Abstract
Background
Diabetes outcomes are worse for underserved patients from certain ethnic/racial minority populations. Telephonic disease management is a cost-effective strategy to deliver self-management services and possibly improve diabetes outcomes for such patients.
Objective
We conducted a trial to test the effectiveness of a supplemental telephonic disease management program compared to usual care alone for patients with diabetes cared for in a community health center.
Design
Randomized controlled trial.
Participants
All patients had type 2 diabetes, and the majority was Hispanic or African American. Most were urban-dwelling with low socioeconomic status, and nearly all had Medicaid or were uninsured.
Measurements
Clinical measures included glycemic control, blood pressure, lipid levels, and body mass index. Validated surveys were used to measure dietary habits and physical activity.
Results
A total of 146 patients were randomized to the intervention and 149 to the control group. Depressive symptoms were highly prevalent in both groups. Using an intention to treat analysis, there were no significant differences in the primary outcome (HbA1c) between the intervention and control groups at 12 months. There were also no significant differences for secondary clinical or behavioral outcome measures including BMI, systolic or diastolic blood pressure, LDL cholesterol, smoking, or intake of fruits and vegetables, or physical activity.
Conclusions
A clinic-based telephonic disease management support for underserved patients with diabetes did not improve clinical or behavioral outcomes at 1 year as compared to patients receiving usual care alone.
KEY WORDS: diabetes, self-management, telehealth, disease management, community health centers, Hispanic Americans
Disease management can improve chronic disease outcomes and reduce health care costs. Currently, 96% of the top 150 commercial payers provide some form of disease management services to their patients1. Published reviews on the impact of such programs for diabetes have been mixed1,2.
Diabetes affects the poor and members of ethnic/racial minorities at a higher rate than other groups3–5. These populations have worse adherence to self care6,7, worse diabetes control, and higher rates of serious diabetes-related complications8. Adherence to a diabetes treatment regimen can be daunting for the most health-literate and motivated patient. For some, such as the poor, the depressed, the low literate, or the non-English speaker, it can be even more challenging.
Disease management, emphasizing self-management, adherence, and frequent contact outside the medical setting, would seem to be ideally suited to improve outcomes for underserved patients with diabetes. To test this hypothesis, we designed a telephonic diabetes disease management protocol in a Community Health Center. This program was implemented within the health center, using health center staff directly connected through an electronic health record to the primary care team. The intervention was tailored to meet the cultural and linguistic needs of a medically underserved, predominantly Hispanic population.
METHODS
Design “Managing the Space Between Visits” (MSBV) was a randomized, controlled trial of telephonic disease management for underserved, largely Hispanic/Latino patients cared for in a Community Health Center. The unit of randomization was the patient. The study was reviewed and approved by Chesapeake Institutional Review Board.
Setting The Community Health Center, Inc. (CHC), is the largest Federally Qualified Health Center in Connecticut, providing services to approximately 50,000 patients in multiple communities across the state. Forty-three percent of the patients are Hispanic, and 13% are African American. Nearly half speak a language other than English at home. Eighty-eight percent are at or below 200% of the poverty level, and 25% have no medical insurance. Primary medical care is provided by internists, family practitioners, pediatricians, and nurse practitioners. Onsite mental health, podiatric, and dental care are available. The intervention was conducted from a centralized call center and was offered to patients with type 2 diabetes at the two largest CHC clinics. Both clinics are similar in size, services, and setting.
Staff Specialized nurses were trained to provide the intervention by a consultant with experience in the commercial disease management industry. Self-management training was conducted by a “master trainer” in the Chronic Disease Self-Management Program from Stanford University. The nursing staff conducted the calls from a call center or from home using a Virtual Private Network (VPN) connection link to the EHR. Patient enrollment and data collection were managed by a research assistant not involved in the provision of the intervention.
EHR Nurses documented their telephone encounters in the patient’s electronic medical record. Templates allowed real-time documentation. Notes were forwarded to the primary care provider for co-signature.
Enrollment All patients with type 2 diabetes age 18 and over from the two participating sites were eligible for the study. Patients were excluded if they were (1) unwilling/unable to give informed consent, (2) spoke primarily a language other than English or Spanish, (3) did not have a telephone, (4) were active substance abusers, or (5) had a mental or physical impairment that would prevent them from engaging in the calls or in diabetes self-management activities. Using a list generated from the practice management system, the research assistant attempted to contact all eligible patients by letter and/or telephone and invite them to participate in the study. Those interested scheduled an appointment with the research assistant. Those who remained interested after the information session signed an informed consent. All patients in the study received a $25 gift card to a local store after completing their 6- and 12-month assessments. Patients were considered lost to follow-up if they transferred care to another location, could not be contacted by telephone after three attempts, or failed to return for their 6- or 12-month assessments.
Randomization Once enrolled, patients were block randomized in groups of four by a computerized algorithm to receive the intervention plus usual care or usual care alone. Patients randomized to usual care continued to receive primary care at CHC.
Assessment Patients were assessed at intake, 6 months, and 12 months into the study. Baseline demographic information was collected at intake. Patients rated their overall health as excellent, very good, good, fair, or poor. The presence of depressive symptoms was assessed using the Patient Health Questionnaire-9 (PHQ-9)9. Validated surveys in English and Spanish were used to assess dietary habits and physical activity, the Brief Dietary Assessment10, and the Rapid Assessment of Physical Activity (RAPA)11, respectively. Height, weight, blood pressure, lipid levels, and hemoglobin A1C levels were measured at each assessment.
Intervention Patients in the intervention received 1 year of telephonic disease management. Call content was semi-structured. Calls were unscripted, allowing the nurse to address each patient’s individual needs, whether related to diabetes or other topics. Nurses had wide latitude to discuss the topics raised by the patient while also covering the following items in a more structured fashion:
brief clinical assessment
self-management: including diet, exercise, stress reduction, smoking cessation, readiness assessment, and development of specific self-management goals
medication adherence: problem solving to help improve adherence
glucose monitoring and review of home glucose monitoring results
Intervention fidelity was monitored by chart review conducted by the project coordinator.
Patients were called weekly, bi-weekly, or monthly depending on their risk stratification (Fig. 1). Patients could be re-assigned to receive more or fewer calls if their risk stratification changed at the 6-month assessment or if the patient requested a change in call frequency. Nurses also mailed educational materials to patients covering various topics discussed during the calls. This material was in English and Spanish, and at a fourth grade reading level.
Figure 1.
Call frequency.
Statistical Methods Analysis was based on the intention-to-treat principle with subjects analyzed in the group to which they were randomized and the modified intent-to-treat analysis population with at least one post randomization HbA1c measure. An enrollment target was made based on power calculations to detect a change in hemoglobin A1C of 0.7 or more. Two hundred seventy-four patients (with 137 in each arm) in total were sufficient to detect a minimum difference in mean changes of 0.7% in HbA1c at 80% power and 5% significance level assuming standard deviations of 2.1% for HbA1c. The total recruitment of 300 patients was needed to account for 10% loss to follow-up. Baseline characteristics were compared between intervention groups using either a Pearson's chi-square test or Fisher's exact test for categorical data, and a Wilcoxon rank sum test or Student’s t test for ordinal or continuous data, respectively.
For the analysis of the primary outcome, HbA1c, a mixed-model repeated-measures analysis of covariance (implemented with the use of SAS PROC MIXED), was used to compare intervention groups at each assessment point (months 6 and 12)12. Models included fixed effects for intervention, time, and their interaction, as well as baseline HbA1c. Residual correlation from repeated measures was accounted for by an unstructured covariance pattern. This analysis assumes that missing data were missing at random (i.e., missing values may be dependent on observed but not on unobserved data) and is more robust than other alternatives such as Last Observation Carried Forward (LOCF)13. Sensitivity analyses were also performed using LOCF, which produced similar conclusions and thus are not presented. Ad hoc subgroup analyses were also performed to determine whether the impact of the intervention was modified by initial HbA1c category, current depression, Spanish speaking only, and lower education level. The interaction test between intervention and these potential moderators was performed.
Secondary clinical or behavioral outcome measures, including BMI, systolic or diastolic blood pressure, and LDL cholesterol, were also assessed with the use of mixed-model repeated-measures analysis of covariance. Generalized estimating equations (GEE) with a negative binomial distribution were used to compare the intake of fruits and vegetables. For the perceived health status and physical assessment outcomes, GEE cumulative logit regressions with the assumption of proportional odds were used to compare intervention groups. Data across all post-randomization measurement times were used with adjustment for baseline values as a covariate.
Analyses were performed with the use of SAS software, version 9.1.3 (SAS Institute, Cary, NC). All comparisons were planned, and tests were two-sided. A P value of less than 0.05 was considered to indicate statistical significance.
RESULTS
From 1,754 patients with type 2 diabetes potentially eligible for the study, 297 signed an informed consent, and 146 were randomized to receive the intervention and 149 to the control group. Figure 2 shows details of the selection process. There were no significant differences in the two groups at baseline with regard to sociodemographic variables. However, the control group had a significantly higher baseline average HbA1c than the intervention group (8.4 ± 2.33 vs. 7.6 ± 1.75, p = 0.006). All other clinical variables were similar between the two groups (Tables 1 and 2). Of those patients who were randomized, 115 (79%) in the control and 94 (64%) in the intervention group completed the 1-year study. The most common reason for not completing the study was the patient’s being lost to follow up.
Figure 2.
Flowchart for enrollment, randomization, and follow-up of study participants.
Table 1.
Demographic and Clinical Characteristics Across Treatment Group
| Characteristic | Treatment group | pc | |
|---|---|---|---|
| Control (N = 149)b | Intervention (N = 146)b | ||
| Gender | 0.747 | ||
| Female | 85 (57.1) | 86 (58.9) | |
| Male | 64 (43.0) | 60 (41.1) | |
| Race | 0.750 | ||
| Black | 12 (8.1) | 15 (10.3) | |
| White | 39 (26.2) | 40 (27.4) | |
| Other | 98 (65.8) | 91 (62.3) | |
| Marital status | 0.820 | ||
| Not married | 93 (62.4) | 93 (63.7) | |
| Married | 56 (37.6) | 53 (36.3) | |
| Education | 0.425 | ||
| 0–6 | 43 (29.1) | 32 (21.9) | |
| 7–8 | 20 (13.5) | 19 (13.0) | |
| 9–12 | 71 (48.0) | 75 (51.4) | |
| ≥13 | 14 (9.5) | 20 (13.7) | |
| DE education | 0.963 | ||
| No | 47 (31.8) | 46 (31.5) | |
| Yes | 101 (68.2) | 100 (68.5) | |
| Language | 0.619 | ||
| Either | 21 (14.1) | 23 (15.8) | |
| English | 37 (24.8) | 42 (28.8) | |
| Spanish | 91 (61.1) | 81 (55.5) | |
| Insurance | 0.693 | ||
| SAGA | 20 (13.4) | 18 (12.3) | |
| Medicaid | 60 (40.3) | 66 (45.2) | |
| Other | 69 (46.3) | 62 (42.5) | |
| CAD | 0.812 | ||
| No | 127 (85.2) | 125 (86.2) | |
| Yes | 22 (14.8) | 20 (13.8) | |
| CHF | 0.977 | ||
| No | 145 (97.3) | 142 (97.3) | |
| Yes | 4 (2.7) | 4 (2.7) | |
| COPD | 0.794 | ||
| No | 143 (96.0) | 140 (96.6) | |
| Yes | 6 (4.0) | 5 (3.5) | |
| Asthma | 0.462 | ||
| No | 123 (82.6) | 114 (79.2) | |
| Yes | 26 (17.4) | 30 (20.8) | |
| Hypertension | 0.686 | ||
| No | 36 (24.2) | 38 (26.2) | |
| Yes | 113 (75.8) | 107 (73.8) | |
| Past depression | 0.680 | ||
| No | 74 (49.7) | 69 (47.3) | |
| Yes | 75 (50.3) | 77 (52.7) | |
| Current depression | 0.996 | ||
| No | 97 (65.1) | 95 (65.1) | |
| Yes | 52 (34.9) | 51 (34.9) | |
| Clinically diagnosed depression | 0.388 | ||
| No | 99 (66.9) | 90 (62.1) | |
| Yes | 49 (33.1) | 55 (37.9) | |
| Current smoker | 0.440 | ||
| No | 119 (80.4) | 112 (76.7) | |
| Yes | 29 (19.6) | 34 (23.3) | |
| Depression (screening) | 0.752 | ||
| No | 112 (75.7) | 112 (77.2) | |
| Yes | 36 (24.3) | 33 (22.8) | |
| Ethnicity | 0.942# | ||
| Central American | 1 (0.7) | 1 (0.7) | |
| Mexican | 8 (5.4) | 6 (4.1) | |
| Puerto Rican | 98 (65.8) | 90 (61.6) | |
| South American | 1 (0.7) | 1 (0.7) | |
| Other Hispanic | 8 (5.4) | 9 (6.2) | |
| Other | 33 (22.2) | 39 (26.7) | |
| Insurance | 0.585 | ||
| Medicaid | 60 (40.3) | 66 (45.2) | |
| Medicare | 34 (22.8) | 28 (19.2) | |
| Medicare/Medicaid | 3 (2.0) | 7 (4.8) | |
| SAGA | 20 (13.4) | 18 (12.3) | |
| Private | 9 (6.0) | 5 (3.4) | |
| Self | 22 (14.8) | 22 (15.1) | |
| Dual eligible | 1 (0.7) | 0 (0) | |
| Disenrolled | 0.007 | ||
| No | 117 (78.5) | 94 (64.4) | |
| Yes (list below) | 32 (21.5) | 52 (35.6) | |
| Communication barrier | 0 (0) | 1 (1.9) | |
| Death or terminal illness | 1 (3.1) | 2 (3.9) | |
| Limited tel. contact | 1 (3.1) | 23 (44.2) | |
| Mental health | 1 (3.1) | 1 (1.9) | |
| Moved | 0 (0) | 1 (1.9) | |
| No 6-month follow-up | 17 (53.1) | 2 (3.9) | |
| No phone contact | 0 (0) | 4 (7.7) | |
| No longer CHC patient | 3 (9.4) | 9 (17.7) | |
| Not adherent to lab protocol | 5 (15.6) | 5 (19.6) | |
| Patient choice | 4 (12.5) | 2 (3.9) | |
| Other | 0 (0) | 2 (3.9) | |
aTable values are n (column %) for categorical variables
bPercentages may not sum to 100% due to rounding
cP-value is from χ2 test (categorical variables) or from Fisher's exact test if marked with #
Note: In the Race category, we have collapsed mixed and Asian into the Other groups; in the Marital Status category, we have collapsed Divorced, Separated, Single, Single with partner and widow into the group of Not Married; Other insurance including Medicare, Private, Self, Dual eligible, Medicare/Medicaid; there is one case in the Asthma category with the value ’o’ that was assumed to be a typo and was changed to ‘No.’ Past depression = patient endorsing the question “have you ever been treated for depression?” Current depression = patient endorsing the question “are you currently being treated for depression?” Clinical diagnosed depression = current diagnosis of depression in the patient’s medical record
Table 2.
Baseline Outcome Measures Comparison Between Two Groups
| Outcome | Treatment group | pc | |
|---|---|---|---|
| Control (N = 149)b | Intervention (N = 146)b | ||
| Clinical outcomes | |||
| HbA1c(average) | 8.4 ± 2.33 | 7.6 ± 1.75 | 0.006* |
| HbA1c <7 | 48 (33.6) | 67 (46.5) | |
| 7≤ HbA1c<9 | 50 (35.0) | 51 (35.4) | |
| HbA1c >9 | 45 (31.5) | 26 (18.1) | |
| Systolic blood pressure | 133.1 ± 19.55 | 132.6 ± 19.30 | 0.845 |
| Diastolic blood pressure | 77.1 ± 14.24 | 78.3 ± 12.49 | 0.445 |
| BMI | 33.7 ± 6.64 | 35.4 ± 8.63 | 0.055 |
| BMI <25 | 7 (4.7) | 13 (8.9) | 0.153 |
| 25≤ BMI < 30 | 43 (28.9) | 31 (21.2) | |
| BMI ≥30 | 99 (66.4) | 102 (69.9) | |
| Cholesterol | 173.9 ± 42.29 | 169.6 ± 47.4 | 0.421 |
| LDL | 94.8 ± 36.11 | 89.5 ± 35.66 | 0.225 |
| HDL | 46.83 ± 13.04 | 46.1 ± 12.64 | 0.644 |
| Triglycerides | 167.8 ± 88.11 | 169.7 ± 108.3 | 0.594* |
| Behavior outcomes | |||
| Physical assessment | 0.900 | ||
| Sedentary | 50 (33.6) | 44 (30.1) | |
| Under-active | 7 (4.7) | 9 (6.2) | |
| Under-active light | 11 (7.4) | 12 (8.2) | |
| Under-active regular | 23 (15.4) | 27 (18.5) | |
| Active | 57 (38.3) | 54 (37.0) | |
| Nutrition outcomes | |||
| Fat intake | 0.599 | ||
| Excellent | 115 (77.7) | 111 (76.0) | |
| Good | 26 (17.6) | 24 (16.4) | |
| Fair | 7 (4.7) | 11 (7.5) | |
| Fruit/vegetable intake (average per day) | 2.4 ± 1.32 | 2.7 ± 1.52 | 0.071 |
| 0–2/day | 82 (55.4) | 71 (48.6) | 0.398 |
| 3–4/day | 55 (37.2) | 59 (40.4) | |
| ≥5/day | 11 (7.4) | 16 (11.0) | |
| N = 50 | N = 55 | ||
| PHQ9 | 12.5 ± 5.99 | 11.09 (5.42) | 0.202 |
aTable values are mean ± standard deviation for continuous variables and n (column %) for categorical variables
bPercentages may not sum to 100% due to rounding
cP-value marked with * are from Wilcoxon test, otherwise from Student’s t-test for continuous variables and χ2 test for categorical variables
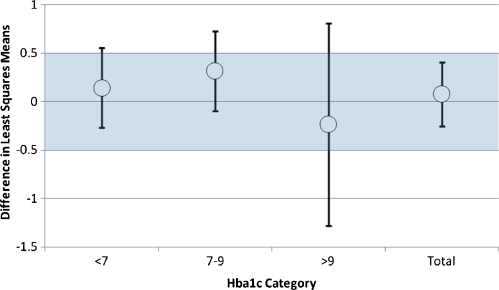
Evaluation of Intervention on HbA1c After adjusting for differences in baseline HbA1c, we found no significant difference in the primary outcome (HbA1c) between the intervention and control groups at 12 months based on the modified intention to treat analysis population [control-intervention 0.083 (−0.25, 0.41)] (Fig. 3 and Table 3). The confidence interval for the treatment difference was consistent with the absence of a clinically relevant intervention benefit (i.e., 0.5 or greater). Analyses by baseline HbA1c group demonstrated no significant difference in the effect of the treatment across these subgroups (Fig. 3, p = 0.989). Group differences at 12 months were: 0.14, (−0.27, 0.56), p = 0.50; 0.32, (−0.10, 0.73), p = 0.14; and −0.23, (−1.28, 0.81), p = 0.66 in those with good (Hba1c <7.0), moderate (Hba1c 7.0–9.0) and poor (Hba1c >9.0) control at baseline, respectively.
Figure 3.
Overall and baseline HbA1c group-specific treatment differences and 95% confidence intervals for HbA1c. Differences in least squares means between control and intervention at 12 months with 95% confidence intervals for the overall treatment effect and the treatment effect for subgroups of baseline HbA1c. The band represents a clinically important difference in HbA1c of ±0.50%.
Table 3.
Comparison of Clinical/Behavioral Outcomes Between Treatment Groups at Month 6 and Month 12 (Data Are Expressed as Least Squares Means)
| Month 6 (N = 256) | Month 12(N = 245) | |||||
|---|---|---|---|---|---|---|
| Outcome | Control | Intervention | Treatment difference (95% CI) | Control | Intervention | Treatment difference (95% CI) |
| HbA1c | 7.64 | 7.66 | −0.01(−0.34,0.31) P = 0.94 | 7.74 | 7.66 | 0.08(−0.25,0.41) P = 0.63 |
| BMI | 34.63 | 34.58 | 0.05(−0.4,0.50) P = 0.82 | 34.69 | 34.50 | 0.19(−0.46,0.84) P = 0.56 |
| DBP | 79.3 | 79.2 | 0.07(−2.7,2.8) P = 0.96 | 78.3 | 78.2 | 0.13(−2.4,2.7) P = 0.92 |
| SBP | 137.1 | 136.6 | 0.5(−4.2,5.2) P = 0.83 | 134.7 | 133.2 | 1.47(−3.04,5.98) P = 0.53 |
| LDL | 89.57 | 92.92 | −3.35(−10.76,4.06) P = 0.38 | 85.31 | 89.48 | −4.16(−11.12,2.80) P = 0.24 |
| Fruits/vegetables | 2.58 | 2.70 | a0.96(0.84,1.08) P = 0.49 | 85.31 | 89.48 | a1.02(0.88,1.16) P = 0.73 |
aRatio of control to intervention mean
Evaluation of Intervention on Secondary Outcomes No significant differences were observed between the intervention and control group at 12 months for secondary clinical or behavioral outcome measures (Table 3). In addition, perceived health status and self-reported rates of physical activity did not vary between treatment and control groups (Table 4).
Table 4.
Comparison of Health Status and Physical Assessment Between Treatment Groups at Month 6 and Month 12 (Data Are Expressed as Odds Ratios)
| Month 6 | Month 12 | |
|---|---|---|
| Outcome | Odds ratio (control/intervention) | Odds ratio (control/intervention) |
| Perceived health status | 1.07 (0.65, 1.74) P = 0.80 | 1.58 (0.92, 2.7) P = 0.10 |
| Physical assessment | 0.83 (0.50, 1.35) P = 0.45 | 0.93 (0.54, 1.61) P = 0.80 |
Subgroup Analyses Additional sub-group analyses were performed to determine whether patients with depression, Spanish speakers, or those with lower educational attainment were more likely to benefit from the intervention (Table 5). None of the interactions between intervention and these potential moderators was significant (p = 0.4, 0.27 and 0.91 respectively). Patients with an established diagnosis of depression did show a trend towards benefit after 12 months of intervention, but this finding did not achieve statistical significance.
Table 5.
Subgroup analysis
| Month 6 | Month 12 | ||||||
|---|---|---|---|---|---|---|---|
| Outcome | Subgroup | Control | Intervention | Treatment difference (95% CI) | Control | Intervention | Treatment difference (95% CI) |
| Current depression | |||||||
| HbA1c | Yes | 7.76 | 7.85 | −0.09 (−0.64, 0.46) p = 0.75 | 8.21 | 7.77 | 0.44(−0.11, 0.99) P = 0.12 |
| No | 7.58 | 7.57 | 0.01 (−0.38, 0.40) p = 0.97 | 7.47 | 7.61 | −0.14(−0.55, 0.27) P = 0.52 | |
| Education | |||||||
| High | 7.61 | 7.65 | −0.04 (−0.46, 0.38) p = 0.85 | 7.74 | 7.60 | 0.14(−0.30, 0.57) p = 0.54 | |
| Low | 7.69 | 7.67 | 0.02 (−0.50, 0.54) p = 0.93 | 7.75 | 7.75 | 0.00(−0.52, 0.52) p = 0.99 | |
| Spanish speaking | |||||||
| Yes | 7.59 | 7.69 | −0.10 (−0.52, 0.32) p = 0.65 | 7.66 | 7.75 | −0.10(−0.53, 0.33) p = 0.65 | |
| No | 7.73 | 7.61 | 0.12 (−0.41, 0.64) p = 0.66 | 7.88 | 7.52 | 0.35(−0.17, 0.88) p = 0.18 | |
DISCUSSION
This telephonic disease management intervention for underserved patients with diabetes showed no significant benefit on a wide variety of primary and secondary clinical and behavioral outcomes. Interval estimates for the primary outcome variable, glycemic control, excluded a clinically significant difference in HbA1c. While not powered to evaluate subgroups, our analysis did not find variation in HbA1C results based on baseline glycemic control.
There are several possible explanations for the lack of treatment effect. It is possible that the 1-year duration was insufficient to realize benefits from an intervention geared at promoting lifestyle changes. Such changes may take time to enact and show benefit, and HbA1c lags behind actual improvement. However, commercial disease management providers have claimed that such benefits accrue rapidly, with interventions similar in design to our own.
The inclusion of medication management and titration might have strengthened the impact of the intervention. We chose to emphasize medication adherence rather than medication management based on our hypothesis that poor adherence was more critical to improving diabetes care in a population already well managed by the primary care providers.
We chose to enroll patients with all levels of glycemic control. Several successful diabetes disease management trials in different settings from ours have focused on patients with elevated HbA1Cs14–16. This decision was made due to uncertainty as to which patients might benefit most from disease management. Patients with good control may be the most motivated to learn self-management skills and better maintain control over time. Alternatively, those with the highest HbA1C might have the most need for disease management but be less motivated or able to engage in self-management.
Patients in both the intervention and the control received high quality “usual” diabetes care, likely superior to that found in many other primary care settings. Usual care included access to diabetes education and self-management, a well-trained, bilingual staff, use of an electronic health record, and strong organizational structure emphasizing quality, continuity, and access. It is possible that an intervention such as this would be more beneficial to patients without access to this level of “usual care.”
It also may be that our intervention was not integrated enough with primary care. Our call center was physically removed from the clinical areas, and nurses did not have face-to-face or scheduled contact with patients, providers, and care teams. The Medicare Care Coordination demonstrations project suggested that substantial face-to-face contact in addition to telephonic contact was needed to improve outcomes17. Rothman et al.14 showed improved intermediate diabetes outcomes in underserved patients with an intervention emphasizing intensive face-to-face support from pharmacists and case managers.
Nearly 35% of the patients carried a diagnosis of depression, and just over 50% had a history of depression. Patients with diabetes and depressive symptoms have worse adherence to self-care guidelines6,7, glycemic control, and higher rates of diabetic complications18–20. Although patients had access to on-site mental health services in both the intervention and control, the intervention itself was not designed to manage depression.
The higher dropout rate in the intervention group could have biased the treatment comparison. However, in this case it is unlikely that the higher dropout rate in the intervention group resulted in the treatment effect equivalence as this requires those dropouts in the intervention group to be the ones improving with intervention. The baseline HbA1c was compared between individuals who dropped out and those were not lost to follow-up, and no significance was found (8.0 ± 2.03 in those not lost to follow-up vs. 8.08 ± 2.44 in those who dropped out).
Randomization failed to achieve an even distribution between groups with regard to HbA1C. This effect was most pronounced in the subgroup with HbA1C > 9, which might have been expected to benefit most from the intervention. While appropriate statistical adjustments were made to account for these baseline differences, this inequity remains a limitation of the study.
The program was grounded on the principals of empowerment and self-management, which have been shown to improve diabetes outcomes in diverse populations21–28. The flexible approach taken by the nurses over the phone in using these techniques was a strength, but also a weakness, making it more difficult to evaluate or replicate. Primary care in a community setting requires a great deal of adaptability to changing circumstances. We chose to embed this concept in the telephonic intervention to give the nurse maximum latitude to address patients' specific needs, even if they were outside the realm of diabetes care. In so doing, it was not possible to assess the fidelity of the intervention through checklists or process measures. We specifically chose to avoid checklists for fear that it would inhibit the nurse from addressing each patient's needs in a patient-centered rather than disease-centered manner.
Lack of blinding of the primary care providers and the presence of control and intervention patients in the same clinics were additional weaknesses, which may have led to contamination.
In summary, our randomized controlled trial provided telephonic disease management to underserved, largely Spanish-speaking patients with diabetes. Despite a strong emphasis on cultural and linguistic competence, a flexible, telephone-based intervention, and use of a common electronic health record to link providers with call center nurses, the intervention did not improve glycemic control or a wide range of other clinical and behavioral outcomes. While the need for new strategies to control the growing diabetes epidemic among underserved, minority populations is great, it is unclear whether telephonic disease management can provide the answer.
Acknowledgements
Funding for this project was provided by a grant from the Connecticut Health Foundation.
Conflict of Interest None disclosed.
Footnotes
Funded by a grant from the Connecticut Health Foundation.
References
- 1.Mattke S, Seid M, Ma S. Evidence for the effect of disease management: is $1 billion a year a good investment? Am J Manag Care. 2007;13:670–676. [PubMed] [Google Scholar]
- 2.Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22:15–38. doi: 10.1016/S0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 3.Self-reported prevalence of diabetes among Hispanics-United States, 1994–1997. MMWR Morb Mortal Wkly Rep. 1999;48:8–12. [PubMed]
- 4.Diabetes among Hispanics-Los Angeles County, California, 2002–2003. MMWR Morb Mortal Wkly Rep. 2003;52:1152–55. [PubMed]
- 5.Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates of diabetes in the United States, 2005. 2005. Atlanta, GA, US Department of Health and Human Services, centers for Disease Control and Prevention. Ref Type: Report.
- 6.Ciechanowski PS, Katon WJ, Russo JE. Depression and diabetes: impact of depressive symptoms on adherence, function, and costs. Arch Intern Med. 2000;160:3278–85. doi: 10.1001/archinte.160.21.3278. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, et al. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care. 2007;30:2222–27. doi: 10.2337/dc07-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28:2280–2288. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 9.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakimoto P, Block G, Mandel S, Medina N. Development and reliability of brief dietary assessment tools for Hispanics. Prev Chronic Dis. 2006;3:A95. [PMC free article] [PubMed] [Google Scholar]
- 11.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 12.Brown H, Prescott R. Applied Mixed Models in Medicine. Chicester, England: Jophn Wiley & Sons, Ltd; 1999. [Google Scholar]
- 13.Allison PD. Missing Data. University Papers Series on Quantitative Applications in the Social Sciences. Thousand Oaks, CA: Sage; 2001. [Google Scholar]
- 14.Rothman RL, Malone R, Bryant B, Shintani AK, Crigler B, Dewalt DA, et al. A randomized trial of a primary care-based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. Am J Med. 2005;118:276–84. doi: 10.1016/j.amjmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Taylor CB, Miller NH, Reilly KR, Greenwald G, Cunning D, Deeter A, et al. Evaluation of a nurse-care management system to improve outcomes in patients with complicated diabetes. Diabetes Care. 2003;26:1058–63. doi: 10.2337/diacare.26.4.1058. [DOI] [PubMed] [Google Scholar]
- 16.Young RJ, Taylor J, Friede T, Hollis S, Mason JM, Lee P, et al. Pro-active call center treatment support (PACCTS) to improve glucose control in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2005;28:278–82. doi: 10.2337/diacare.28.2.278. [DOI] [PubMed] [Google Scholar]
- 17.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301:603–18. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 18.de GM, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–30. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Katon W, von KM, Ciechanowski P, Russo J, Lin E, Simon G, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care. 2004;27:914–20. doi: 10.2337/diacare.27.4.914. [DOI] [PubMed] [Google Scholar]
- 20.Lustman PJ, Anderson RJ, Freedland KE, de GM, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23:934–42. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- 21.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, et al. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 22.Lorig K, Ritter PL, Villa F, Piette JD. Spanish diabetes self-management with and without automated telephone reinforcement: two randomized trials. Diabetes Care. 2008;31:408–14. doi: 10.2337/dc07-1313. [DOI] [PubMed] [Google Scholar]
- 23.Norris SL, Lau J, Smith SJ, Schmid CH, Engelgau MM. Self-management education for adults with type 2 diabetes: a meta-analysis of the effect on glycemic control. Diabetes Care. 2002;25:1159–71. doi: 10.2337/diacare.25.7.1159. [DOI] [PubMed] [Google Scholar]
- 24.Anderson D, Christison-Lagay J. Diabetes self-management in a community health center: improving health behaviors and clinical outcomes for underserved patients. Clin Diabetes. 2008;26:22–27. doi: 10.2337/diaclin.26.1.22. [DOI] [Google Scholar]
- 25.Gilmer TP, Philis-Tsimikas A, Walker C. Outcomes of Project Dulce: a culturally specific diabetes management program. Ann Pharmacother. 2005;39:817–22. doi: 10.1345/aph.1E583. [DOI] [PubMed] [Google Scholar]
- 26.Glazier RH, Bajcar J, Kennie NR, Willson K. A systematic review of interventions to improve diabetes care in socially disadvantaged populations. Diabetes Care. 2006;29:1675–88. doi: 10.2337/dc05-1942. [DOI] [PubMed] [Google Scholar]
- 27.Metghalchi S, Rivera M, Beeson L, Firek A, De LM, Balcazar H, et al. Improved clinical outcomes using a culturally sensitive diabetes education program in a Hispanic population. Diabetes Educ. 2008;34:698–706. doi: 10.1177/0145721708320913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007;64:101S–56S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]