Abstract
Summary
Sugammadex 16 mg kg−1 can be used for the immediate reversal of neuromuscular block 3 min after administration of rocuronium and could be used in place of succinylcholine for emergency intubation. We have systematically reviewed the efficacy and cost-effectiveness and made an economic assessment of sugammadex for immediate reversal. The economic assessment investigated whether sugammadex appears cost-effective under various assumptions about the value of any reduction in recovery time with sugammadex, the likelihood of a ‘can't intubate, can't ventilate’ (CICV) event, the age of the patient, and the length of the procedure. Three trials were included in the efficacy review. Sugammadex administered 3 or 5 min after rocuronium produced markedly faster recovery than placebo or spontaneous recovery from succinylcholine-induced block. No published economic evaluations were found. Our economic analyses showed that sugammadex appears more cost-effective, where the value of any reduction in recovery time is greater, where the reduction in mortality compared with succinylcholine is greater, and where the patient is younger, for lower probabilities of a CICV event and for long procedures which do not require profound block throughout. Because of the lack of evidence, the value of some parameters remains unknown, which makes it difficult to provide a definitive assessment of the cost-effectiveness of sugammadex in practice. The use of sugammadex in combination with high-dose rocuronium is efficacious. Further research is needed to clarify key parameters in the analysis and to allow a fuller economic assessment.
Keywords: complications, intubation tracheal; neuromuscular block, recovery; neuromuscular block, rocuronium; neuromuscular block, succinylcholine
Key points.
The efficacy of sugammadex reversal of rocuronium is established.
The economic evaluation model used is new in this clinical setting.
Further research is required to clarify some of the parameters in the model.
The model is a useful starting point which may be adapted with increased clinical experience.
Sugammadex, a modified γ-cyclodextrin, forms a tight one-to-one complex with rocuronium, encapsulating the drug in the plasma and hence reducing its concentration at the neuromuscular junction and rapidly terminating block.1 Different doses of sugammadex (2, 4, and 16 mg kg−1) are available to reverse different levels of block. Sugammadex 16 mg kg−1 is indicated for the immediate reversal of neuromuscular block 3 min after administration of rocuronium.2 The availability of this dose and indication for sugammadex enable high-dose rocuronium to be used safely to achieve rapid onset of block in place of succinylcholine, which would avoid the well-recognized adverse effects of the depolarizing agent.3
Although some of the adverse effects of succinylcholine are acceptable in current patient management, the availability of an alternative could be expected to reduce morbidity and would be welcomed by clinicians. However, the cost of reversing neuromuscular block with sugammadex in a typical (75 kg) individual is £59.74 for 2 mg kg−1, £119.28 for 4 mg kg−1, and £357.84 for 16 mg kg−1 compared with only £0.71 for spontaneous recovery from succinylcholine (Table 1). It is therefore unclear whether its use is viable in clinical practice. We carried out systematic reviews of clinical efficacy (time to recovery from block) and cost-effectiveness and an economic assessment of the use of sugammadex for the reversal of neuromuscular block from high-dose rocuronium.
Table 1.
Strategies considered in the economic assessment
Where a ‘can't intubate, can't ventilate’ event occurs:
|
Where a ‘can't intubate, can't ventilate’ event does not occur and the subsequent procedure is very short:
|
Where a ‘can't intubate, can't ventilate’ event does not occur and the subsequent procedure is short (<60 min) or requires profound block throughout:
|
Where a ‘can't intubate, can't ventilate’ event does not occur and the subsequent procedure is long (>60 min) and does not require profound block throughout:
|
Methods
Systematic review
Studies were eligible for the efficacy review if they were randomized controlled trials (RCTs) that compared sugammadex 16 mg kg−1 with placebo or an active comparator for the reversal of neuromuscular block in surgical patients. Studies were required to administer sugammadex 3 or 5 min after an intubating dose of rocuronium. The primary outcome was time to recovery from block as determined by objective monitoring of the response to train-of-four (TOF) stimulation. Other outcomes of interest were adverse events, clinical signs of recovery, patient-reported outcomes, and measures of costs or resource use.
We searched bibliographic databases (including MEDLINE, EMBASE, CINAHL, Science Citation Index, BIOSIS, and CENTRAL), conference proceedings, Internet sites, and clinical trial registers to identify published and unpublished studies of the clinical and cost-effectiveness of sugammadex. Supplementary searches for economic evaluations of sugammadex were undertaken in NHS Economic Evaluation Database (NHS EED) and Health Economic Evaluations Database (HEED). We also searched the manufacturer's submission to the US Food and Drug Administration (FDA)4 and the European Medicines Agency (EMEA) assessment report for sugammadex5 and contacted the manufacturer directly. The main searches were carried out in May 2008 and supplemented by current awareness updates up to November 2008.
Studies were assessed for inclusion by two independent reviewers. Full texts of study publications were obtained for inclusion screening and data extraction where available. For unpublished studies, decisions were taken based on the data from published abstracts and the manufacturer's FDA submission4 and EMEA assessment5 after checking the completeness of these documents with the manufacturer. These sources were also used for extraction of data on study characteristics and outcomes using standard forms. Study quality was assessed using a checklist based on the recommendations from the Centre for Reviews and Dissemination, covering randomization, allocation concealment, blinding of outcome assessors, comparability of treatment groups, and reporting of withdrawals/drop-outs.6 Data extraction and quality assessment were performed by one reviewer and checked by another. Discrepancies were resolved by discussion with referral to a third reviewer if necessary.
We did not perform any meta-analyses because of the small number and clinical heterogeneity of the included studies. Instead, studies were described using text and tables (an approach known as narrative synthesis),6 and the resulting information was used to assess the robustness of the evidence for the clinical efficacy of sugammadex 16 mg kg−1. Visual inspection suggested that the data for time to recovery from block might be skewed and we therefore reported medians and ranges and also means and standard deviations where available.
Economic assessment
The systematic search found no relevant economic evaluations. Because of the lack of published evidence, a de novo economic assessment was carried out into strategies for the rapid induction and subsequent reversal of neuromuscular block. The assessment took the perspective of the NHS and Personal Social Services as described in the accompanying paper.7
The economic assessment was severely hindered by the lack of suitable evidence to inform many of the parameters. In particular, there appeared to be no evidence linking measures of clinical efficacy, such as time to recovery of the TOF ratio to 0.9, to patients’ health-related quality of life, and to mortality risks. As a result, direct cost-effectiveness modelling was not considered feasible. Rather, a series of analyses was undertaken to establish the extent of the mortality reduction (relative to succinylcholine) required for sugammadex to appear cost-effective under various assumptions about: (i) the location of any savings in recovery time achieved by administering sugammadex rather than neostigmine/glycopyrrolate; (ii) the age of the patient; (iii) the probability of a ‘can't intubate, can't ventilate’ (CICV) event occurring; and (iv) the length of the procedure. To establish cost-effectiveness, we used a cost-effectiveness threshold of £20 000 per quality-adjusted life-year (QALY) in line with that adopted by the National Institute for Health and Clinical Excellence (NICE).8 This is a benchmark indicating the maximum the NHS would be willing to pay to achieve an extra QALY worth of health benefit. Full details of the analyses are reported elsewhere.9
The economic assessment assumed that patients requiring rapid sequence induction (RSI) of anaesthesia followed by surgery would initially have neuromuscular block induced by succinylcholine (1 mg kg−1) or rocuronium (1.2 mg kg−1). The strategies considered for neuromuscular block and subsequent reversal are summarized in Table 1. To simplify the modelling, it was assumed that the rapid reversal of neuromuscular block in such circumstances would only be required in the very rare circumstance of a CICV event, in which case it was assumed that surgery would not be performed. In the typical situation of a CICV event not occurring, it was assumed that surgery would proceed as usual. The drugs and doses administered would depend on the length of the procedure and/or whether the procedure required profound block throughout. Since the sugammadex 16 mg kg−1 dose is specifically indicated for the immediate reversal of rocuronium-induced block, only rocuronium-induced block was considered in the RSI setting. Other assumptions made for the assessment (e.g. staff costs and value of time saved in the operating theatre and recovery room) were similar to those made in comparing sugammadex with neostigmine/glycopyrrolate for the routine reversal of neuromuscular block.7
Each analysis sought to derive the number of administrations of sugammadex over which at least one death must be prevented for sugammadex to appear cost-effective. Adverse events other than death were not considered because of a lack of evidence to inform the expected costs and quality-of-life effects associated with them. In the absence of a CICV event, the number of minutes of recovery time potentially saved by adopting sugammadex and the value of each minute saved were incorporated.
It was assumed that each minute of recovery time saved through using sugammadex was valued either at £4.44 (on the basis that all time savings would be in the operating theatre) or at £0.33 (on the basis that all time savings would be in the recovery room), and that the amount of recovery time saved for each procedure was 23.37 min for the reversal of moderate block and 66.80 min for the reversal of profound block, as reported in the accompanying paper.7 Costs and other parameters used in the model are summarized in Table 2.
Table 2.
Parameters used in the economic assessment (Paton and colleagues)7
|
Cost of drugs (per dose) | |||||
|---|---|---|---|---|---|
| Drug | Average dose | Vial size (cost) | Cost per dose | ||
| Rocuronium (1.2 mg kg−1) | 90 mg | 100 mg (£6.01) | £6.01 | ||
| Succinylcholine (1 mg kg−1) | 75 mg | 100 mg (£0.71) | £0.71 | ||
| Neostigmine/glycopyrrolate | 2.5 mg/0.5 mg | 2.5/0.5 mg (£1.01) | £1.01 | ||
| Sugammadex (2 mg kg−1) | 150 mg | 200 mg (£59.64) | £59.64 | ||
| Sugammadex (4 mg kg−1) | 300 mg | 2×200 mg (£119.28) | £119.28 | ||
| Sugammadex (16 mg kg−1) | 1200 mg | 2×500 mg (£298.20) and 1×200 mg (£59.64) | £357.84 | ||
| Estimated staff costs associated with the recovery period | |||||
| Staff member | Annual salary | Annual NI and pension | Working time | Cost per minute | |
| Consultant surgeon | £117 450 | £29 686 | 41.4 weeks, 43.4 h | £1.36 | |
| SpR surgeon | £48 038 | £11 084 | 42.4 weeks, 40.0 h | £0.58 | |
| Consultant anaesthetist | £117 450 | £29 686 | 41.4 weeks, 43.4 h | £1.36 | |
| Nurse (band 5) | £22 900 | £4793 | 41.3 weeks, 37.5 h | £0.30 | |
| Nurse (band 6) | £29 200 | £6249 | 41.3 weeks, 37.5 h | £0.38 | |
| Nurse (band 7) | £34 000 | £7357 | 41.3 weeks, 37.5 h | £0.45 | |
| Weighted average | £25 075 | £5296 | £0.33 | ||
| Total | £369 038 | £88 855 | £4.44 | ||
| HRQoL index by age (adapted from Table A in Kind and colleagues)15 | Discounted, quality-adjusted life expectancy (QALYs) lost due to a fatality at age 20 and 60 yr | ||||
| Age range (yr) | Average HRQoL weight | Example age (yr) | Life expectancy (yr) | Discounted, quality-adjusted life expectancy (QALYs) | |
| 0–24 | 0.94 | 20 | 59.96 | 22.91 | |
| 25–34 | 0.93 | 60 | 22.52 | 12.33 | |
| 35–44 | 0.91 | ||||
| 45–54 | 0.85 | ||||
| 55–64 | 0.8 | ||||
| 65–74 | 0.78 | ||||
| 75+ | 0.73 | ||||
Results
Systematic review of efficacy
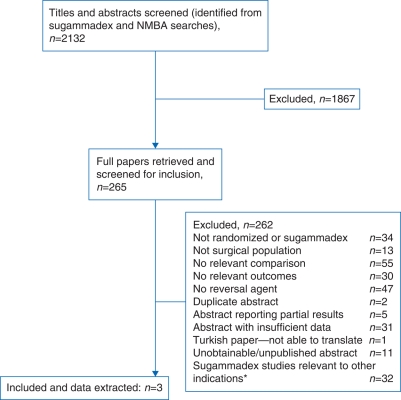
After screening of titles and abstracts, and full papers where available (Fig. 1), we included three completed trials of sugammadex 16 mg kg−1 in the review: two placebo-controlled trials10,11 and one active-control trial (Table 3).12 No relevant ongoing trials were identified. All the trials involved patients undergoing elective surgery; as expected, we did not find any RCTs in patients undergoing RSI of anaesthesia or of the use of sugammadex in a CICV situation. Instead, the trials sought to establish that sugammadex 16 mg kg−1 could reverse neuromuscular block induced by high-dose rocuronium quickly enough to be useful in this setting.
Fig 1.
Flow chart of studies through the review process. *Multiple publications of some studies.
Table 3.
Characteristics of included trials. ASA, American Society of Anesthesiologists; roc, rocuronium; sug, sugammadex
| Trial | Number of patients | Age of population | Gender | ASA physical status | Weight | Treatment arms (n treated) | Outcome measures |
|---|---|---|---|---|---|---|---|
| Placebo-controlled trials | |||||||
| de Boer and colleagues10 | 45 | Mean 42 (sd 15) yr (43 patients) | 22/43 (51%) males | ASA I 32/43 (74%) | Overall weight, mean 76 (sd 18) kg | 1. Roc 1.2 mg kg−1+sug 16 mg kg−1 (n=7) | Time to TOF ratio=0.9 from administration of sugammadex or placebo (5 min after rocuronium) |
| ASA II 11/43 (26%) | 2. Roc 1.2 mg kg−1+placebo (n=4) | ||||||
| Puhringer and colleagues11 | 176 | Mean 50 (sd 16) yr | 93/173 (54%) males | ASA I 66/173 (38%) | Overall weight, mean 77 (sd 15) kg | 1. Roc 1 mg kg−1+sug 16 mg kg−1 (n=10) | Time to TOF ratio=0.7, 0.9 from administration of sugammadex or placebo (3 min after rocuronium) |
| ASA II 88/173 (51%) | 2. Roc 1 mg kg−1+placebo (n=5) | ||||||
| ASA III 19/173 (11%) | 3. Roc 1.2 mg kg−1+sug 16 mg kg−1 (n=11) | ||||||
| 4. Roc 1.2 mg kg−1+placebo (n=5) | |||||||
| Active-control trial | |||||||
| Lee and colleagues12 | 115 randomized | Mean 42 yr (range 18–65 yr) | 42% males (46/110 calculated) | ASA I 70/110 (64%) calculated | Not reported. Mean BMI 25 (sd 3) kg/m2 | 1. Roc 1.2 mg kg−1+sug 16 mg kg−1 (n=55) | Time to T1/T0=0.1 and 0.9 from administration of NMBA |
| ASA II 40/110 (36%) calculated | 2. Succinylcholine 1 mg kg−1 (n=55) | Time from administration of sugammadex (3 min after rocuronium) to TOF ratio=0.7, 0.8, 0.9 (sugammadex group only) | |||||
| Clinical signs of recovery | |||||||
The two placebo-controlled studies met all quality criteria except reporting of a power calculation.10,11 The absence of a power calculation is not surprising as these studies were designed as dose-finding and safety trials rather than to assess efficacy. Details of randomization were unclear for the active-control study.12
In the two placebo-controlled trials, sugammadex or placebo was administered 3 or 5 min after an intubating dose (1 or 1.2 mg kg−1) of rocuronium. In both studies, time to recovery of the TOF ratio to 0.9 was substantially shorter with sugammadex 16 mg kg−1 than with placebo, with differences of the order of 90–120 min.10,11
In the active-control trial,12 patients requiring short duration neuromuscular block were randomized to receive an intubating dose of rocuronium 1.2 mg kg−1 followed by sugammadex 16 mg kg−1 3 min after the start of rocuronium administration, or an intubating dose of succinylcholine (1 mg kg−1) followed by spontaneous recovery. The primary efficacy endpoint was time to recovery of T1 (first twitch of the TOF response) to 10% of the control value (T1/T0=0.1). This endpoint was chosen as a surrogate for the appearance of signs of clinical recovery such as diaphragmatic movement or the return of spontaneous respiration on the capnogram, which are expected to occur 4.5 min or more after administration of succinylcholine.4 Recovery of T1 to 90% of control (T1/T0=0.9) was a secondary endpoint.
For time from administration of rocuronium or succinylcholine to T1/T0 of 0.1 and 0.9, the study authors’ statistical analysis indicated that the difference between the groups was significant (P<0.0001) for both outcomes (Table 4). However, there was a degree of overlap in the range of recovery times. The time from the start of administration of sugammadex to recovery of the TOF ratio to 0.9 was measured in the rocuronium+sugammadex group (Table 4). This ancillary analysis also showed that most patients (87%) had recovered to a TOF ratio of 0.9 by 3 min after administration of sugammadex.12 Clinical signs of recovery did not show any differences between the groups.12
Table 4.
Time to recovery in active-control trial of sugammadex for the rapid reversal of rocuronium-induced neuromuscular block.5,12,13 *Sugammadex was administered 3 min after rocuronium; **P<0.0001 (study authors’ analysis)
| Rocuronium+sugammadex (16 mg kg−1) (n=55)* | Succinylcholine (1 mg kg−1) (n=55) | |
|---|---|---|
| Time from start of NMBA administration to T1/T0=0.1** | ||
| Mean (sd) | 4.4 (0.7) | 7.1 (1.6) |
| Median (range) | 4.2 (3.5–7.7) | 7.1 (3.7–10.5) |
| Time from start of NMBA administration to T1/T0=0.9** | ||
| Mean (sd) | 6.2 (1.83) | 10.9 (2.42) |
| Median (range) | 5.7 (4.2–13.6) | 10.7 (5.0–16.2) |
| Time from start of sugammadex administration to T4/T1=0.9 | ||
| Mean (sd) | 2.2 (2.2) | |
| Median (range) | 1.73 (0.48–14.3) | |
None of the studies reported on outcomes related to quality of life, costs, or resource use. A Quality of Recovery questionnaire was used in the active-control study,13 but the results were not reported in the published paper.12
Economic assessment
Sugammadex is more likely to be cost-effective if any savings in recovery time are achieved in the operating theatre rather than the recovery room, since the value of any such savings is greater in terms of staff time in that setting (Table 5). In addition, sugammadex becomes more cost-effective as the reduction in mortality by administering sugammadex increases, since the number of QALYs saved increases. For younger patients, a smaller reduction in mortality is required for sugammadex to appear cost-effective since the number of QALYs saved by preventing death is greater. Furthermore, sugammadex appears slightly more cost-effective for lower probabilities of a CICV event due to the beneficial reduction in recovery time when surgery goes ahead.
Table 5.
Results of economic assessment. Number of administrations of sugammadex over which at least one death must be prevented for sugammadex to appear cost-effective. CICV, ‘can't intubate, can't ventilate’. *Since no reduction in recovery time is realized, the location of any possible reduction is irrelevant. †Short procedures (<60 min) or long procedures (>60 min) which require profound block throughout. ‡Long procedures (>60 min) which do not require profound block throughout
| Location of any reduction in recovery time | Operating theatre |
Recovery room |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age of patient | 20 yr |
60 yr |
20 yr |
60 yr |
||||||||
| Probability of CICV event (%) | 0 | 0.5 | 1 | 0 | 0.5 | 1 | 0 | 0.5 | 1 | 0 | 0.5 | 1 |
| Very short procedures* | 3678 | 3643 | 3609 | 1979 | 1960 | 1942 | 3678 | 3643 | 3609 | 1979 | 1960 | 1942 |
| Short procedures† | Sugammadex cost-effective without mortality benefit | 4696 | 4633 | 4571 | 2527 | 2493 | 2460 | |||||
| Long procedures‡ | Sugammadex cost-effective without mortality benefit | 8790 | 8535 | 8295 | 4730 | 4593 | 4463 | |||||
For very short procedures—where the subsequent use of rocuronium (followed by neostigmine/glycopyrrolate) is not necessary—any benefit of sugammadex reducing the recovery time associated with neostigmine/glycopyrrolate is not realized. For 60-yr-old patients, where the risk of CICV events is considered to be effectively zero, sugammadex appears cost-effective if it prevents at least one death for every 1979 patients administered with sugammadex (Table 5). Where a positive risk of a CICV event is assumed to exist, a greater reduction in overall mortality is required for sugammadex to appear cost-effective due to the greater cost of administering a 16 mg kg−1 dose of sugammadex where necessary; for example, if 1% of the cases are assumed to result in a CICV event, then sugammadex must prevent at least one death for every 1942 patients in order to appear cost-effective. For 20-yr-old patients, these values are considerably higher (3678 and 3609, respectively) since each prevented death is associated with a much greater QALY benefit.
For short or long procedures, if the saved recovery time with sugammadex is achieved in the operating theatre, then the value of this saved time is sufficient in itself for sugammadex to appear cost-effective, regardless of any reduction in mortality risk. However, where the saved recovery time with sugammadex is achieved in the recovery room (and so has lower value), sugammadex appears cost-effective only if its use results in a reduction in mortality. Short procedures require a greater reduction in mortality than long procedures for sugammadex to appear cost-effective since a larger and more expensive dose of sugammadex is required to reverse profound block rather than moderate block.
For short (long) procedures, where the possibility of a CICV event is not considered, sugammadex appears cost-effective for 60-yr-olds if it prevents at least one death for every 2527 (4730) patients. However, if 1% of the cases are assumed to result in a CICV event, sugammadex appears cost-effective if it avoids at least one mortality for every 2460 (4463) patients. Where the likelihood of a CICV event is between 0 and 1%, the number of cases over which a mortality reduction must be realized for sugammadex to appear cost-effective lies between these figures.
Discussion
The available evidence indicates that sugammadex 16 mg kg−1 administered 3 or 5 min after an intubating dose of rocuronium produces a markedly faster recovery than placebo.10,11 In addition, one randomized trial indicates that recovery from rocuronium-induced block after administration of sugammadex 16 mg kg−1 is faster than spontaneous recovery from succinylcholine.12 On the basis of these trials, average recovery times appear to be fast enough to allow high-dose rocuronium to potentially replace succinylcholine in RSI as the block could be reversed rapidly with sugammadex if required. This could have important benefits in terms of reducing the morbidity and possibly mortality associated with the continuing use of succinylcholine. The rapid reversal of rocuronium-induced neuromuscular block by sugammadex 16 mg kg−1 could also be potentially life saving in a CICV situation during preparation for elective surgery. However, the evidence has important limitations as discussed below. The effectiveness of high-dose sugammadex in terms of facilitating the handling of emergencies and avoiding catastrophic events such as hypoxic brain damage is difficult to assess until the drug has been widely used in clinical practice.
The economic assessment was limited by a lack of suitable evidence and by uncertainty about where time savings associated with the use of sugammadex may be realized. As a result, we were not able to provide a definitive assessment of cost-effectiveness. Instead, a series of analyses was undertaken to explore the reduction in mortality required for sugammadex to appear cost-effective. Sugammadex appears more cost-effective for lower probabilities of a CICV event. This may seem counterintuitive but can be explained by the fact that the main economic benefit of sugammadex relates to the potential reductions in recovery time it can generate when surgery goes ahead. When a CICV event occurs, it is assumed that surgery is postponed and this benefit is not realized. Sugammadex also appears more cost-effective for younger patients and/or when the saved recovery time with sugammadex is achieved in the operating theatre rather than the recovery room. Except for those procedures where some saving in recovery time is achieved in the operating theatre, at least some reduction in mortality is required for sugammadex to appear cost-effective. This ranges from the prevention of one death in every 8790 uses of sugammadex (for 20-yr-old patients undertaking long procedures without the need for profound block, where there is no likelihood of a CICV event occurring and where the savings in recovery time are achieved in the recovery room) to the prevention of one death in every 1942 uses of sugammadex (for 60-yr-old patients undertaking very short procedures, where there is a 1% probability of a CICV event occurring). These results may be compared with the underlying risk of mortality with succinylcholine: where there is less than one mortality for every 8790 uses of succinylcholine, sugammadex does not appear cost-effective in any of these circumstances. However, where this underlying risk of mortality with succinylcholine is greater, sugammadex may appear cost-effective if the relative risk of mortality with sugammadex is sufficiently low. Unfortunately, there is little available evidence to inform either the underlying risk of mortality with succinylcholine or the relative risk of mortality with sugammadex compared with succinylcholine. It is not yet feasible to assess whether reductions in mortality risks necessary for sugammadex to be cost-effective (Table 5) are realistic, as there have been too few administrations of the product for such estimates to be validated.
We used rigorous and systematic methods for searching for relevant studies, study selection, validity assessment, and data extraction. Study inclusion and exclusion criteria were specified in advance to reduce the risk of bias. Similarly, rigorous methods were used for the economic assessment. The limitations of both assessments reflect the limitations of the available evidence: more data were available to estimate costs than clinical events.
As a result of this lack of evidence, a decision about whether sugammadex is cost-effective requires an assessment of parameters whose value is unknown: for example, the underlying risk of mortality associated with the use of succinylcholine. We therefore attempted to establish the extent of the mortality reduction (relative to succinylcholine) required for sugammadex to be cost-effective. We modelled the probability of a CICV event across the range from 0 to 1%. It is recognized that, in practice, such events are very rare, particularly in specialist settings (e.g. a recent small UK study reported no failed intubations and only 23 difficult intubations in 3430 obstetric general anaesthetics).14 However, even with a very low risk, such events can be economically important and are therefore considered explicitly in the modelling.
As discussed elsewhere, there is also uncertainty about the extent to which time savings achieved by the use of sugammadex can be put to productive use.7 For example, there may be times when the patient is ready to be discharged from the operating theatre, but no bed is available in the recovery area. Therefore, to get the full economic benefit out of the potential reduction in recovery time associated with sugammadex, attention should be given to optimizing the flow of patients from the operating theatre to recovery.
The evaluation of sugammadex for immediate reversal after rapid induction of anaesthesia using rocuronium depends heavily on the single trial comparing rocuronium followed by sugammadex with succinylcholine followed by spontaneous recovery.12 Several uncertainties remain after publication of this trial. The primary outcome of the trial was time to recovery of T1/T0 to 0.1, but the clinical relevance of this endpoint is uncertain: although some signs of breathing may be present, T1/T0 of 0.1 does not represent a sufficient degree of recovery to allow safe extubation. However, the more clinically relevant endpoint of recovery of the TOF ratio to 0.9 was also measured in this study in the sugammadex group, giving a median of 1.7 min (range 0.48–14.3 min), similar to times reported in the placebo-controlled dose-finding studies. The wide range of times required to reach this endpoint could be of concern in clinical practice.
A potential issue with the use of sugammadex 16 mg kg−1 in an emergency is that the relevant clinical trials were only simulations of this situation and the appropriate dose of sugammadex was drawn up and ready for immediate administration. In routine practice, drawing up this dose in advance in anticipation of a very rare event would be wasteful and expensive. On the other hand, the time required to prepare the dose, including opening three ampoules and drawing the contents into a syringe, would increase the time the patient was exposed to hypoxia. The exact time this might take under the stress of an emergency situation is difficult to estimate.
Sugammadex 16 mg kg−1 can reverse the block induced with high-dose rocuronium shortly after it has been established. This cannot be achieved with any other reversal agent. Hence, sugammadex 16 mg kg−1 immediately after high-dose rocuronium could be considered a replacement for succinylcholine for RSI. This would avoid the morbidity associated with succinylcholine. The economic assessment suggests, however, that the cost-effectiveness of sugammadex will be highly sensitive to a given patient's underlying mortality risk during the procedure, so the drug may not be a cost-effective option in some patients at the current list prices for sugammadex. This option could be considered in the context of a clinical study at a limited number of centres.
Further research is needed to evaluate the effects of replacing succinylcholine by high-dose rocuronium and sugammadex on morbidity, mortality, patient-reported outcomes, and resource use. Further monitoring of adverse events associated with treatment with sugammadex is also needed, as the data available are limited for all doses but particularly for the 16 mg kg−1 dose. The range of recovery times recorded in the trials after administration of sugammadex 16 mg kg−1 suggests that an analysis of the proportion of patients who do not recover within, say, 5 min of administration of sugammadex, could be valuable.
The economic assessment of sugammadex for reversal after rapid induction of neuromuscular block was based on a series of analyses rather than a full cost-effectiveness model. Limited evidence was available to inform the modelling, and in particular, further research is required to define the baseline risk of mortality associated with succinylcholine in patients undergoing RSI and the relative risk of mortality with sugammadex. It should be emphasized that it is not yet feasible to assess whether reductions in mortality risks necessary for sugammadex to be cost-effective are realistic. This is because there have been too few administrations of the product for such estimates to be possible. A fuller economic assessment of sugammadex should be undertaken when more evidence is available, including evidence on resource use and the effects of sugammadex on health-related quality of life.
Conflict of interest
In the past (more than 3 yr ago), J.M.H. has had funding for clinical trials of sugammadex from Organon/Schering-Plough. She has no current funding related to sugammadex.
Funding
This project was funded by the NIHR Health Technology Assessment Programme (project number 08/10/01). It will be published in full in Health Technology Assessment, Vol. [14], No. [39]. See the HTA Programme website (www.hta.ac.uk) for further project information. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health.
Acknowledgement
We would like to thank Jonathan Wilson at York NHS Trust for his help and advice when writing the full report.
References
- 1.Naguib M. Sugammadex: another milestone in clinical neuromuscular pharmacology. Anesth Analg. 2007;104:575–81. doi: 10.1213/01.ane.0000244594.63318.fc. [DOI] [PubMed] [Google Scholar]
- 2.Schering-Plough Corporation. Bridion® (Sugammadex). Dose and Administration [Internet] Available from http://www.bridion.com/HCP/About_Bridion/Dosage_and_Administration/index.asp. (cited February 10, 2009) [Google Scholar]
- 3.Hunter JM. New neuromuscular blocking drugs. N Engl J Med. 1995;332:1691–9. doi: 10.1056/NEJM199506223322507. [DOI] [PubMed] [Google Scholar]
- 4.Organon, Schering-Plough. FDA Anesthetic and Life Support Advisory Committee Meeting. Sugammadex Sodium Injection (NDA 22-225). March 11, 2008. Briefing Document (Background Package); Kenilworth, NJ: Organon USA, Schering-Plough Corporation; 2008. [Google Scholar]
- 5.European Medicines Agency. Assessment Report for Bridion. International Nonproprietary Name: Sugammadex. Procedure No. EMEA/H/C/000885. London: European Medicines Agency; 2008. [Google Scholar]
- 6.Centre for Reviews and Dissemination. Systematic Reviews: CRD's Guidance for Undertaking Reviews in Health Care. York: University of York; 2009. [Google Scholar]
- 7.Paton F, Paulden M, Chambers D, et al. Sugammadex compared with neostigmine/glycopyrrolate for routine reversal of neuromuscular block: a systematic review and economic evaluation. Br J Anaesth. 2010;105:558–67. doi: 10.1093/bja/aeq269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Institute for Health and Clinical Excellence. Guide to the Methods of Technology Appraisal. London: National Institute for Health and Clinical Excellence; 2008. [PubMed] [Google Scholar]
- 9.Chambers D, Paulden M, Paton F, et al. Sugammadex for the reversal of muscle relaxation in general anaesthesia: a systematic review and economic assessment. Health Technol Assess. 2010;14 doi: 10.3310/hta14390. 39) [DOI] [PubMed] [Google Scholar]
- 10.de Boer HD, Driessen JJ, Marcus MAE, Kerkkamp H, Heeringa M, Klimek M. Reversal of rocuronium-induced (1.2 mg/kg) profound neuromuscular block by sugammadex: a multicenter, dose-finding and safety study. Anesthesiology. 2007;107:239–44. doi: 10.1097/01.anes.0000270722.95764.37. [DOI] [PubMed] [Google Scholar]
- 11.Puhringer FK, Rex C, Sielenkamper AW, et al. Reversal of profound, high-dose rocuronium-induced neuromuscular blockade by sugammadex at two different time points: an international, multicenter, randomized, dose-finding, safety assessor-blinded, phase II trial. Anesthesiology. 2008;109:188–97. doi: 10.1097/ALN.0b013e31817f5bc7. [DOI] [PubMed] [Google Scholar]
- 12.Lee C, Jahr JS, Candiotti KA, Warriner B, Zornow MH, Naguib M. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology. 2009;110:1020–5. doi: 10.1097/ALN.0b013e31819dabb0. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration, Center for Drug Evaluation and Research, Division of Anesthesia Analgesia and Rheumatology Products. Briefing Document for the Anesthesia and Life Support Drug Advisory Committee meeting. March 11, 2008. Bridion. NDA 22-225. Silver Spring, MD: Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Division of Anesthesia, Analgesia and Rheumatology Products; 2008. [Google Scholar]
- 14.Djabatey EA, Barclay PM. Difficult and failed intubation in 3430 obstetric general anaesthetics. Anaesthesia. 2009;64:1168–71. doi: 10.1111/j.1365-2044.2009.06060.x. [DOI] [PubMed] [Google Scholar]
- 15.Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. York: Centre for Health Economics, University of York; 1999. [Google Scholar]