. 2006;9(2):102–14. doi: 10.1089/dis.2006.9.102. [DOI] [PubMed] [Google Scholar]
- 52.Marquez-Contreras E, Martell-Claros N, Gil-Guillen V, et al. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24(1):169–75. doi: 10.1097/01.hjh.0000198023.53859.a2. [DOI] [PubMed] [Google Scholar]
- 53.McKenney JM, Munroe WP, Wright JT. Impact of an electronic medication compliance aid on long-term blood-pressure control. J Clin Pharmacol. 1992;32(3):277–83. doi: 10.1002/j.1552-4604.1992.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 54.Saunders LD, Irwig LM, Gear JSS, Ramushu DL. A randomized controlled trial of compliance improving strategies in Soweto hypertensives. Med Care. 1991;29(7):669–78. doi: 10.1097/00005650-199107000-00007. [DOI] [PubMed] [Google Scholar]
- 55.Schneider PJ, Murphy JE, Pederson CA. Impact of medication packaging on adherence and treatment outcomes in older ambulatory patients. J Am Pharm Assoc. 2008;48(1):58–63. doi: 10.1331/JAPhA.2008.07040. [DOI] [PubMed] [Google Scholar]
- 56.Simkins CV, Wenzloff NJ. Evaluation of a computerized reminder system in the enhancement of patient medication refill compliance. Drug Intell Clin Pharm. 1986;20(10):799–802. doi: 10.1177/106002808602001017. [DOI] [PubMed] [Google Scholar]
- 57.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and health care expenditures for hypertension. J Hum Hypertens. 1993;7(5):515–8. [PubMed] [Google Scholar]
- 58.Skaer TL, Sclar DA, Markowski DJ, Won JKH. Effect of value-added utilities on prescription refill compliance and Medicaid health-care expenditures—a study of patients with non-insulin-dependent diabetes-mellitus. J Clin Pharm Ther. 1993;18(4):295–9. doi: 10.1111/j.1365-2710.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 59.Stewart A, Noakes T, Eales C, Shepard K, Becker P, Veriawa Y. Adherence to cardiovascular risk factor modification in patients with hypertension. Cardiovasc J South Afr. 2005;16(2):102–7. [PubMed] [Google Scholar]
- 60.Marquez Contreras E, Vegazo Garcia O, Claros NM, et al. Efficacy of telephone and mail intervention in patient compliance with antihypertensive drugs in hypertension. ETECUM-HTA study. Blood Press. 2005;14(3):151–8. doi: 10.1080/08037050510008977. [DOI] [PubMed] [Google Scholar]
- 61.Santschi V, Wuerzner G, Schneider MP, Bugnon O, Burnier M. Clinical evaluation of IDAS II, a new electronic device enabling drug adherence monitoring. Eur J Clin Pharmacol. 2007;63(12):1179–84. doi: 10.1007/s00228-007-0364-7. [DOI] [PubMed] [Google Scholar]
- 62.Ogedegbe G, Chaplin W, Schoenthaler A, et al. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21(10):1137–43. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eshelman FN, Fitzloff J. Effect of packaging on patient compliance with an antihypertensive medication. Curr Ther Res Clin Exp. 1976;20(2):215–9. [PubMed] [Google Scholar]
- 64.Emmett CL, Montgomery AA, Peters TJ, Fahey T. Three-year follow-up of a factorial randomised controlled trial of two decision aids for newly diagnosed hypertensive patients. Br J Gen Pract. 2005;55(516):551–3. [PMC free article] [PubMed] [Google Scholar]
- 65.Takala J. Screening, treatment and adherence to treatment for hypertension. Scand J Prim Health Care. 1983;1(3–4):114–9. doi: 10.3109/02813438309038478. [DOI] [PubMed] [Google Scholar]
- 66.Morisky DE, DeMuth NM, Field-Fass M, Green LW, Levine DM. Evaluation of family health education to build social support for long-term control of high blood pressure. Health Educ Q. 1985;12(1):35–50. doi: 10.1177/109019818501200104. [DOI] [PubMed] [Google Scholar]
- 67.Powell KM, Edgren B. Failure of educational videotapes to improve medication compliance in a health maintenance organization. Am J Health Syst Pharm. 1995;52(20):2196–9. doi: 10.1093/ajhp/52.20.2196. [DOI] [PubMed] [Google Scholar]
- 68.Polack J, Jorgenson D, Robertson P. Evaluation of different methods of providing medication-related education to patients following myocardial infarction. Canadian Pharmacists Journal. 2008;141(4):241–7. doi: 10.3821/1913-701X(2008)141[241:EODMOP]2.0.CO;2. [DOI] [Google Scholar]
- 69.Bosworth HB, Olsen MK, Neary A, et al. Take Control of Your Blood pressure (TCYB) study: a multifactorial tailored behavioral and educational intervention for achieving blood pressure control. Patient Educ Couns. 2008;70(3):338–47. doi: 10.1016/j.pec.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elliott RA, Barber N, Clifford S, Horne R, Hartley E. The cost effectiveness of a telephone-based pharmacy advisory service to improve adherence to newly prescribed medicines. Pharm World Sci. 2008;30(1):17–23. doi: 10.1007/s11096-007-9134-y. [DOI] [PubMed] [Google Scholar]
- 71.Faulkner MA, Wadibia EC, Lucas BD, Hilleman DE. Impact of pharmacy counseling on compliance and effectiveness of combination lipid-lowering therapy in patients undergoing coronary artery revascularization: a randomized, controlled trial. Pharmacotherapy. 2000;20(4):410–6. doi: 10.1592/phco.20.5.410.35048. [DOI] [PubMed] [Google Scholar]
- 72.Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients clinical condition in heart-failure. Br J Clin Pract. 1995;49(4):173–6. [PubMed] [Google Scholar]
- 73.Hamet P, Campbell N, Curnew G, Eastwood C, Pradhan A. Avapromise: a randomized clinical trial for increasing adherence through behavioural modification in essential hypertension. Exp Clin Cardiol. 2003;7(4):165–72. [PMC free article] [PubMed] [Google Scholar]
- 74.Haynes RB, Sackett DL, Gibson ES, et al. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1(7972):1265–8. doi: 10.1016/S0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 75.Kirscht JP, Kirscht JL, Rosenstock IM. A test of interventions to increase adherence to hypertensive medical regimens. Health Educ Q. 1981;8(3):261–72. doi: 10.1177/109019818100800303. [DOI] [PubMed] [Google Scholar]
- 76.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol—a randomized controlled trial. JAMA- J Am Med Assoc. 2006;296(21):2563–71. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 77.Lee SSC, Cheung PYP, Chow MSS. Benefits of individualized counseling by the pharmacist on the treatment outcomes of hyperlipidemia in Hong Kong. J Clin Pharmacol. 2004;44(6):632–9. doi: 10.1177/0091270004265364. [DOI] [PubMed] [Google Scholar]
- 78.Lopez Cabezas C, Falces Salvador C, Cubi Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp. 2006;30(6):328–42. doi: 10.1016/S1130-6343(06)74004-1. [DOI] [PubMed] [Google Scholar]
- 79.Mengden T, Vetter H, Tousset E, Uen S. Management of patients with uncontrolled arterial hypertension–the role of electronic compliance monitoring, 24-h ambulatory blood pressure monitoring and Candesartan/HCTZ. BMC Cardiovasc Disord. 2006;6:36. doi: 10.1186/1471-2261-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nessman DG, Carnahan JE, Nugent CA. Increasing compliance—patient-operated hypertension groups. Arch Intern Med. 1980;140(11):1427–33. doi: 10.1001/archinte.140.11.1427. [DOI] [PubMed] [Google Scholar]
- 81.Rich MW, Gray DB, Beckham V, Wittenberg C, Luther P. Effect of a multidisciplinary intervention on medication compliance in elderly patients with congestive heart failure. Am J Med. 1996;101(3):270–6. doi: 10.1016/S0002-9343(96)00172-6. [DOI] [PubMed] [Google Scholar]
- 82.Schectman G, Hiatt J, Hartz A. Telephone contacts do not improve adherence to niacin or bile-acid sequestrant therapy. Ann Pharmacother. 1994;28(1):29–35. doi: 10.1177/106002809402800104. [DOI] [PubMed] [Google Scholar]
- 83.Schroeder K, Fahey T, Hollinghurst S, Peters TJ. Nurse-led adherence support in hypertension: a randomized controlled trial. Fam Pract. 2005;22(2):144–51. doi: 10.1093/fampra/cmh717. [DOI] [PubMed] [Google Scholar]
- 84.Sclar DA, Chin A, Skaer TL, Okamoto MP, Nakahiro RK, Gill MA. Effect of health-education in promoting prescription refill compliance among patients with hypertension. Clin Ther. 1991;13(4):489–95. [PubMed] [Google Scholar]
- 85.Spector R, McGrath P, Uretsky N, Newman R, Cohen P. Does intervention by a nurse improve medication compliance. Arch Intern Med. 1978;138(1):36–40. doi: 10.1001/archinte.138.1.36. [DOI] [PubMed] [Google Scholar]
- 86.Tsuyuki RT, Fradette M, Johnson JA, et al. A multicenter disease management program for hospitalized patients with heart failure. J Card Fail. 2004;10(6):473–80. doi: 10.1016/j.cardfail.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 87.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22(12):1533–40. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 88.Vrijens B, Belmans A, Matthys K, Klerk E, Lesaffre E. Effect of intervention through a pharmaceutical care program on patient adherence with prescribed once-daily atorvastatin. Pharmacoepidemiol Drug Saf. 2006;15(2):115–21. doi: 10.1002/pds.1198. [DOI] [PubMed] [Google Scholar]
- 89.Phumipamorn S, Pongwecharak J, Soorapan S, Pattharachayakul S. Effects of the pharmacist's input on glycaemic control and cardiovascular risks in Muslim diabetes. Prim Care Diabetes. 2008;2(1):31–7. doi: 10.1016/j.pcd.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Riesen WF, Noll G, Dariolo R. Impact of enhanced compliance initiatives on the efficacy of rosuvastatin in reducing low density lipoprotein cholesterol levels in patients with primary hypercholesterolaemia. Swiss Med Wkly. 2008;138(29–30):420–6. doi: 10.4414/smw.2008.12120. [DOI] [PubMed] [Google Scholar]
- 91.Gabriel M, Gagnon JP, Bryan CK. Improved patients compliance through use of a daily drug reminder chart. Am J Public Health. 1977;67(10):968–9. doi: 10.2105/AJPH.67.10.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kerr JA. Adherence and self-care. Heart Lung. 1985;14(1):24–31. [PubMed] [Google Scholar]
- 93.Rehder TL, McCoy LK, Blackwell B, Whitehead W, Robinson A. Improving medication compliance by counseling and special prescription container. Am J Hosp Pharm. 1980;37(3):379–85. [PubMed] [Google Scholar]
- 94.Burrelle T. Evaluation of an interdisciplinary compliance service for elderly hypertensives. J Geriatr Drug Ther. 1986;1(2):23–51. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Physician-Active Adherence Interventions (DOC 55 kb)
Physician-Passive Adherence Interventions (DOC 67 kb)
Physician-Noninvolved Adherence Interventions: Reminder/Reinforcement, Behavioral and Educational (DOC 72 kb)
Physician-Noninvolved Adherence Interventions: Complex/Combination Interventions (DOC 71 kb)