Summary
The cost-effectiveness of sugammadex for the routine reversal of muscle relaxation produced by rocuronium or vecuronium in UK practice is uncertain. We performed a systematic review of randomized controlled trials of sugammadex compared with neostigmine/glycopyrrolate and an economic assessment of sugammadex for the reversal of moderate or profound neuromuscular block (NMB) produced by rocuronium or vecuronium. The economic assessment aimed to establish the reduction in recovery time and the ‘value of time saved’ which would be necessary for sugammadex to be potentially cost-effective compared with existing practice. Three trials indicated that sugammadex 2 mg kg−1 (4 mg kg−1) produces more rapid recovery from moderate (profound) NMB than neostigmine/glycopyrrolate. The economic assessment indicated that if the reductions in recovery time associated with sugammadex in the trials are replicated in routine practice, sugammadex would be cost-effective if those reductions are achieved in the operating theatre (assumed value of staff time, £4.44 per minute), but not if they are achieved in the recovery room (assumed value of staff time, £0.33 per minute). However, there is considerable uncertainty in these results. Sugammadex has the potential to be cost-effective compared with neostigmine/glycopyrrolate for the reversal of rocuronium-induced moderate or profound NMB, provided that the time savings observed in trials can be achieved and put to productive use in clinical practice. Further research is required to evaluate the effects of sugammadex on patient safety, predictability of recovery from NMB, patient outcomes, and efficient use of resources.
Keywords: clinical trials; neuromuscular block, recovery; neuromuscular block, rocuronium
Key points.
An innovative economic approach to evaluating the use of a new drug.
There are potential time savings using sugammadex for reversal.
The clinical value of any time saved is less clear.
Further studies are needed to allow development of the model.
Sugammadex (Bridion®, Organon/Schering-Plough USA) is a modified γ-cyclodextrin that forms tight one-to-one complexes with rocuronium and, to a slightly lesser extent, vecuronium, reducing the free plasma concentration of these neuromuscular blocking agents (NMBAs) and rapidly terminating NMB.1 Potential clinical benefits of sugammadex are a fast and predictable reversal of any degree of block, which is not achievable with neostigmine/glycopyrrolate.1,2 There are also potential benefits in terms of increased patient safety and reduced incidence of residual block on recovery, and more efficient use of health-care resource.1,2
We present our assessment of the available literature on the clinical effectiveness and cost-effectiveness of sugammadex for the routine reversal of moderate or profound NMB, relative to UK practice. This assessment includes a systematic review of effectiveness and, within the constraints of the available evidence, an economic assessment of strategies for the onset and subsequent reversal of NMB. An assessment on the use of rocuronium and sugammadex compared with succinylcholine during rapid sequence induction is presented in a parallel article3 and a full Health Technology Assessment Database (HTA) report.4
Methods
The following electronic databases were searched to identify relevant published and unpublished clinical studies: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Science Citation Index, BIOSIS, Cochrane Database of Systematic Reviews (CDSR), CENTRAL, Database of Abstracts of Reviews of Effect (DARE), HTA, conference proceedings, internet sites, and clinical trial registers. We also searched the manufacturer's submission to the US Food and Drug Administration (FDA)5 and the European Medicines Agency (EMEA) assessment report for sugammadex.6 The main searches were carried out in May 2008 and supplemented by current awareness updates up to November 2008. There were no restrictions by study design, country of origin, language, or publication date.
A broader search to identify economic studies about NMBAs was also undertaken. The economic evaluation databases, NHS Economic Evaluation Database (NHS EED) and Health Economic Evaluations Database (HEED), were searched. In addition, the following databases were searched using an economic methodological search filter: MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, EMBASE, CINAHL, Science Citation Index, ISI Proceedings: Science and Technology, and CENTRAL. Full details of all search strategies are available on request.
Clinical studies were eligible for inclusion in the review if they were randomized controlled trials (RCTs) of human patients of any age and health status, and undergoing in-hospital surgery requiring NMB. Studies were required to assess the reversal of moderate or profound rocuronium (using sugammadex 2 or 4 mg kg−1, respectively) or moderate vecuronium-induced NMB (using sugammadex 4 mg kg−1) compared with the reversal of rocuronium, vecuronium, atracurium, cisatracurium, or mivacurium-induced block using neostigmine/glycopyrrolate. Methods of stimulation included post-tetanic count (PTC) and train-of-four (TOF) stimulation. Trials of reversal agents administered at the return of T2 (second twitch of the TOF response; the point at which sugammadex was given in studies of moderate block) or at an alternative point (T1 20% or 25%) based on TOF monitoring and considered to represent an equivalent degree of recovery were eligible for inclusion in the review. For profound block, trials of reversal agents administered at PTC 1–2 were eligible for inclusion (PTC varies between 1 and 12, with a PTC of 1–2 representing profound NMB). Full-text articles were assessed for inclusion by two independent reviewers. Discrepancies were resolved by discussion and, if necessary, through consultation with a third reviewer.
The primary outcome was the time from administration of the reversal agent to a TOF ratio (TOFR)=0.9; secondary outcomes included time to a TOFR=0.7 and 0.8, and clinical signs of recovery.7,8 Studies reporting outcomes relating to the patient's experience of recovery, and any outcomes relating to reduced recovery time or resource use, were also eligible for inclusion.
Data on study and patient characteristics, outcomes, and study quality were extracted using a standardized data extraction form. The quality of the RCTs was assessed using a checklist based on the Centre for Reviews and Dissemination (CRD) recommendations,9 covering randomization, allocation concealment, blinding of outcome assessors, comparability of treatment groups, and reporting of withdrawals/dropouts. Data extraction and quality assessment were performed by one reviewer and checked by a second reviewer. Disagreements were resolved by consensus, with referral to a third reviewer if necessary. Meta-analyses across all studies were not possible due to the small number of studies included. The data were therefore presented as a narrative synthesis, retaining the original summary statistics.
Economic evaluation
The systematic search uncovered a number of papers related to the cost-effectiveness of NMBAs but none related to the costs of the reversal of NMB. A de novo economic evaluation was therefore carried out into strategies for the onset and reversal of NMB. The evaluation took the perspective of the NHS and Personal Social Services (NHS and PSS), with costs expressed in UK pounds sterling at a 2008–9 price base. It was assumed that there would be no health-related quality-of-life differences between strategies—this was consistent with the clinical evidence. The issue is, therefore, one of assessing the net cost of sugammadex (i.e. the product's acquisition cost minus the value of any reduced recovery times with the product). Since all costs considered in the assessment are incurred on the day that the NMBA is administered, costs are not discounted.
Owing to the lack of suitable evidence, it was decided that a definitive cost-effectiveness analysis would not be possible. Rather, pair-wise threshold analyses were undertaken which essentially ask the question ‘how much reduction in recovery time would sugammadex need to achieve, and with what value per minute of staff time, to justify its additional acquisition price?’ These analyses compared: (i) rocuronium 0.6 mg kg−1 followed by reversal using neostigmine 2.5 mg with glycopyrrolate 0.5 mg (hereafter referred to as ‘rocuronium with neostigmine/glycopyrrolate’) with rocuronium 0.6 mg kg−1 followed by reversal with sugammadex 2 or 4 mg kg−1 (hereafter referred to as ‘rocuronium with sugammadex’); (ii) vecuronium 0.1 mg kg−1 followed by reversal using neostigmine 2.5 mg with glycopyrrolate 0.5 mg (hereafter referred to as ‘vecuronium with neostigmine/glycopyrrolate’) with vecuronium 0.1 mg kg−1 followed by sugammadex 2 or 4 mg kg−1 (hereafter referred to as ‘vecuronium with sugammadex’).
The routine reversal of moderate block was considered separately from that of profound (deep) block. It was assumed that a dose of sugammadex 2 mg kg−1 would be used in the former scenario and a dose of 4 mg kg−1 would be used in the latter scenario. It was also assumed that the choice of NMBA or reversal agent had no impact on surgery itself (i.e. time spent in surgery, adverse events resulting from surgery, etc.) or on the staff mix in the operating theatre. It was assumed that the anaesthetist was equally proficient at administering each strategy and used good anaesthetic practice to control all components of anaesthesia that contribute to wakening from anaesthesia (e.g. avoiding potential respiratory depression from opioids and cessation of the inhalational agent). The possible drivers for differences between the costs and health outcomes of each strategy were identified as: the cost of acquiring each drug; the time spent in recovery; and rates of recurrence of block or residual block associated with the anaesthetic strategies. The aim of the modelling was to integrate as many of these possible drivers as was feasible, given the evidence constraints faced.
The prices for rocuronium, vecuronium, and neostigmine with glycopyrrolate were taken from the British National Formulary (BNF) 56. The cost per average dose of sugammadex was calculated on the assumption that the average patient had a weight of 75 kg, the cheapest combination of vials specified by the BNF was used, and any unused drug in a vial was wasted (Table 1).
Table 1.
Parameter values used in the economic evaluation. The additional hour of recovery time therefore represented a resource cost of £19.61
| Cost of drugs (per dose) |
Reduction in recovery time associated with sugammadex and rate of recurrence of block or residual block |
|||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Average dose | Vial size (cost) | Cost per dose | Strategy | Arithmetic mean time to recovery (min) (derived from Blobner and colleagues12 and Jones and colleagues14) |
Recurrence of block or residual block |
||
| Moderate block | Profound block | Probability | Resource cost | |||||
| Rocuronium (0.6 mg kg−1) | 45 mg | 50 mg (£3.01) | £3.01 | Rocuronium with neostigmine/glycopyrrolate | 25.39 | 70.69 | 0.059 (2/34)15 | £1.15 |
| Rocuronium with sugammadex | 2.02 | 3.90 | — | |||||
| Reduction associated with sugammadex | 23.37 | 66.80 | ||||||
| Vecuronium (0.1 mg kg−1) | 7.5 mg | 10 mg (£3.95) | £3.95 | Vecuronium with neostigmine/glycopyrrolate | 27.27 | 71.99 | 0.055 (13/230)16 | £1.11 |
| Vecuronium with sugammadex | 3.03 | 4.76 | — | |||||
| Reduction associated with sugammadex | 24.24 | 67.23 | ||||||
| Neostigmine/glycopyrrolate | 2.5 mg/0.5 mg | 2.5 mg/0.5 mg (£1.01) | £1.01 | |||||
| Sugammadex (2 mg kg−1) | 150 mg | 200 mg (£59.64) | £59.64 | |||||
| Sugammadex (4 mg kg−1) | 300 mg | 2×200 mg (£119.28) | £119.28 | |||||
| Estimated staff costs associated with the recovery period | ||||||||
| Staff member | Annual salary | Annual NI and pension | Working time | Cost per minute | ||||
| Consultant surgeon | £117 450 | £29 686 | 41.4 weeks, 43.4 h | £1.36 | ||||
| SpR surgeon | £48 038 | £11 084 | 42.4 weeks, 40.0 h | £0.58 | ||||
| Consultant anaesthetist | £117 450 | £29 686 | 41.4 weeks, 43.4 h | £1.36 | ||||
| Nurse (band 5) | £22 900 | £4793 | 41.3 weeks, 37.5 h | £0.30 | ||||
| Nurse (band 6) | £29 200 | £6249 | 41.3 weeks, 37.5 h | £0.38 | ||||
| Nurse (band 7) | £34 000 | £7357 | 41.3 weeks, 37.5 h | £0.45 | ||||
| Weighted average | £25 075 | £5296 | £0.33 | |||||
| Total | £369 038 | £88 855 | £4.44 | |||||
For the economic analysis, it was assumed that time to a TOFR=0.9 was a meaningful measure of time to recovery and that any reduction in recovery time achieved through adopting sugammadex could potentially represent a resource saving to the NHS. For the purposes of the economic model, data had to be included as arithmetic means rather than geometric means; where necessary, the arithmetic mean times to recovery were calculated in each instance using previously reported methodology,10 assuming that the data followed an exponential distribution. In each pair-wise comparison, the sugammadex strategy was associated with the shorter arithmetic mean time to recovery. However, given the uncertainty and the anticipated heterogeneity around these estimates, the arithmetic mean time to recovery was modelled as a variable taking values from 0 to 90 min inclusive. Thus, a wide range of possible recovery times was modelled.
The value of a minute of saved recovery time is also highly uncertain and variable. It depends on which clinical staff would have additional time available due to reduced recovery and whether this additional time would be used productively. Given this uncertainty, the per-minute value of reductions in recovery time was also modelled across a wide range. To contextualize this range, two specific valuations of these productivity benefits were estimated and represent particular points in the range. In the first, the value of each minute of recovery time saved was estimated as being the pro-rata cost of using the operating theatre staff (on the basis that all time savings would be achieved in the operating theatre); in the second, the value of each minute saved was estimated as the pro-rata cost of using a single nurse in the recovery room (on the basis that all time savings would be achieved in the recovery room). After expert clinical opinion, it was assumed that the operating theatre staff comprised a consultant surgeon, a specialist registrar surgeon, a consultant anaesthetist, a nurse team manager (band 7), and two staff nurses (one band 5 and one band 6), whereas the recovery room nurse was assumed to be of band 5, band 6, or band 7. The cost associated with this time was calculated on a per-minute basis by taking the annual cost of using each member of staff (including salary, national insurance, and pension costs) from the Personal Social Services Research Unit11 (Table 1).
It was assumed that any incidence of recurrence of block or residual block in patients who had been considered to have recovered would represent a resource cost. After expert clinical opinion, it was assumed that patients suffering from recurrence of block or residual block were monitored by a single nurse and that the additional time associated with caring for a patient in such circumstances was 1 h (valued at the pro-rata cost of using the nurse over that time; Table 1). Owing to a lack of suitable evidence, it was assumed that there was no decrement in patients’ health-related quality of life associated with recurrence of block or residual block.
A threshold analysis was undertaken, with the critical variables being the reduction in (arithmetic mean) recovery time from using sugammadex and the value of each minute of recovery time saved. The threshold analysis sought to derive the minimum value of each minute of recovery time saved with sugammadex for it to generate a net cost saving compared with neostigmine/glycopyrrolate at the current list price.
Results
Clinical efficacy
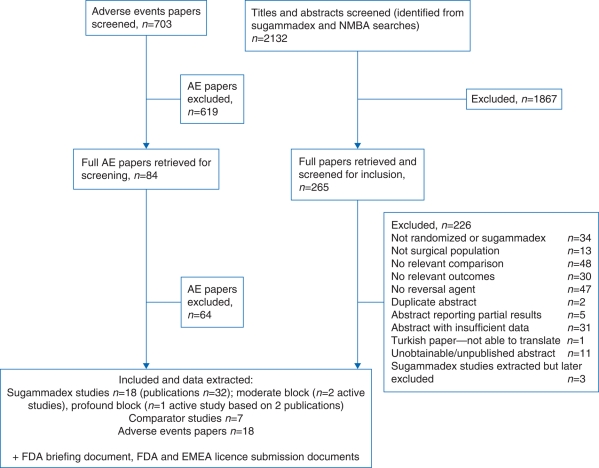
After screening of the retrieved papers, three RCTs met the inclusion criteria for the assessment of clinical effectiveness (see Fig. 1 for flow chart). Two studies were included in the assessment of sugammadex for the reversal of moderate block12,13 and one for profound block (based on two publications).14,15
Fig 1.
Flow chart of studies through the review process.
The studies included in the assessment of sugammadex for the reversal of moderate block12,13 used acceleromyography (TOF-Watch®). One study compared rocuronium and sugammadex with rocuronium and neostigmine/glycopyrrolate, and also compared vecuronium and sugammadex with vecuronium and neostigmine/glycopyrrolate.13 The other12 compared rocuronium and sugammadex with cisatracurium and neostigmine/glycopyrrolate (Table 2). The two studies largely conformed to the expected quality criteria, bearing in mind the lack of blinded primary outcome assessment, and it was unclear whether the safety assessments were performed blind to treatment allocation in one study.13
Table 2.
Study characteristics in studies of sugammadex for the reversal of moderate and profound NMB. *Number in relevant treatment arms
| Author | Number of Patients* | Age of population | Gender | ASA physical status | Weight | Treatment arms (n treated) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Moderate block | |||||||
| Blobner and colleagues13 | 189 | Not reported | Not reported | Not reported in detail. All were ASA classes I–III | Not reported | 1. Roc (0.6 mg kg−1)+sugammadex (2 mg kg−1) (n=48) | Time to TOFR=0.9 |
| 2. Roc (0.6 mg kg−1)+neostigmine (0.05 mg kg−1)/glycopyrrolate (0.01 mg kg−1) (n=48) | |||||||
| 3. Vec (0.1 mg kg−1)+sugammadex (2 mg kg−1) (n=48) | |||||||
| 4. Vec (0.1 mg kg−1)+neostigmine (0.05 mg kg−1)/glycopyrrolate (0.01 mg kg−1) (n=45) | |||||||
| Flockton and colleagues12 | 73 | Mean 45 yr (calculated) | 37/73 (41%) males | ASA I: 34/73 (47%) | Mean 75 kg (calculated) | 1. Rocuronium (0.6 mg kg−1)+sugammadex (2 mg kg−1) (n=34) | Time to TOFR=0.7 and 0.9 |
| ASA II: 36/73 (49%) | |||||||
| ASA III: 3/73 (4%) | 2. Cisatracurium (0.15 mg kg−1)+neostigmine (0.05 mg kg−1 (maximum of 5 mg kg−1)/glycopyrrolate 0.01 mg kg−1) (n=39) | ||||||
| Profound block | |||||||
| Lemmens and colleagues,14 Jones and colleagues15 | 187 (187 randomized, 157 treated) | Adults aged ≥18 yr | Not reported | Not reported. All were ASA classes I–III | Not reported | 1. Rocuronium (0.6 mg kg−1)+sugammadex (n=37) | Time to TOFR=0.9 |
| 2. Rocuronium (0.6 mg kg−1)+N&G (n=37) | Measured from reversal at PTC 1–2 | ||||||
| 3. Vecuronium (0.1 mg kg−1)+sugammadex (n=47) | |||||||
| 4. Vecuronium (0.1 mg kg−1)+N&G (n=36) | |||||||
Statistical analysis conducted by the primary study authors12,13 (two-way analysis of variance) for recovery from moderate block indicated significantly faster recovery times after rocuronium or vecuronium with sugammadex compared with neostigmine/glycopyrrolate (Table 3).13 A significant difference in moderate block recovery times was also reported between rocuronium with sugammadex compared with cisatracurium with neostigmine/glycopyrrolate.12 Similar trends were also observed for recovery from moderate block to a TOFR=0.8 and 0.7, P<0.00001 (primary authors’ analysis).12
Table 3.
Summary of time (min) from the start of administration of sugammadex or neostigmine/glycopyrrolate to the recovery of TOFR to 0.7, 0.8, or 0.9 in active-control studies of sugammadex for the reversal of moderate NMB. *CIs not reported
| Blobner and colleagues13 | Rocuronium+sugammadex (2 mg kg−1) (n=48) | Rocuronium+neostigmine/glycopyrrolate (0.05 mg kg−1) (n=48) | Vecuronium+sugammadex (2 mg kg−1) (n=48) | Vecuronium+neostigmine/glycopyrrolate (0.05 mg kg−1) (n=45) |
|---|---|---|---|---|
| Time to TOFR=0.9 | ||||
| Geometric mean (95% CI) | 1.5 (1.3–1.7) | 18.5 (14.3–23.9) | 2.8 (2.3–3.4) | 16.8 (12.9–21.9) |
| Median (range) | 1.4 (0.9–5.4) | 17.6 (3.7–106.9) | 2.1 (1.2–64.2) | 18.9 (2.9–76.2) |
| Flockton and colleagues12 | Rocuronium+sugammadex (2 mg kg−1) (n=34) | Cisatracurium+neostigmine/glycopyrrolate (0.05 mg kg−1) (n=39) | ||
| Time to TOFR=0.9 | ||||
| Geometric mean (95% CI) | 1.9 (1.6–2.2) | 9.0 (7.5–10.8) | ||
| Median (range) | 1.9 (0.7–6.4) | 7.3 (4.2–28.2) | ||
| Time to TOFR=0.8 | ||||
| Geometric mean (95% CI) | 1.6* | 6.5* | ||
| Median (range) | 1.5 (0.7–3.4) | 5.9 (3.2–15.6) | ||
| Time to TOFR=0.7 | ||||
| Geometric mean (95% CI) | 1.4* | 5.1* | ||
| Median (range) | 1.2 (0.7–2.9) | 4.7 (2.4–10.9) | ||
Clinical signs of recovery from moderate block were reported for the comparison of rocuronium/sugammadex and cisatracurium/neostigmine/glycopyrrolate,12 with 22 out of 34 patients (65%) in the sugammadex group and 27 of 39 patients (69%) in the neostigmine/glycopyrrolate group awake and orientated before transfer to the recovery room. The majority of patients in both treatment groups were reported to be co-operative, able to perform a five second head lift, and did not experience muscle weakness before transfer to, or before discharge from, the recovery room.
The key comparative study for profound block was a multicentre trial reported in two publications (Table 2).14,15 Sugammadex 4 mg kg −1 was administered when recovery had reached a PTC of 1–2 (PTC 1–2) after rocuronium or vecuronium. Statistical analysis by the study authors using two-way analysis of variance on log-transformed recovery times indicated that there was a significant difference between sugammadex and neostigmine/glycopyrrolate in both the rocuronium and vecuronium groups (P<0.001) (Table 4). Within the rocuronium and vecuronium groups, recovery times were faster after reversal with sugammadex compared with neostigmine/glycopyrrolate. However, there was a greater inter-individual variation in recovery times for vecuronium with sugammadex than for rocuronium with sugammadex.
Table 4.
Summary of time (min) from the start of administration of sugammadex or neostigmine/glycopyrrolate to the recovery of the TOFR to 0.9 in active-control studies for the reversal of profound NMB (reversed at PTC 1–2). *Number in relevant treatment arms. **Geometric mean (95% CI). P<0.0001 for comparisons of NMBA+sugammadex vs NMBA+neostigmine/glycopyrrolate
| Study | Number of patients* | Time to TOFR=0.9 |
|
|---|---|---|---|
| Mean (sd) | Median (min–max) | ||
| Lemmens and colleagues,14 Jones and colleagues15 | 1. Rocuronium (0.6 mg kg−1)+sugammadex (n=37) | 2.9 (2.5–3.4)** | 2.7 (1.2–16.1) |
| 2. Rocuronium (0.6 mg kg−1)+neostigmine/glycopyrrolate (n=37) | 50.4 (43.5–58.4)** | 49.0 (13.3–145.7) | |
| 3. Vecuronium (0.1 mg kg−1)+sugammadex (n=47) | 4.5 (3.3–6.0)** | 3.3 (1.4–68.4) | |
| 4. Vecuronium (0.1 mg kg−1)+neostigmine/glycopyrrolate (n=36) | 66.2 (55.6–78.9)** | 49.9 (46.0–312.7) | |
None of the patients in the controlled trials comparing sugammadex and neostigmine for the reversal of moderate or profound NMB had residual block or recurrence of block, based on acceleromyographic monitoring. No patients showed clinical evidence of recurrence of block or residual block.
Economic evaluation
In each of the pair-wise comparisons, the trial evidence suggested that the sugammadex strategy was associated with a shorter geometric mean time to recovery. As such, the model considered the reduction in recovery time associated with sugammadex but reflected the uncertainty in how these trial estimates might be reflected in routine practice.
In patients with moderate block, the single RCT comparing rocuronium and sugammadex with rocuronium and neostigmine/glycopyrrolate13 suggests that sugammadex reduces the geometric mean time to a TOFR=0.9 by 17 min for rocuronium-induced block and 14 min for vecuronium-induced block. In patients with profound block, the RCT comparing rocuronium and sugammadex with rocuronium and neostigmine/glycopyrrolate14,15 suggests that sugammadex reduces the geometric mean time to a TOFR=0.9 by 47.5 min for rocuronium-induced block and 61.7 min for vecuronium-induced block. In order to incorporate reduced recovery time into the economic model, these geometric means were converted into arithmetic means on the assumption that the recovery times are exponentially distributed. Full details are given in the HTA report.4
In addition to the extent of reduction in recovery time, whether sugammadex is cost-effective also depends on the value of each minute of recovery time saved (in terms of staff productivity). The economic modelling found that both reductions in recovery time and the value per minute of that time are varied across a wide range (Table 5 and Fig. 2). This demonstrates that if sugammadex provides no reduction in recovery time, then it is not cost-effective at the current list price. As the reduction in recovery time increases, the minimum value of each minute of saved recovery time required for sugammadex to be cost-effective decreases. The results are broadly similar for rocuronium- and vecuronium-induced block, with any differences driven by the small differences between the prices of rocuronium and vecuronium and the rates of recurrence of block or residual block. However, the results differ substantially between moderate block and profound block (Table 5).
Table 5.
Threshold analysis comparing the reversal of block with sugammadex vs reversal with neostigmine/glycopyrrolate. The table shows the minimum value of each minute of recovery time saved for sugammadex to be considered cost-effective under the base-case assumptions
| Reduction in recovery time (min) | Minimum value of each minute of reduced recovery time for sugammadex to be considered cost saving |
|||
|---|---|---|---|---|
| Moderate block |
Profound block |
|||
| Rocuronium | Vecuronium | Rocuronium | Vecuronium | |
| 0 | N/A | N/A | N/A | N/A |
| 1 | £57.47 | £57.55 | £117.11 | £117.19 |
| 2 | £28.74 | £28.78 | £58.56 | £58.60 |
| 3 | £19.16 | £19.18 | £39.04 | £39.06 |
| 4 | £14.37 | £14.39 | £29.28 | £29.30 |
| 5 | £11.49 | £11.51 | £23.42 | £23.44 |
| 10 | £5.75 | £5.76 | £11.71 | £11.72 |
| 15 | £3.83 | £3.84 | £7.81 | £7.81 |
| 20 | £2.87 | £2.88 | £5.86 | £5.86 |
| 25 | £2.30 | £2.30 | £4.68 | £4.69 |
| 30 | £1.92 | £1.92 | £3.90 | £3.91 |
| 35 | £1.64 | £1.64 | £3.35 | £3.35 |
| 40 | £1.44 | £1.44 | £2.93 | £2.93 |
| 50 | £1.15 | £1.15 | £2.34 | £2.34 |
| 60 | £0.96 | £0.96 | £1.95 | £1.95 |
| 70 | £0.82 | £0.82 | £1.67 | £1.67 |
| 80 | £0.72 | £0.72 | £1.46 | £1.46 |
| 90 | £0.64 | £0.64 | £1.30 | £1.30 |
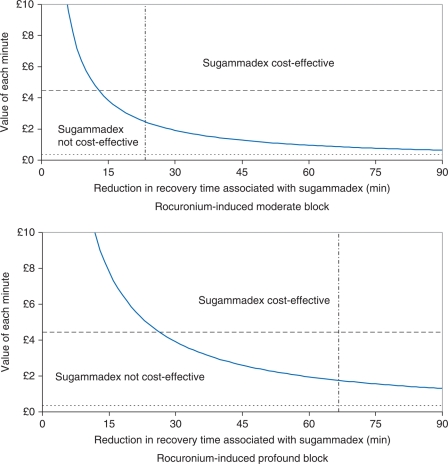
Fig 2.
Threshold analysis comparing the reversal of rocuronium-induced block with sugammadex vs reversal with neostigmine/glycopyrrolate. The region above (below) the bold line represents the combinations of reduction in recovery time associated with sugammadex and value of each minute of recovery time saved at which sugammadex is (is not) cost saving under the base-case assumptions for each scenario. Separate graphs are plotted for moderate and profound block. The horizontal dashed (dotted) line represents an estimate of the value of each minute saved were all the time savings to occur in the operating theatre (recovery room), while the dotted and dashed vertical line represents an estimate of the reduction in recovery time associated with sugammadex.
If the time savings achieved in the RCTs transfer into routine clinical practice, sugammadex can be cost-effective in patients with moderate block where the value of each minute of recovery time saved with sugammadex is approximately £2.40 or greater, and in profound block where the value is £1.75 or greater. We estimated that time saved in the operating theatre has a value of £4.44 per minute when it is assumed that all medical and nursing staff have their time freed up by the shorter recovery time and that they use this for a productive activity. The time saved in the recovery room was estimated to have a value of £0.33 per minute. If these values are realistic, sugammadex 2 mg kg−1 (4 mg kg−1) would appear cost-effective for the routine reversal of rocuronium-induced moderate or profound block at the current list price if all reductions in recovery time associated with sugammadex are achieved in the operating theatre, but not if all reductions in recovery time are achieved in the recovery room (Fig. 2). Another way of interpreting Table 5 is that if the actual time saving achieved in practice is, for example, 15 min, sugammadex is cost-effective if the value of the time saved is £3.83 per minute or more for moderate block or £7.81 per minute or more for profound block.
Discussion
The available evidence from RCTs suggests that sugammadex produces a substantially faster and more predictable recovery from rocuronium- or vecuronium-induced moderate NMB than neostigmine/glycopyrrolate12,13 and can produce a rapid recovery from profound NMB,14,15 a facility not available with any other drug combination. Thus, sugammadex represents an efficacious and potentially useful new agent for the reversal of NMB. However, the findings are based on limited evidence, and considerable uncertainties remain concerning its clinical effectiveness in practice and especially its cost-effectiveness.
First, the patients in the sugammadex trials were mainly relatively young and in ASA classes I–II, and may not be fully representative of those who would receive sugammadex in routine clinical practice. Secondly, the reductions in recovery time with sugammadex seen in the clinical trials may represent the maximum that can be achieved and the benefits in normal clinical practice will remain uncertain pending wider adoption and evaluation of sugammadex.
Thirdly, the available trials did not compare sugammadex–rocuronium or sugammadex–vecuronium combination with all the commonly used NMBA/reversal agent combinations. Although trials making these direct comparisons are not available, statistical methods have been developed which would have allowed us to combine data from comparisons between other NMB drugs/reversal agent combinations and aminosteroids with sugammadex, namely a mixed treatment comparison.18,19 The application of such methods is, however, subject to certain requirements and unfortunately, due to a lack of access to the necessary data on sugammadex, only limited data being available from older trials that were comparable with those from the newer sugammadex trials, and the nature of the available data (the primary studies elected to report outcomes using a mix of arithmetic mean, geometric mean, and median), we were unable to include this analysis in our review.
To demonstrate cost-effectiveness for sugammadex, two things need to be established. First, that some reduction in patient recovery time can be achieved by using sugammadex compared with neostigmine/glycopyrrolate for the routine reversal of NMB. Secondly, that any such time saving would have value in terms of freeing up staff to work on productive activities. The key economic uncertainties surround the productivity benefits of a reduced time in recovery, that is, the extent to which any time saved in recovery could be put to alternative productive use, for example, in caring for another patient or some other activity. The proportion of recovery time saved which could be put to productive use is ultimately unknown—no evidence was identified in the literature. There is also the possibility that extra operations could be scheduled as a result of any reduced recovery time, but again there is a lack of suitable evidence on the associated impact on costs and health effects. Similarly, there are no data to inform any possible differences between anaesthetic strategies in health-related quality of life and it should also be noted that the estimates reported for this represent the opinion of a single clinical expert only. These and other data weaknesses need to be considered when the results presented here are being interpreted.
The clinical trials of sugammadex were not sufficiently powered to estimate the rates of significant adverse events (including death) with any level of precision, nor were there any observational data to inform these rates. As such, in the absence of clear evidence to the contrary, it was assumed that there were no differences in rates of adverse events between the strategies. This is a limitation of the modelling and should be considered when interpreting the results. One scenario not modelled in the routine setting (due to the lack of available data) is that where a ‘cannot intubate, cannot ventilate’ event occurs. In current practice, such an event has potentially serious consequences (for both patient health and resource use) due to the inability to quickly reverse profound block with neostigmine/glycopyrrolate. It remains unclear whether administering sugammadex 16 mg kg−1 is cost-effective in such circumstances, due to uncertainty over the time it would take to draw up the sugammadex in a high-pressure situation, or alternatively the cost associated with preparing such a dose beforehand for every listed patient.3
If the estimates of the reduction in recovery time derived from the clinical trials were replicated in routine clinical practice, the analysis suggests that sugammadex would be cost-effective if all reductions in recovery time associated with sugammadex were to be achieved in the operating theatre, but not if all reductions in recovery time were to be in the recovery room. Other factors will also affect the cost-effectiveness of sugammadex, for example, where there is additional value in reducing recovery times (e.g. in preventing operations from being delayed or cancelled). The results are broadly similar for rocuronium and vecuronium with any differences driven by the small differences between the prices of these two products and the rates of recurrence of block or residual block.
If sugammadex is to be recommended for wider use in routine surgery, the overall cost of reversal agents would be expected to increase. Also, the use of rocuronium and vecuronium for NMB would increase relative to other non-depolarizing NMBAs. In addition, there would be some requirement for training of staff during the introduction of sugammadex, but this is not expected to involve significant costs.
The implications for the use of objective monitoring if sugammadex was more commonly used in practice are uncertain. In the clinical trials, sugammadex was administered at specific points determined by TOF monitoring and if anaesthetists always follow this practice, the use of monitoring would increase. However, as sugammadex appears effective at all levels of block, anaesthetists may feel able to reduce levels of monitoring as they become more experienced in its use, with resultant savings in equipment costs. There could be an overall deterioration in practice associated with decreased monitoring, although this would be difficult to quantify.
A recently published Cochrane Review20 appears to support the clinical findings of this review, but this review is the first to discuss the cost-effectiveness of sugammadex in UK clinical practice.
In conclusion, the evidence suggests that there are potential benefits of sugammadex in terms of increased patient safety, increased predictability of recovery from NMB, and more efficient use of theatre time and staff. However, whether this potential is realized routinely is unclear: the evidence base is small and the potential benefits of sugammadex have yet to be explored further in clinical practice. A wider range of outcomes, including patient-reported outcomes and effects on costs and resource use (e.g. time in the operating theatre or efficient operating list management), may have to be assessed before the full benefits of sugammadex can be evaluated. These implications relate to UK practice and may not apply to other countries and different health-care systems, and the clinical and economic uncertainties should be taken into consideration when interpreting the findings
Conflict of interest
In the past (over 3 yr ago), J.M.H. has had funding for clinical trials of sugammadex from Organon/Schering-Plough. She has no current funding related to sugammadex.
Funding
This project was funded by the NIHR Health Technology Assessment Programme (project number 08/10/01). It will be published in full in Health Technology Assessment, Vol. 14, No. 39. See the HTA Programme website (www.hta.ac.uk) for further project information. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Acknowledgements
We would like to thank Jonathan Wilson at York NHS Trust for his help and advice when writing the full report.
References
- 1.Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–81. doi: 10.1213/01.ane.0000244594.63318.fc. [DOI] [PubMed] [Google Scholar]
- 2.Welliver M, McDonough J, Kalynych N, Redfern R. Discovery, development, and clinical application of sugammadex sodium, a selective relaxant binding agent. Drug Des Devel Ther. 2008;2:49–59. doi: 10.2147/dddt.s2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers D, Paulden M, Paton F, et al. Sugammadex for reversal of neuromuscular blockade following rapid sequence intubation: a systematic review and economic assessment. Br J Anaesth. 2010;105:568–75. doi: 10.1093/bja/aeq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers D, Paulden M, Paton F, Heirs M, Duffy S, Craig D, et al. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess. 2010;14:39. doi: 10.3310/hta14390. [DOI] [PubMed] [Google Scholar]
- 5.Organon, Schering-Plough. FDA Anesthetic and Life Support Advisory Committee Meeting. Sugammadex Sodium Injection (NDA 22-225). March 11, 2008. Briefing Document (Background Package) Kenilworth, NJ: Organon USA, Schering-Plough Corporation; 2008. [Google Scholar]
- 6.European Medicines Agency. Assessment report for Bridion. International Nonproprietary Name: Sugammadex. Procedure No. EMEA/H/C/000885. London: European Medicines Agency; 2008. [Google Scholar]
- 7.Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block. I. Br J Anaesth. 1971;43:473–7. doi: 10.1093/bja/43.5.473. [DOI] [PubMed] [Google Scholar]
- 8.Ali HH, Utting JE, Gray TC. Quantitative assessment of residual antidepolarizing block. II. Br J Anaesth. 1971;43:478–85. doi: 10.1093/bja/43.5.478. [DOI] [PubMed] [Google Scholar]
- 9.Centre for Reviews and Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: University of York; 2009. [Google Scholar]
- 10.Jones L, Hawkins N, Westwood M, Wright K, Richardson G, Riemsma R. Systematic review of the clinical effectiveness and cost-effectiveness of capecitabine (Xeloda) for locally advanced and/or metastatic breast cancer. Health Technol Assess. 2004;8:1–143. doi: 10.3310/hta8050. [DOI] [PubMed] [Google Scholar]
- 11.Curtis L. Unit Costs of Health and Social Care 2008. Canterbury: Personal Social Services Research Unit, University of Kent; 2008. [Google Scholar]
- 12.Flockton EA, Mastronardi P, Hunter JM, et al. Reversal of rocuronium-induced neuromuscular block with sugammadex is faster than reversal of cisatracurium-induced block with neostigmine. Br J Anaesth. 2008;100:622–30. doi: 10.1093/bja/aen037. [DOI] [PubMed] [Google Scholar]
- 13.Blobner M, Eriksson L, Scholz J, Hillebrand H, Pompei L. Sugammadex (2.0 mg kg−1) significantly faster reverses shallow rocuronium-induced neuromuscular blockade compared with neostigmine (50 mcg kg−1) [abstract] Eur J Anaesthesiol. 2007;24:125. [Google Scholar]
- 14.Lemmens HJM, El-Orbany MI, Berry J, Martin G. Sugammadex reverses profound vecuronium blockade more rapidly than neostigmine [abstract]. American Society of Anesthesiologists. Annual Meeting; San Francisco, CA: 2007. p. A1578. October 13–17, 2007. [Google Scholar]
- 15.Jones RK, Caldwell JE, Brull SJ, Soto RG. Reversal of profound rocuronium-induced blockade with sugammadex: a randomized comparison with neostigmine. Anesthesiology. 2008;109:816–24. doi: 10.1097/ALN.0b013e31818a3fee. [DOI] [PubMed] [Google Scholar]
- 16.Murphy GS, Szokol JW, Franklin M, Marymont JH, Avram MJ, Vender JS. Postanesthesia care unit recovery times and neuromuscular blocking drugs: a prospective study of orthopedic surgical patients randomized to receive pancuronium or rocuronium. Anesth Analg. 2004;98:193–200. doi: 10.1213/01.ANE.0000095040.36648.F7. [DOI] [PubMed] [Google Scholar]
- 17.Berg H, Roed J, Viby-Mogensen J, Mortensen CR, Engbaek J, Skovgaard LT, et al. Residual neuromuscular block is a risk factor for postoperative pulmonary complications: a prospective, randomised, and blinded study of postoperative pulmonary complications after atracurium, vecuronium and pancuronium. Acta Anaesthesiol Scand. 1997;41:1095–103. doi: 10.1111/j.1399-6576.1997.tb04851.x. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–92. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrishami A, Ho J, Yin L, Wong J, Chung F. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009:CD007362. doi: 10.1002/14651858.CD007362.pub2. doi:10.1002/14651858.CD007362.pub2. [DOI] [PubMed] [Google Scholar]