Abstract
Background
Extracorporeal circuit priming and intravascular volume expansion during cardiopulmonary bypass (CPB) may lead to dilutional coagulopathy and excessive diffuse postoperative bleeding. Prothrombin complex concentrate (PCC) containing clotting factors II (FII), VII (FVII), IX (FIX), and X (FX) could be of potential value in correcting dilutional coagulopathy and reducing blood loss.
Methods
Anaesthetized pigs underwent CPB with hypothermia for 2 h at 25°C followed by 1 h of normothermia. Approximately 1 h after CPB, animals randomly received either isotonic saline 1 ml kg−1 or PCC 30 IU kg−1 in a volume of 1 ml kg−1. Diffuse coagulopathic bleeding was assessed as suture hole blood loss from a Gore-Tex patch placed over a full-thickness incision in the left carotid artery.
Results
After CPB, levels of FII, FVII, FIX, and FX declined from baseline by 32% to 48%, and PCC fully or partially reversed those deficits. Median suture hole blood loss after administration of saline placebo was 74 ml. PCC reduced suture hole bleeding by a median of 54 ml with a 95% confidence interval of 6–112 ml (P=0.026) compared with saline. PCC, but not saline, normalized skin bleeding time. Peak thrombin generation markedly decreased after CPB, but then returned in PCC-treated animals to a level higher than baseline by 28.7 nM (14.5–41.1 nM; P=0.031).
Conclusions
PCC was effective in correcting dilutional coagulopathy and reducing diffuse bleeding in an in vivo large-animal CPB model. Further research is warranted on PCC as a haemostatic agent in CPB.
Keywords: blood coagulation disorders, cardiopulmonary bypass, haemodilution, haemorrhage, prothrombin complex concentrates
Key points.
Patients undergoing cardiopulmonary bypass (CPB) may develop dilutional coagulopathy.
The value of prothrombin complex concentrate in reducing suture hole bleeding from the carotid artery was assessed in pigs after CPB.
The prothrombin complex concentrate reduced bleeding.
This agent may be useful in the clinical setting.
Excessive postoperative bleeding remains a frequent, serious, and unpredictable complication of cardiac surgery with cardiopulmonary bypass (CPB).1–4 Such bleeding is often non-surgical. For instance, diffuse oozing with no identifiable surgical site of bleeding was present in 42 of 191 consecutive CPB patients (22%) undergoing re-exploration for excessive blood loss.2
Dilution of coagulation factors may contribute to excessive haemorrhage post-CPB.5 Commencement of CPB precipitates an abrupt major haemodilution due to extracorporeal circuit priming with fluid volumes typically of ∼2000 ml or more. More than 2000 ml of additional fluids may be administered during surgery, further exacerbating haemodilution. As a consequence, the estimated dilution of plasma clotting factors resulting from CPB surgery is ∼50%.5
Prothrombin complex concentrate (PCC) could be of potential utility in correcting dilutional coagulopathy arising during CPB. The ability of PCC to ameliorate diffuse coagulopathic bleeding has already been demonstrated in a study of general surgery patients.6 In that study, 27 patients with life-threatening diffuse bleeding and an international normalized ratio (INR) above 1.1 received Beriplex P/N, a biochemically well-characterized balanced PCC containing coagulation factors II (FII), VII (FVII), IX (FIX), and X (FX), and the anticoagulant proteins C and S.7 Cessation of bleeding was attained in 26 patients (96%) within 3 h of PCC administration. Clinical evidence on the use of Beriplex P/N in cardiac surgery has also been provided by case reports, a retrospective study, and a randomized trial.8–10
A conventional option for correcting haemodilution-induced coagulation factor deficiencies after CPB is transfusion of fresh-frozen plasma (FFP), which contains all the coagulation factors. However, since FII, FVII, FIX, and FX are present in Beriplex P/N at ∼25 times the corresponding plasma concentrations, smaller volumes and less time are needed to correct dilutional coagulopathy compared with FFP. Also, FFP requires thawing, and blood type matching may also be needed. Furthermore, most FFP preparations have not been virally inactivated. In the purification of Beriplex P/N, such inactivation is accomplished by pasteurization and nanofiltration of plasma screened by polymerase chain reaction.7,11 There has been no evidence in clinical trials of viral transmission related to Beriplex P/N infusion.12,13 The present study evaluated the ability of Beriplex P/N to attenuate diffuse post-CPB bleeding in a porcine model.
Methods
Animals
Seventeen castrated male pigs (large white×German noble) weighing 24–40 kg were procured from a local breeding farm (Willi Schlosser, Schwalmtal, Germany) at age 3–4 months. The animals were housed at 18–21°C in stables with straw bedding under ambient day–night cycles and fed ad libitum with Deuka V pig chow (Deutsche Tiernahrung Cremer GmbH & Co., KG, Düsseldorf, Germany). Tap water was supplied ad libitum. Animal husbandry and study procedures complied with the German Animal Welfare law and European Union regulations. The study was approved by the regional government authorities.
Anaesthesia
Anaesthetic procedures have been previously described.14 Briefly, after an overnight fast with unrestricted access to water and i.m. premedication using a mixture of azaperone 2 mg kg−1 (Stresnil®, Janssen-Cilag GmbH, Neuss, Germany), ketamine 15 mg kg−1 (Ketavet, Pharmacia & Upjohn, Erlangen, Germany), and atropine sulphate 0.02 mg kg−1 (Atropinsulfate, B. Braun Melsungen AG, Melsungen, Germany), the pigs were anaesthetized with thiopental sodium 10 mg kg−1 via an ear vein. After tracheal intubation, respiration was supported via a Heyer Access ventilator. Inhaled anaesthesia was maintained with isoflurane 1–2% (Isofluran CP®, CP Pharma GmbH, Burgdorf, Germany). Attainment and maintenance of deep anaesthesia were confirmed by an absent pedal withdrawal reflex and lack of any response to surgery. A 1.4×2.1 mm catheter was advanced into a carotid artery for collection of blood samples and a 0.5×0.9 mm catheter into a femoral artery for continuous arterial pressure measurements. Ringer's solution at 4 ml kg−1 h−1 to satisfy basal fluid requirements and test fluids were infused via an indwelling 1.4×2.1 mm catheter in an external jugular vein. Body temperature was monitored by rectal thermometry.
Cardiopulmonary bypass
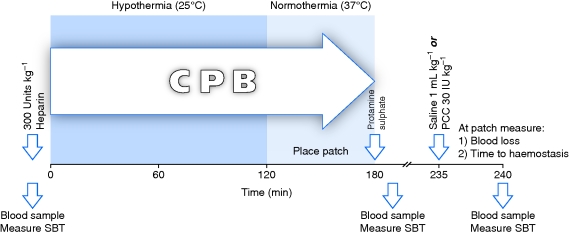
The experimental design is outlined in Figure 1. Sternotomy was performed with an oscillating saw, and the heart was exposed. The pericardium was opened longitudinally and secured to the chest wall with four sutures. Two purse-string sutures each were placed in the ascending aorta and right atrium. An i.v. bolus of heparin 300 U kg−1 was administered. After 10 min, a 5.2 mm diameter arterial catheter and a 32 Fr venous catheter were placed and secured with tourniquets. Both catheters were connected to a small adult hollow fibre oxygenator with a hard shell venous reservoir (D905 EOS, Sorin SpA, Milan, Italy). The extracorporeal circuit was primed with a solution consisting of isotonic saline 500 ml, 6% hydroxyethyl starch 200/0.5 1000 ml (Infukoll, Schwarz Pharma AG, Mannheim, Germany), 15% mannitol 2 ml kg−1 (Osmofundin®, B. Braun) and heparin 1000 units. Hydroxyethyl starch has been extensively investigated for priming in cardiac surgery randomized trials15 and is a common choice in clinical practice.16,17 The venous and arterial lines were opened in succession and the venous blood allowed to flow into the venous reservoir by gravity. An arterial pump conveyed the blood into the oxygenator. The oxygenated blood was equilibrated to target temperature and returned via the arterial line to the aorta. Hypothermia was maintained at 25°C for 2 h followed by 1 h of normothermia (37°C).
Fig 1.
Study design. CPB, cardiopulmonary bypass; PCC, prothrombin complex concentrate; SBT, skin bleeding time.
Patch placement
Polytetrafluorethylene (PTFE) is a commonly used graft material in vascular surgery. Gore-Tex is a PTFE-based material. Oozing from the multiple suture holes created when securing PTFE grafts can often lead to substantial cumulative blood loss.18 Suture hole bleeding is thus similar in nature to the diffuse oozing typical of non-surgical postoperative haemorrhage in cardiac surgery patients.
Methods of patch placement have been detailed elsewhere.19 During the period of normothermia, the left carotid artery was occluded with two clamps 5 cm apart. After a full-thickness incision into the arterial wall, a Gore-Tex patch 4 mm wide and ∼30 mm long (W.L. Gore & Associates GmbH, Putzbrunn, Germany) was sutured to the incision site with 5/0 monofilament (Ethicon GmbH, Norderstedt, Germany).
Treatment
Upon termination of CPB, the blood remaining in the oxygenator was returned to the animal. Anticoagulation was reversed with protamine sulphate 1 mg per 100 units of administered heparin. Study treatment fluids were infused 55 min thereafter. Six animals each were randomly assigned to receive isotonic saline 1 ml kg−1 or PCC 30 IU kg−1 (Beriplex® P/N, CSL Behring GmbH, Marburg, Germany) in a volume of 1 ml kg−1. As an additional control group, five animals were subjected to carotid patch placement but not CPB.
Measurements
Immediately after test fluid infusion, the carotid clamps were released. The volume of blood shed from the suture holes was measured. Additionally, the time elapsed from clamp release until cessation of observable suture hole bleeding was recorded as the time to haemostasis.
Blood samples for laboratory assays were collected at (i) baseline before the commencement of CPB, (ii) 5 min after reversal of anticoagulation with protamine, and (iii) 5 min after test fluid infusion. At those time points, determinations were also made of skin bleeding time (SBT), defined as the time until cessation of blood loss from a standardized 5 mm long by 1 mm deep inner ear incision created using a Surgicutt® cutting device (International Technidyne Corp., Edison, NJ, USA).
Concentrations of coagulation factors were measured in coagulation factor-deficient plasma (Dade Behring, Marburg, Germany) with a Schnitger and Gross coagulometer (Heinrich Amelung GmbH, Lemgo, Germany). Measurement of FII, FVII, FIX, and FX in porcine plasma using assays designed for human samples has been previously described.14,20–22 Prothrombin time (PT) was determined using a Schnitger & Gross coagulometer and the Thromborel reagent (Dade Behring).
Peak thrombin generation was measured by calibrated automated thrombinography (Thrombinoscope B.V., Maastricht, The Netherlands) in diluted plasma.23 The respective concentrations of recombinant relipidated tissue factor and phospholipids were 5 pM and 4 µM. The peak molar quantity of thrombin present in clotting plasma was computed using the Thrombinoscope software version 3.0.0.29.
Statistical analysis
On the basis of previously reported suture hole blood loss measurements in the pig with or without fibrin sealant,19 it was assumed that an effective treatment would reduce blood loss on average by 50 ml with a standard deviation of 25 ml. On the basis of that assumption, a sample size of six animals per group was calculated to furnish >80% statistical power for detecting a between-group difference at the 0.05 α-level.
Median differences and their 95% confidence intervals (CI) were calculated by exact Hodges–Lehmann estimation. The statistical significance of differences was evaluated by the exact Wilcoxon test. Computer programs used for statistical analysis were R version 2.7.2 (The R Foundation for Statistical Computing, Vienna, Austria) and StatXact 7.0 (Cytel Software Corp., Cambridge, MA, USA).
Results
Median PT was prolonged after CPB compared with baseline (Table 1). After saline infusion, PT returned to approximately the baseline level. PT after PCC administration was shorter by a median 2.6 s (CI, 2.1–4.2 s) than at baseline and by a median 2.6 s (CI, 1.5–4.7 s) than after saline infusion. CPB decreased median platelet concentration to 254×109 from 393×109 litre−1 at baseline, and platelets remained low both after saline and PCC infusion (Table 1).
Table 1.
PT, platelets, and SBT. CPB, cardiopulmonary bypass; IQR, inter-quartile range; PCC, prothrombin complex concentrate; PT, prothrombin time; SBT, skin bleeding time
| Category | n | Median (IQR) |
||
|---|---|---|---|---|
| PT (s) | Platelets (×109 litre−1) | SBT (s) | ||
| Baseline | 12 | 13.6 (13.0–14.4) | 393 (347–482) | 122 (110–130) |
| Post-CPB | 12 | 15.4 (14.5–16.4) | 254 (210–270) | 172 (159–206) |
| Saline | 6 | 13.8 (13.4–14.4) | 284 (252–301) | 166 (157–169) |
| PCC | 6 | 11.3 (10.7–11.7) | 239 (216–272) | 138 (130–143) |
Coagulation factors
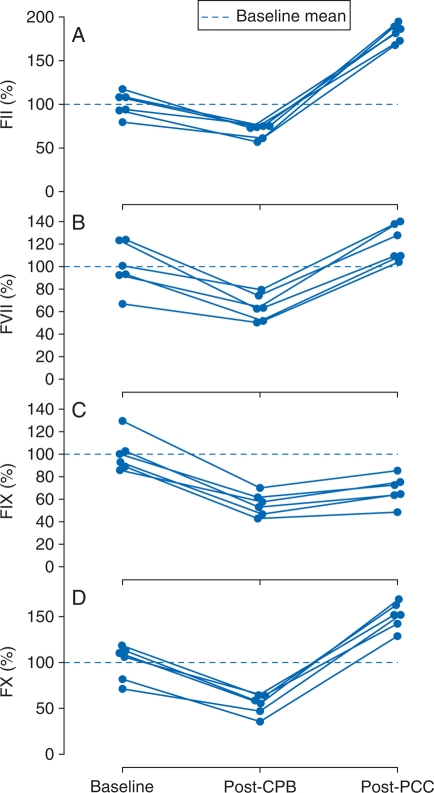
After CPB, circulating levels of FII, FVII, FIX, and FX declined from baseline by median values of 31.7% (CI, 18.4–44.4%), 35.5% (CI, 16.6–60.6%), 46.2% (CI, 28.2–59.4%), and 48.2% (CI, 24.5–55.1%), respectively (Fig. 2). In response to PCC administration, FII, FVII, and FX rebounded to levels, respectively, 82.5% (CI, 64.1–109.4%), 20.4% (CI, 3.8–42.7%), and 51.7% (CI, 29.6–80.5%) above baseline. Compared with the post-CPB levels, FIX increased 13.0% (CI, 5.7–18.0%) after PCC administration, but nevertheless remained 33.8% (CI, 10.6–44.1%) below baseline.
Fig 2.
Individual animal levels of (a) FII, (b) FVII, (c) FIX, and (d) FX at baseline and after CPB and subsequent administration of PCC. Concentration values expressed as percentages of the baseline means, shown as dashed lines. CPB, cardiopulmonary bypass; FII, factor II; FVII, factor VII; FIX, factor IX; FX, factor X; PCC, prothrombin complex concentrate.
As expected, the median decreases in the concentrations of the four coagulation factors due to CPB-related haemodilution were similar, all falling within the comparatively narrow range of 31.7–48.2%. However, the concentration changes in response to PCC infusion differed widely. Those differences reflect both the relative proportions of the four factors in the PCC and disparities in endogenous coagulation factor concentrations between humans and pigs. The respective mean (standard deviation) concentrations of FII, FVII, FIX, and FX in Beriplex P/N are 31.0 (3.4), 16.2 (1.9), 28.9 (2.2), and 40.5 (3.3) IU ml−1.12 The reported mean concentrations of those four coagulation factors in pigs, as percentages of normal human pooled plasma, are 45 (3), 83 (8), 330 (15), and 72 (4), respectively.20 Consequently, when a fixed PCC dose was infused, its FIX component was added to a high endogenous FIX concentration in the pig equalling 3.4-fold the human level, and the resulting per cent increase in total measured FIX concentration was relatively small. Similarly, the smaller median increase from baseline in FVII (20.4%) than FII (82.5%) reflects both the two-fold higher content of FII than FVII in the administered PCC and the two-fold higher endogenous concentration of FVII than FII in the pig.
Haemostasis
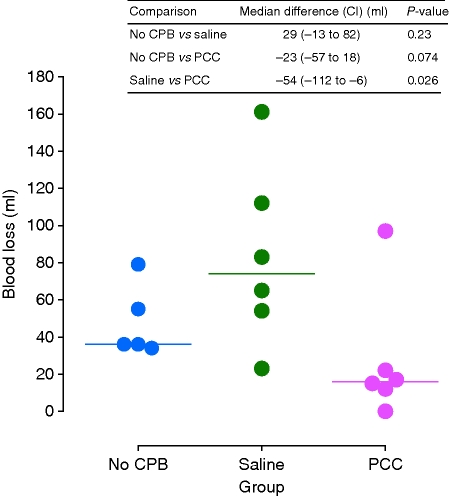
Figure 3 displays suture hole blood loss in animals treated post-CPB either with saline placebo or PCC, and also in a control group not undergoing CPB. Median suture hole blood loss after administration of saline placebo was 74 ml. Compared with saline, PCC reduced blood loss by a median of 54 ml. The median time to suture hole haemostasis was 143 min (inter-quartile range, IQR, 142–172 min) in the control group not subjected to CPB compared, respectively, with 292 min (IQR, 247–328 min) and 217 min (IQR, 142–288 min) after CPB and infusion of saline placebo or PCC. Although time to haemostasis was shorter by a median of 69 min (CI, −110 to 212 min) in the PCC than the saline placebo group, the difference was not statistically significant.
Fig 3.
Individual animal suture hole blood losses after CPB and administration of saline or PCC in comparison with those of a control group which did not undergo CPB. Horizontal lines indicate median values. CI, 95% confidence interval; CPB, cardiopulmonary bypass; PCC, prothrombin complex concentrate.
Median SBT was prolonged after CPB (172 s) vs baseline (122 s), as summarized in Table 1. PCC normalized SBT (P=0.25 for paired comparison with baseline), whereas after saline placebo administration, SBT remained significantly prolonged vs baseline by a median of 50 s (CI, 33–68 s; P=0.031).
Thrombin generation
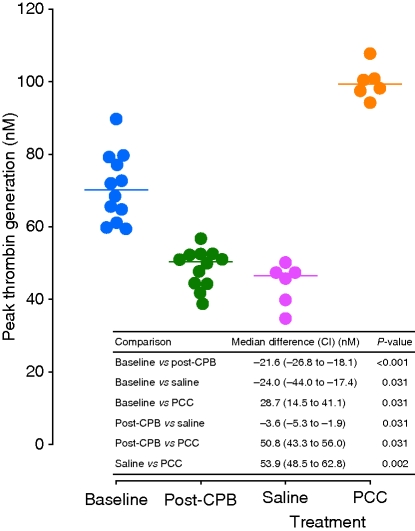
CPB resulted in a marked attenuation of peak thrombin generation (Fig. 4). Infusion of saline exacerbated this effect, whereas PCC increased peak thrombin generation to a level higher than baseline by a median of 28.7 nM.
Fig 4.
Peak thrombin generation in individual animals at baseline and after CPB and subsequent administration of saline or PCC. Medians depicted as horizontal lines. In the table inset, grouped comparison indicated between saline and PCC; all other comparisons paired. CI, 95% confidence interval; CPB, cardiopulmonary bypass; PCC, prothrombin complex concentrate.
Discussion
In this randomized placebo-controlled animal model study, PCC significantly alleviated suture hole bleeding after CPB. Such bleeding may simulate the diffuse oozing occurring in many CPB patients who experience excessive postoperative blood loss. The PCC-mediated reduction in suture hole bleeding was accompanied by normalization of SBT and a pronounced increase in peak thrombin generation.
This pilot study is limited by its relatively small size. The results need to be confirmed in further investigations, and the optimal PCC regimen for use in CPB remains to be delineated. The thrombogenic potential of PCC in CPB must also be characterized.
PCC displayed haemostatic efficacy in this study notwithstanding a reduction in median platelet concentration to 254×109 litre−1. In a recent study of haemodilutional coagulopathy and trauma in pigs,22 PCC displayed haemostatic efficacy, despite an even more pronounced platelet reduction to a mean of 133×109 litre−1. Additionally, in a clinical study of 16 consecutive intensive care unit patients, cessation of bleeding was achieved after PCC administration in all cases, despite a mean nadir platelet level of 149×109 litre−1.24 Since decreases in platelet function and number are a hallmark of CPB, one potential attraction of PCC might be that its effectiveness does not appear to be limited by comparatively low platelet counts.
A report of two cardiac surgery patients with liver dysfunction documented successful normalization of INR and cessation of excessive bleeding after infusion of Beriplex P/N.8 In a retrospective study, Beriplex P/N was administered to seven cardiac surgery patients, five undergoing coronary artery bypass graft and two valve procedures, who were experiencing bleeding refractory to FFP, platelets, and cryoprecipitate.9 Beriplex P/N shortened PT by 25% and activated partial thromboplastin time by 38%, and partial or complete haemostasis was achieved in all six patients with recorded results for bleeding. In a newly reported randomized trial of 40 patients undergoing semi-urgent cardiac surgery with CPB, Beriplex P/N was more effective than FFP for reversal of oral anticoagulant therapy.10
In previous clinical trials, Beriplex P/N has also proven effective for emergency reversal of oral anticoagulant therapy outside the setting of CPB.13,25 Additionally, Beriplex P/N showed haemostatic efficacy among patients with clotting factor deficits due to severe liver disease who required rapid haemostasis because of bleeding or the need for urgent surgical or invasive diagnostic procedures.26
In trauma, as in CPB, administered fluids can produce dilutional coagulopathy. In a porcine trauma model, PCC reduced blood loss from traumatic bone and spleen injuries compared with FFP14 and accelerated haemostasis after spleen trauma compared with recombinant activated FVII (rFVIIa).22 In those studies, PCC also increased peak thrombin generation vs FFP and rFVIIa. Reduction in blood loss also resulted from combination treatment with PCC and fibrinogen in a porcine model of haemodilutional coagulopathy and uncontrolled liver bleeding.27 Similarly, in a rabbit model of dilutional coagulopathy, PCC reduced blood loss and augmented peak thrombin generation after kidney trauma compared either with saline placebo or rFVIIa.28 Importantly, at effective dosages, PCC, unlike rFVIIa, showed no evidence of thrombogenicity in that study, as evaluated using the Wessler stasis model. Available clinical evidence also suggests that the thrombogenic potential of PCC is low. In a pharmacovigilance study, the estimated incidence of thrombotic events among patients receiving PCC was 1.0 per 105 infusions (CI, 0.1–3.6 per 105 infusions).29
rFVIIa has been evaluated in a recent randomized clinical trial of 179 cardiac surgery patients with postoperative bleeding.30 The primary study endpoint was the incidence of critical serious adverse events, defined as either death or thromboembolic complications. Owing to a trend towards increasing critical serious adverse events after rFVIIa treatment (odds ratio, 1.67; CI, 0.50–5.47), the investigators cautioned that further clinical trials are required.
The pig has been extensively utilized as a model for evaluating haemostasis after administration of human PCC14,21,22 and human rFVIIa.22,31–38 Certain uncertainties are inherent in such studies. The activity, bioavailability, or both of the human protein might be attenuated in the porcine host.34 Assays of PT or thrombin generation optimized for measurements of human samples may yield disparate results in the pig. For instance, PT measured with a porcine-specific assay is greater by over three-fold than with the standard assay designed for human specimens.37,39 The porcine-specific assay revealed a nearly 50% prolongation of PT during hypothermia, while no effect was detectable by the standard assay method.39 It is possible that the use of the standard assay may account for the relatively small impact of CPB on PT in the present study, despite the substantial increase in blood loss observed after CPB and saline infusion. In any case, large absolute differences in PT assay values may not translate into major differences in the observed relative effects of human haemostatic proteins in the pig model. Thus, despite an over three-fold absolute PT difference at baseline, the average relative shortening of PT by 90 µg kg−1 human rFVIIa was comparable when measured by standard assay (64%) vs the porcine-specific method (51%).37
The thrombin generation assay used in the present study was optimized for human samples, and the possibility cannot be excluded of differences in results compared with those that might have been generated by a porcine-specific method. Nonetheless, assay differences could not have affected bleeding, and the observed increase in thrombin generation by PCC relative to saline was accompanied by a corresponding decrease in blood loss. Furthermore, in two pig studies of dilutional coagulopathy and trauma, human PCC augmented thrombin generation compared with human rFVIIa and with both standard- and high-dose porcine PCC.14,22 In both studies, corresponding acceleration of haemostasis by PCC vs those control agents was demonstrated.
In light of the data reviewed above, PCC appears to be a versatile haemostatic agent suitable for use in indications involving multiple clotting factor deficiencies. Further clinical studies are needed to define the appropriate role for PCC in CPB. The present results provide encouragement to embark upon such studies.
Conflict of interest
Prothrombin complex concentrate for use in this study was furnished by CSL Behring GmbH, Marburg, Germany, the commercial supplier of that product and sponsor of the study.
Funding
This study was supported through an unrestricted grant from CSL Behring GmbH, Marburg, Germany.
References
- 1.Frankel TL, Stamou SC, Lowery RC, et al. Risk factors for hemorrhage-related reexploration and blood transfusion after conventional versus coronary revascularization without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2005;27:494–500. doi: 10.1016/j.ejcts.2004.11.021. doi:10.1016/j.ejcts.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Choong CK, Gerrard C, Goldsmith KA, Dunningham H, Vuylsteke A. Delayed re-exploration for bleeding after coronary artery bypass surgery results in adverse outcomes. Eur J Cardiothorac Surg. 2007;31:834–8. doi: 10.1016/j.ejcts.2007.02.001. doi:10.1016/j.ejcts.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Salis S, Mazzanti VV, Merli G, et al. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:814–22. doi: 10.1053/j.jvca.2008.08.004. doi:10.1053/j.jvca.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RH, Sheng S, O'Brien SM, et al. Reoperation for bleeding in patients undergoing coronary artery bypass surgery: incidence, risk factors, time trends, and outcomes. Circ Cardiovasc Qual Outcomes. 2009;2:583–90. doi: 10.1161/CIRCOUTCOMES.109.858811. doi:10.1161/CIRCOUTCOMES.109.858811. [DOI] [PubMed] [Google Scholar]
- 5.Bull BS, Hay KL, Herrmann PC. Postoperative bypass bleeding: a bypass-associated dilutional (BAD) coagulopathy? Blood Cells Mol Dis. 2009;43:256–9. doi: 10.1016/j.bcmd.2009.07.002. doi:10.1016/j.bcmd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Schick KS, Fertmann JM, Jauch K-W, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Crit Care. 2009;13:R191. doi: 10.1186/cc8186. doi:10.1186/cc8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Römisch J, Gröner A, Bernhardt D, et al. Nanofiltration bei der Herstellung von Beriplex® P/N: Erhöhung der Kapazität zur Viruseliminierung unter Beibehaltung der Produktqualität. Beitr Infusionsther Transfusionsmed. 1996;33:220–4. [PubMed] [Google Scholar]
- 8.Stuklis RG, O'Shaughnessy DF, Ohri SK. Novel approach to bleeding in patients undergoing cardiac surgery with liver dysfunction. Eur J Cardiothorac Surg. 2001;19:219–20. doi: 10.1016/s1010-7940(00)00641-2. doi:10.1016/S1010-7940(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 9.Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit Care. 2008;12:R105. doi: 10.1186/cc6987. doi:10.1186/cc6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demeyere R, Gillardin S, Arnout J, Strengers PFW. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sang. doi: 10.1111/j.1423-0410.2010.01339.x. DOI:10.1111/j.1423-0410.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- 11.Weimer T, Streichert S, Watson C, Gröner A. High-titer screening PCR: a successful strategy for reducing the parvovirus B19 load in plasma pools for fractionation. Transfusion. 2001;41:1500–4. doi: 10.1046/j.1537-2995.2001.41121500.x. doi:10.1046/j.1537-2995.2001.41121500.x. [DOI] [PubMed] [Google Scholar]
- 12.Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98:790–7. [PubMed] [Google Scholar]
- 13.Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H. Prothrombin complex concentrate (Beriplex® P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost. 2008;6:622–31. doi: 10.1111/j.1538-7836.2008.02904.x. doi:10.1111/j.1538-7836.2008.02904.x. [DOI] [PubMed] [Google Scholar]
- 14.Dickneite G, Pragst I. Prothrombin complex concentrate vs fresh frozen plasma for reversal of dilutional coagulopathy in a porcine trauma model. Br J Anaesth. 2009;102:345–54. doi: 10.1093/bja/aen391. doi:10.1093/bja/aen391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkes MM, Navickis RJ, Sibbald WJ. Albumin versus hydroxyethyl starch in cardiopulmonary bypass surgery: a meta-analysis of postoperative bleeding. Ann Thorac Surg. 2001;72:527–33. doi: 10.1016/s0003-4975(01)02745-x. doi:10.1016/S0003-4975(01)02745-X. [DOI] [PubMed] [Google Scholar]
- 16.Canver CC, Nichols RD. Use of intraoperative hetastarch priming during coronary bypass. Chest. 2000;118:1616–20. doi: 10.1378/chest.118.6.1616. doi:10.1378/chest.118.6.1616. [DOI] [PubMed] [Google Scholar]
- 17.Wiesen P, Canivet JL, Ledoux D, Roediger L, Damas P. Effect of hydroxyethylstarch on renal function in cardiac surgery: a large scale retrospective study. Acta Anaesthesiol Belg. 2005;56:257–63. [PubMed] [Google Scholar]
- 18.Glickman M, Gheissari A, Money S, Martin J, Ballard JL. A polymeric sealant inhibits anastomotic suture hole bleeding more rapidly than gelfoam/thrombin: results of a randomized controlled trial. Arch Surg. 2002;137:326–31. doi: 10.1001/archsurg.137.3.326. [DOI] [PubMed] [Google Scholar]
- 19.Dickneite G, Metzner H, Nicolay U. Prevention of suture hole bleeding using fibrin sealant: benefits of factor XIII. J Surg Res. 2000;93:201–5. doi: 10.1006/jsre.2000.5985. doi:10.1006/jsre.2000.5985. [DOI] [PubMed] [Google Scholar]
- 20.McLoughlin TM, Fontana JL, Alving B, Mongan PD, Bünger R. Profound normovolemic hemodilution: hemostatic effects in patients and in a porcine model. Anesth Analg. 1996;83:459–65. doi: 10.1097/00000539-199609000-00003. doi:10.1097/00000539-199609000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Dickneite G, Doerr B, Kaspereit F. Characterization of the coagulation deficit in porcine dilutional coagulopathy and substitution with a prothrombin complex concentrate. Anesth Analg. 2008;106:1070–7. doi: 10.1213/ane.0b013e318165dfbb. doi:10.1213/ane.0b013e318165dfbb. [DOI] [PubMed] [Google Scholar]
- 22.Dickneite G, Dörr B, Kaspereit F, Tanaka KA. Prothrombin complex concentrate versus recombinant factor VIIa for reversal of hemodilutional coagulopathy in a porcine trauma model. J Trauma. 2010;68:1151–7. doi: 10.1097/TA.0b013e3181b06364. doi:10.1097/TA.0b013e3181b06364. [DOI] [PubMed] [Google Scholar]
- 23.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. doi:10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 24.Staudinger T, Frass M, Rintelen C, et al. Influence of prothrombin complex concentrates on plasma coagulation in critically ill patients. Intensive Care Med. 1999;25:1105–10. doi: 10.1007/s001340051019. doi:10.1007/s001340051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116:619–24. doi: 10.1046/j.0007-1048.2001.03295.x. doi:10.1046/j.0007-1048.2001.03295.x. [DOI] [PubMed] [Google Scholar]
- 26.Lorenz R, Kienast J, Otto U, et al. Efficacy and safety of a prothrombin complex concentrate with two virus-inactivation steps in patients with severe liver damage. Eur J Gastroenterol Hepatol. 2003;15:15–20. doi: 10.1097/00042737-200301000-00004. doi:10.1097/00042737-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Fries D, Haas T, Klingler A, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy—a porcine model. Br J Anaesth. 2006;97:460–7. doi: 10.1093/bja/ael191. doi:10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 28.Pragst I, Kaspereit F, Dörr B, Dickneite G. Prothrombin complex concentrate (Beriplex P/N) for control of bleeding after kidney trauma in a rabbit dilutional coagulopathy model. Thromb Res. 2010;125:272–7. doi: 10.1016/j.thromres.2009.10.011. doi:10.1016/j.thromres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Rodewald L, Scharrer I. Protein chemical investigation of 30 consecutive lots of double-virus eliminated PCC (Beriplex P/N) and summary of safety data. Hämostaseologie. 2004;24:A46. [Google Scholar]
- 30.Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo-controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–7. doi: 10.1161/CIRCULATIONAHA.108.834275. doi:10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 31.Martinowitz U, Holcomb JB, Pusateri AE, et al. Intravenous rFVIIa administered for hemorrhage control in hypothermic coagulopathic swine with grade V liver injuries. J Trauma. 2001;50:721–9. doi: 10.1097/00005373-200104000-00021. doi:10.1097/00005373-200104000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Jeroukhimov I, Jewelewicz D, Zaias J, et al. Early injection of high-dose recombinant factor VIIa decreases blood loss and prolongs time from injury to death in experimental liver injury. J Trauma. 2002;53:1053–7. doi: 10.1097/00005373-200212000-00004. doi:10.1097/00005373-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Lynn M, Jerokhimov I, Jewelewicz D, et al. Early use of recombinant factor VIIa improves mean arterial pressure and may potentially decrease mortality in experimental hemorrhagic shock: a pilot study. J Trauma. 2002;52:703–7. doi: 10.1097/00005373-200204000-00016. doi:10.1097/00005373-200204000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber MA, Holcomb JB, Hedner U, Brundage SI, Macaitis JM, Hoots K. The effect of recombinant factor VIIa on coagulopathic pigs with grade V liver injuries. J Trauma. 2002;53:252–7. doi: 10.1097/00005373-200208000-00011. doi:10.1097/00005373-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Sondeen JL, Pusateri AE, Hedner U, Yantis LD, Holcomb JB. Recombinant factor VIIa increases the pressure at which rebleeding occurs in porcine uncontrolled aortic hemorrhage model. Shock. 2004;22:163–8. doi: 10.1097/01.shk.0000129202.76706.bd. doi:10.1097/01.shk.0000129202.76706.bd. [DOI] [PubMed] [Google Scholar]
- 36.Klemcke HG, Delgado A, Holcomb JB, et al. Effect of recombinant FVIIa in hypothermic, coagulopathic pigs with liver injuries. J Trauma. 2005;59:155–61. doi: 10.1097/01.ta.0000174557.89804.a2. doi:10.1097/01.TA.0000174557.89804.A2. [DOI] [PubMed] [Google Scholar]
- 37.Pusateri AE, Ryan KL, Delgado AV, et al. Effects of increasing doses of activated recombinant factor VII on haemostatic parameters in swine. Thromb Haemost. 2005;93:275–83. doi: 10.1160/TH04-03-0200. [DOI] [PubMed] [Google Scholar]
- 38.Howes DW, Stratford A, Stirling M, Ferri CC, Bardell T. Administration of recombinant factor VIIa decreases blood loss after blunt trauma in noncoagulopathic pigs. J Trauma. 2007;62:311–5. doi: 10.1097/01.ta.0000229704.06991.9d. doi:10.1097/01.ta.0000229704.06991.9d. [DOI] [PubMed] [Google Scholar]
- 39.Martini WZ, Pusateri AE, Uscilowicz JM, Delgado AV, Holcomb JB. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J Trauma. 2005;58:1002–9. doi: 10.1097/01.ta.0000156246.53383.9f. doi:10.1097/01.TA.0000156246.53383.9F. [DOI] [PubMed] [Google Scholar]