Abstract
Background
A promising strategy to create stimuli-responsive gene delivery systems is to exploit the redox gradient between the oxidizing extracellular milieu and the reducing cytoplasm in order to disassemble DNA/cationic lipid complexes (lipoplexes). On these premises, we previously described the synthesis of SS14 redox-sensitive gemini surfactant for gene delivery. Although others have attributed the beneficial effects of intracellular reducing environment to reduced glutathione (GSH), these observations cannot rule out the possible implication of the redox milieu in its whole on transfection efficiency of bioreducible transfectants leaving the determinants of DNA release largely undefined.
Methodology/Principal Findings
With the aim of addressing this issue, SS14 was here formulated into binary and ternary 100 nm-extruded liposomes and the effects of the helper lipid composition and of the SS14/helper lipids molar ratio on chemical-physical and structural parameters defining transfection effectiveness were investigated. Among all formulations tested, DOPC/DOPE/SS14 at 25∶50∶25 molar ratio was the most effective in transfection studies owing to the presence of dioleoyl chains and phosphatidylethanolamine head groups in co-lipids. The increase in SS14 content up to 50% along DOPC/DOPE/SS14 liposome series yielded enhanced transfection, up to 2.7-fold higher than that of the benchmark Lipofectamine 2000, without altering cytotoxicity of the corresponding lipoplexes at charge ratio 5. Secondly, we specifically investigated the redox-dependent mechanisms of gene delivery into cells through tailored protocols of transfection in GSH-depleted and repleted vs. increased oxidative stress conditions. Importantly, GSH specifically induced DNA release in batch and in vitro.
Conclusions/Significance
The presence of helper lipids carrying unsaturated dioleoyl chains and phosphatidylethanolamine head groups significantly improved transfection efficiencies of DOPC/DOPE/SS14 lipoplexes. Most importantly, this study shows that intracellular GSH levels linearly correlated with transfection efficiency while oxidative stress levels did not, highlighting for the first time the pivotal role of GSH rather than oxidative stress in its whole in transfection of bioreducible vectors.
Introduction
Gene delivery using non-viral approaches has been extensively studied as a basic tool for intracellular gene transfer and gene therapy [1]. In the past, the primary focus has been on application of physical, chemical, and biological principles to development of a safe and efficient method that delivers a transgene into target cells for appropriate expression. Nowadays, the development of non-viral-based approaches to deliver nucleic acids to cells (transfection) is an inherently interdisciplinary endeavor and a rapidly advancing area of research [2]. Polymeric and lipidic vectors rely on the basics of supramolecular chemistry termed “self-assembling”: at physiological pH, they are cations and, after removal of small counterions, spontaneously form complexes with anionic nucleic acids [3]. Such vectors must be able to (i) complex nucleic acids in stable, nanoscaled and positively charged aggregates, (ii) promote the internalization of DNA by cells, (iii) prevent the intracellular DNA degradation and, finally, (iv) induce exogenous gene expression [4]. In this scenario, DNA/cationic lipid complexes (lipoplexes) have drawn significant attention since their use in gene therapy clinical trials is rapidly increasing (http://www.wiley.co.uk/genmed/clinical/) although their cytotoxicity and low efficiency remain major drawbacks.
Hence, in order to overcome limitations of currently available non-viral vectors, the use of stimuli-responsive carriers offer novel alternatives for the optimization of this therapy [5], [6]. Redox potential has been proposed as an efficient stimuli mechanism in gene delivery because of the high difference (102–103 fold) existing between the reducing intracellular space and the oxidizing extracellular milieu [7]. Indeed, the versatility of reducible disulfide carriers has been shown in many different approaches [8]–[12] but the underlying biological mechanism and physiological mediator(s) remain poorly understood [7], [13].
Since their introduction as gene carriers in 1987 [14], liposomes have become one of the most studied non-viral vectors, featuring remarkable flexibility at molecular, formulation and dimension level [15], [16]. Along this line, over the last twenty years cationic liposomes containing 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) [17], [18], 1,2-dimyristoyl-sn-glycero-3-phosphatidylcholine (DMPC) [19], as main constituents, 1,2-dimyristoyl-sn-glycero-3-phosphatidylethanolamine (DMPE) [20], 1,2-dioleoyl-sn-glycero-3-phosphatidylethanolamine (DOPE) [17], [21], as helper lipids, and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) [18], [21] and 3β-[N-(N',N'-dimethylaminoethane)-carbamoyl]cholesterol (DC-Chol) [21] as cationic lipids have been extensively used for gene delivery purposes.
In the panorama of cationic amphiphiles, gemini surfactants are a relatively new class of molecules with peculiar physicochemical properties, composed by two or more head groups and two aliphatic chains, linked by a spacer [22]. Moreover, recent studies have pointed out that suitably tailored cationic geminis are able to yield high transfection efficiency [23]. Nevertheless, there are only a few reports on the transfection properties of gemini lipids [24]–[28].
This study ensues from our report concerning the synthesis and characterization of a new redox-sensitive triazine-based gemini surfactant, SS14 (Fig. 1A), for gene delivery [27]. The aim of this study was twofold. First, we studied the effects of the helper lipid composition and of the SS14 to helper lipids molar ratio on liposome dimension and overall charge (ζ-potential), parameters that all contribute in defining transfection efficiency and cytotoxicity. Second and most important, we sought to determine in vitro the physiological mechanism leading to lipoplex disassembly and gene delivery by bioreducible SS14-containing liposomes.
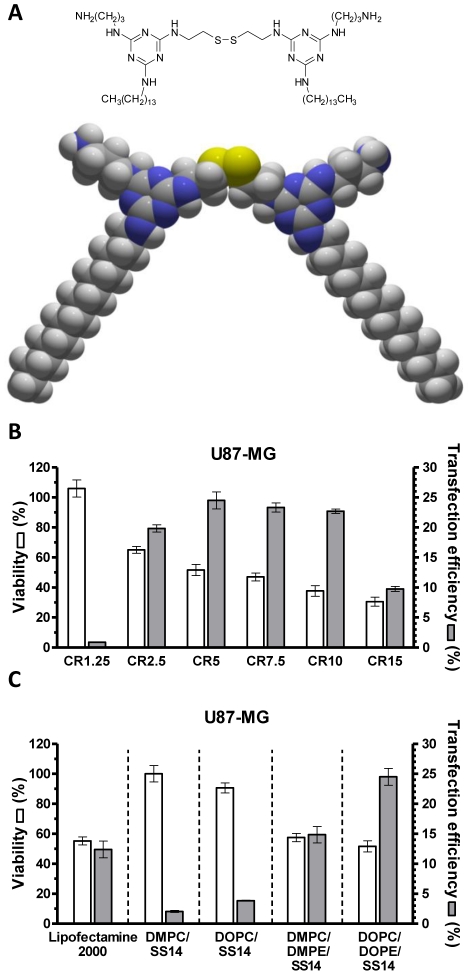
Figure 1. SS14 gemini surfactant molecule and evaluation of transfection effectiveness of SS14-containg liposome formulations.
(A) Chemical structure and space-filling molecular model of gemini surfactant SS14. Color coding: yellow = sulfur; purple = nitrogen; grey = carbon; white = hydrogen. (B) Cytotoxicity (viability, left axis, white bars) and transfection efficiency (% of EGFP-positive cells, right axis, grey bars) of DOPC/DOPE/SS14 (25∶50∶25 molar ratio) lipoplexes on U87-MG cell line as a function of charge ratio (CR, +/−). (C) Cytotoxicity and transfection efficiency of binary DMPC/SS14, DOPC/SS14 (75∶25 molar ratio each), ternary DMPC/DMPE/SS14, and DOPC/DOPE/SS14 (25∶50∶25 molar ratio each) lipoplexes at CR5 on U87-MG cell line. Lipofectamine 2000 was used as positive control in transfection experiments. All results are expressed as mean ± SEM (n = 3).
Results and Discussion
Preparation and characterization of bioreducible liposomes and lipoplexes
First, binary DOPC/SS14, DMPC/SS14 (75∶25 molar ratio each) and ternary DMPC/DMPE/SS14, DOPC/DOPE/SS14 (25∶50∶25 molar ratio each) unilamellar vesicles were designed following a number of considerations: i) the chosen co-lipids should differ both in their headgroup structure (phosphatidylethanolamine vs. phosphatidylcholine groups), acyl chain length and saturation degree (dimyristoyl vs. dioleoyl chains), to assess the effect of these components on transfection; ii) multi-component liposomes should be preferred to binary ones because of their well documented, superior transfection efficiency ; iii) SS14 content should be optimized in terms of transfection effectiveness represented by the best compromise between high transfection efficiency and low cytotoxicity.
All liposome formulations were extruded with 100 nm pore membranes. The size distribution of DOPC/SS14, DMPC/SS14, and DOPC/DOPE/SS14 liposomes was markedly narrower than that of DMPC/DMPE/SS14 formulation for which a main population with mean diameter centered at 110 nm could still be evidenced (70% by integrated intensity). On the other hand, the measured ζ-potential of two-component liposomes and DOPC/DOPE/SS14 formulations were, within experimental error, the same (Table 1).
Table 1. Hydrodynamic diameter, ζ-potential and polydispersity index (P.I.) of each liposome formulation.
| Liposomes | |||
| Diameter (nm)a | ζ-potential (mV)a | P.I. | |
| DMPC/SS14 (75∶25 molar ratio) | 109±3 | +39±7 | 0.12 |
| DOPC/SS14 (75∶25 molar ratio) | 112±3 | +40±6 | 0.07 |
| DMPC/DMPE/SS14 (25∶50∶25 molar ratio) | 110±25 | +55±8 | 0.36 |
| DOPC/DOPE/SS14 (25∶50∶25 molar ratio) | 120±3 | +46±8 | 0.07 |
Mean ± Standard Deviation.
Based on these results all developed formulations were considered suitable candidates for further investigations as potential gene delivery vectors. We next evaluated by fluorescence titration assay the ability of all liposome formulations to complex the DNA at increasing charge ratio (CR, +/−). Interestingly, all liposomes shared the same affinity towards DNA template, represented by the lowest fluorescence values for CR≥5 (not shown). However, DNA condensation is not sufficient to ensure significant transfection levels [29]. On this ground, we decided to investigate transfection ability (evaluated as % of EGFP-positive cells) and cytotoxicity (measured by viability assay) of DOPC/DOPE/SS14 at increasing CR in U87-MG cell line commonly used in transfection experiments [30], [31]. Since use of serum cannot be avoided in long-term cultures of eukaryotic cells in vitro [29], transfection experiments were carried out in complete medium (D-MEM with 10% fetal bovine serum, FBS). Although this experimental condition is far from in vivo situation, transfections carried out in serum-complete medium are commonly used to check serum resistance of lipoplexes prior to performing animal studies [16], [31]. As expected, transfection effectiveness of lipoplexes was dramatically affected by cationic lipid to DNA ratio in that transfection efficiency followed a bell-shape trend and cell viability dramatically decreased as CR increased, as previously reported by others [32]–[34]. Among all CR tested, maximal transfection efficiency and reasonable cytotoxicity for the aims of the present work were obtained with the minimal dose of liposomes corresponding to lipoplexes at CR5 (Fig. 1B). Hence, CR5 was chosen for a comparative evaluation of all formulations. Of note, although transfection efficiencies seemed lower than for other reported transfectants [35]–[37], the method of analysis here used and firstly described by Walker and colleagues [38] allows very stringent discrimination between intrinsic autofluorescence of mock-transfected cells and truly EGFP-positive ones, as also exemplified in upper panels of Fig. 2C illustrating EGFP-positive U87-MG cells after transfection. Indeed, Lipofectamine 2000 yielded 12.4±1.4% of EGFP-positive U87-MG cells (Fig. 1C), similar or lower than transfection efficiencies observed with DMPC/DMPE/SS14 and DOPC/DOPE/SS14 formulations, using this analytical method.
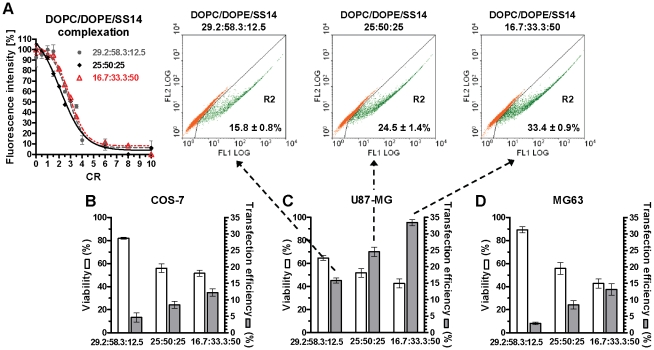
Figure 2. Complexation abilities of DOPC/DOPE/SS14 liposome formulations and evaluation of their transfection effectiveness.
(A) Fluorophore-exclusion titration of DOPC/DOPE/SS14 liposomes at 29.2∶58.3∶12.5 (grey circles), 25∶50∶25 (black rhombus), and 16.7∶33.3∶50 molar ratios (red triangles) as a function of CR. All curves underlying data simply represent a guide to the eye and were drawn to better evidence trend variations. Cytotoxicity (viability, left axis, white bars) and transfection efficiency (% of EGFP-positive cells, right axis, grey bars) of the three different DOPC/DOPE/SS14 lipoplexes at CR5 on COS-7 (B), U87-MG (C), and MG63 (D) cell lines at CR5. Results are expressed as mean ± SEM (n = 3). Examples of cytofluorimetric analysis are reported as FL1 (green fluorescence) vs. FL2 (orange fluorescence) dot plot of U87-MG transfected cells (C, upper panels). Mock-transfected (pCMV-GLuc) but autofluorescent population of cells lies along the 0, 0; 104, 104 diagonal. EGFP-expressing cells appear as an additional population delineated by region 2 (R2), where FL1>FL2.
In DOPC/SS14 lipoplexes, the presence of unsaturated acyl dioleoyl chains conferred higher transfection efficiency compared to saturated dimyristoyl chains (3.8±0.1% vs. 2.0±0.2%, p<0.05) with no appreciable difference in cytotoxicity (viability: 90.6±3.3% vs. 100.1±5.4%, not statistically significant). Our results are in agreement with Felgner et al. that firstly showed that the transfection efficacies in a series of homologous lipids with symmetric saturated hydrophobic moieties were Coleyl>C16>C18>C14 [39]. In parallel, the introduction in our liposome formulations of helper lipids bearing phosphatidylethanolamine polar heads increased transfection efficiency up to 7.4- and 6.5-fold in DMPC/DMPE/SS14 and DOPC/DOPE/SS14 lipoplexes, with respect to each binary counterpart. These results highlight the superior transfection efficiency of multicomponent lipoplexes with respect to binary ones, as previously reported by Caracciolo and colleagues [40]. Although some speculative arguments have been proposed, the exact physico-chemical reasons why multicomponent lipoplexes are more efficient than binary lipoplexes have never been stated unambiguously [41]. On the other hand, viability of cells transfected with phosphatidylethanolamine-containing lipoplexes decreased by 1.7- and 1.9-fold, respectively. The strong transfection efficiency-dependence on DOPE and DMPE presence in liposome formulations supports the role of the phosphatidylethanolamine headgroup as membrane fusion or destabilization agent due to its ability to promote transition from the bilayer phase (Lα) into the inverted hexagonal phase (HII) [39], [42]. Altogether, DOPC/DOPE/SS14 represented the best compromise between the highest transfection efficiency (24.5±1.4% vs. 14.8±1.3% for DMPC/DMPE/SS14, p<0.05) and acceptable cytotoxicity (viability: 51.7±3.7% vs. 57.5±2.8 for DMPC/DMPE/SS14, not statistically significant) (Fig. 1c). Cytotoxicities were in line with those reported by others with DOPE-containing liposomes evaluated 48 h post-transfection [43] and comparable to that of the gold standard Lipofectamine 2000 (Viability: 55.2±2.7%), as reported in Fig. 1C. Noteworthy, our DOPC/DOPE/SS14 lipoplex formulation yielded almost two-fold improved transfection efficiency compared to Lipofectamine 2000 (24.5±1.4% vs. 12.4±1.4% for Lipofectamine 2000, p<0.05).
Thus, we focused on developing and optimizing the DOPC/DOPE/SS14 liposome formulation with the aim of determining its specific mechanism of transfection. In order to find the optimal SS14 ratio for transfection experiments, three DOPC/DOPE/SS14 formulations at different SS14 molar fractions (29.2∶58.3∶12.5, 25∶50∶25, and 16.7∶33.3∶50 molar ratios) were prepared. Next, chemical-physical properties and the corresponding transfection efficiencies were examined (Fig. 2).
As reported above, an asymptotic decrease in fluorescence was observed for all liposome formulations at CR5 (Fig. 2A), which still represented the best compromise between high transfection efficiency and low cytotoxicity in vitro (not shown). Therefore, we evaluated both size and ζ-potential for all liposomes and for corresponding lipoplexes at CR5 (Table 2).
Table 2. Hydrodynamic diameter, ζ-potential and polydispersity index (P.I.) of DOPC/DOPE/SS14 liposomes and lipoplexes at CR5.
| Liposomes | Lipoplexes at CR5 | |||||
| Diameter (nm)a | ζ-potential (mV)a | P.I. | Diameter (nm)a | ζ-potential (mV)a | P.I. | |
| DOPC/DOPE/SS14 (29.2∶58.3∶12.5 molar ratio) | 134±4 | +44±9 | 0.05 | 295±12 | +20±4 | 0.32 |
| DOPC/DOPE/SS14 (25∶50∶25 molar ratio) | 120±3 | +46±8 | 0.07 | 259±3 | +30±3 | 0.04 |
| DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) | 129±3 | +50±9 | 0.03 | 291±7 | +26±4 | 0.09 |
Mean ± Standard Deviation.
Liposomes extruded at 100 nm had a hydrodynamic diameter of circa 130 nm and a ζ-potential ranging from +44 to +50 mV. As expected, after complexation with DNA at CR5, the hydrodynamic diameter increased on average by 2.2-fold and the overall charge decreased by almost twofold. Since transfection effectiveness depends to a great extent on the cell type and the lipid composition [29], [41], we tested DOPC/DOPE/SS14 formulations on three different cell lines. In spite of similar homogeneities in size and ζ-potential, DOPC/DOPE/SS14 lipoplexes with the highest SS14 content (16.7∶33.3∶50 molar ratio) showed the highest transfection efficiency in all cell lines tested (p<0.05), reaching up to circa 35% of EGFP-positive U87-MG cells (Fig. 2C upper panels), while the cytotoxicity was similar to that of DOPC/DOPE/SS14 at 25∶50∶25 molar ratio (not statistically significant) (Fig. 2B–D). Noteworthy, in U87-MG cell line the transfection efficiency of DOPC/DOPE/SS14 liposomes at 29.2∶58.3∶12.5, 25∶50∶25, and 16.7∶33.3∶50 molar ratios, were 1.3-, 1.9-, and 2.7-fold higher than that of the benchmark Lipofectamine 2000, as shown in Fig. 1C and 2C. In agreement with a previous report [41], we found that both the number of lipid components and their relative molar ratio in multicomponent liposomes altered transfection activity. In particular, transfection efficiency of DOPC/DOPE/SS14 lipoplexes peaked at 16.7∶33.3∶50 molar ratio when both SS14 cationic and neutral lipid species were mixed in equimolar ratio.
Hence, neither size nor ζ-potential of the complexes was clearly associated with transfection efficiency while lipid composition and relative molar ratios affected it. In agreement with our findings, Farhood and colleagues previously reported that, in cationic DOPE/DC-Chol liposome formulations, transfection efficiency was proportional to DC-Chol content, with the optimum at 50–60% of cationic lipid [44]. Moreover, in accordance with our results, Pinnaduwage et al. showed, in three different DOPE-containing liposomes, that cytotoxicity was directly related to the amount of the cationic component [45].
Effect of GSH on bioreducible lipoplexes
An important feature of our liposome formulations is the disulfide linker moiety in SS14 that, in suitable reducing environment, might promote lipoplex disassembly by reversion of the gemini surfactant to single-chain amphiphiles. According to the existing literature, the intracellular reduction of disulfide bonds in lipo/polyplexes is most likely mediated by small redox molecules [46]. Among antioxidants, glutathione (L-γ-glutamyl-L-cysteinyl-glycine) is the most abundant non-protein thiol in mammalian cells, typically present in the reduced form (GSH) and oxidized glutathione disulfide (GSSG) [47], with an overall cellular GSH/GSSG ratio ranging from 30∶1 to 100∶1 [48]. Although glutathione is ubiquitous, it is present in high levels (1–11 mM) intracellularly and at low concentration (10 µM) in the extracellular milieu [13]. Since the increasing content of redox-sensitive surfactant, SS14, along DOPC/DOPE/SS14 series correlated with higher transfection efficiency, we next examined by fluorescence titration assay whether GSH might lead to lipoplex disassembly and DNA release. As shown in Fig. 3A, in test tube, only reducing GSH triggered DNA release from DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) lipoplexes.
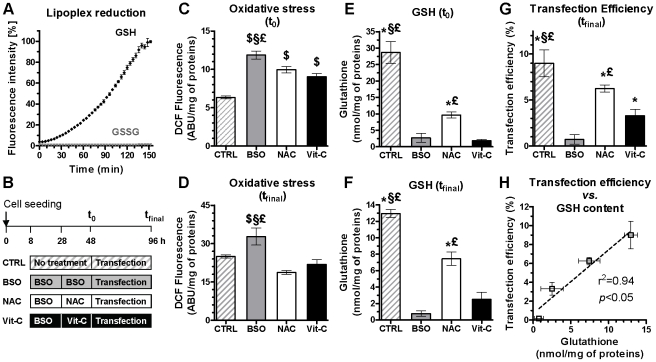
Figure 3. GSH-mediated lipoplex disassembly in batch and effect of intracellular GSH levels on transfection efficiency.
(A) Stability of DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) lipoplexes at CR5 in presence of GSH or GSSG. Results are presented as % of fluorescence emitted with respect to DNA. (B) Experimental procedure. MG63 cells were divided in four groups: untreated CTRL, BSO-, NAC-, and Vit-C-treated cells. Following pharmacological treatment (t0), cells underwent 48 h transfection (tfinal) with DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) lipoplexes at CR5. Oxidative stress and GSH content were measured at t0 ((C) and (E), respectively) and after transfection ((D) and (F), respectively). Transfection efficiency, expressed as % of EGFP-positive cells, was also evaluated (G). A linear correlation between GSH content and transfection efficiency was observed (H). Results are expressed as mean ± SEM (n = 3). $ p<0.05 vs. CTRL; * p<0.05 vs. BSO; § p<0.05 vs. NAC; £ p<0.05 vs. Vit-C.
Although others have conferred the beneficial effects of intracellular reducing environment to glutathione only [46], [49], [50], the possible implication of the reducing milieu in its whole on transfection efficiency of bioreducible transfectants has been overlooked. In particular, the thioredoxin (Trx) system, composed of thioredoxin reductases (TrxR), thioredoxins and NADPH [51] is known to participate in modulating the intracellular redox environment and thiol/disulfide exchange, rendering difficult to single out the effect of GSH per se in the context of the overall redox state [52]. Most studies have relied on the use of the glutathione depletor L-buthionine-sulfoximine (BSO) to demonstrate the link between glutathione content and transfection efficiency of bioreducible transfectants. However, although BSO does modulate glutathione synthesis inhibiting γ-glutamylcysteine synthetase [53], it has also been described to alter expression profiles of several genes involved in redox homeostasis [54], [55]. As a consequence, the unique role of glutathione in physiological disulfide-containing lipoplex reduction and disassembly has never been strikingly demonstrated. For this reason we here evaluated the contribution of both the overall redox status as well as GSH only on transfection efficiency.
In this context, we studied how the intracellular redox status and GSH content separately modulate the transfection activity of DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) bioreducible lipoplexes. To this end, MG63 cells were supplemented with the GSH depletor BSO for 20 h, after which cells were treated with either BSO, the glutathione repletor N-acetyl-L-cysteine (NAC) or the antioxidant L-ascorbic acid (Vitamin C, Vit-C) for another 20 h before transfection (t0) (Fig. 3B). At t0 BSO treatment increased by almost twofold oxidative stress levels with respect to untreated cells (CTRL) (p<0.05), while the antioxidant treatment with NAC and Vit-C equally alleviated BSO effects (Fig. 3C).
Noteworthy, antioxidants cannot indiscriminately be lumped together. Although Vit-C is part of an antioxidant network where GSH plays a pivotal role, recycling other antioxidants and keeping them in their active state, it does not compensate for GSH depletion [56]–[58]. In this regard, a few studies supported the use of supplemental Vit-C in individuals predisposed to reduced GSH levels, either due to age [59] or diseases [60], [61]. Although dietary supplementation with Vit-C restored resistance to oxidative stress and its sequelae, it did not replenish GSH levels [62]. Indeed, in our study only preincubation of GSH-depleted cells with NAC partially restored GSH levels (t0; 9.7±0.9 vs. 2.7±1.3 and 1.9±0.4 nmol/mg of proteins for BSO and Vit-C groups, respectively, p<0.05) (Fig. 3E). Noteworthy, both NAC- and Vit-C-treated cells shared the same oxidative stress levels (not statistically significant) (Fig. 3C) significantly lower than those of BSO group (t0; 11.9±0.5 vs. 9.9±0.4 and 9.1±0.4 ABU/mg of proteins for NAC and Vit-C groups, respectively, p<0.05), highlighting the unspecific antioxidant effects of both. Afterwards, cells underwent 48 h transfection (tfinal). 9.0±1.5% of EGFP-positive cells were observed in the CTRL group compared to 6.3±0.4% in NAC- and 3.3±0.7% in Vit-C-treated groups (Fig. 3G). Remarkably, after transfection, oxidative stress levels in NAC- and Vit-C-treated groups were equal to CTRL (not statistically significant) (Fig. 3D), as previously shown by Shang and co-workers in an in vitro study dealing with effects of GSH and Vit-C in BSO-treated epithelial cells [58]. However, only the NAC-treated group showed a significantly high, 57% repletion in GSH content compared to BSO-untreated CTRL (tfinal; 7.4±0.8 vs. 0.8±0.3 and 2.5±0.8 nmol/mg of proteins for BSO and Vit-C groups, respectively, p<0.05) (Fig. 3F) yielding improved transfection efficiency (p<0.05 vs. BSO and Vit-C) (Fig. 3G). Finally we found a linear correlation between GSH content and transfection efficiency (r2 = 0.94, p<0.05) in cells transfected with bioreducible DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio)-based lipoplexes (Fig. 3H). Inversely, oxidative stress levels and transfection efficiency did not correlate at all (r2 = 0.35, p = 0.21, not statistically significant). As a proof of concept, transfection efficiency of non-reducible transfectant Lipofectamine 2000 did not rely on either oxidative stress status (r2 = 0.37, p = 0.39, not statistically significant) or GSH content (r2 = 0.39, p = 0.38, not statistically significant), specifically attributing the above demonstrated crucial role of GSH to bioreducible transfectants.
In summary, we have developed novel effective ternary DOPC/DOPE/SS14 liposomes for gene delivery formulated with the bioreducible cationic gemini surfactant, SS14, previously synthesized by our group [27]. In such formulations, the presence of unsaturated acyl (dioleoyl) chains and phosphatidylethanolamine polar heads conferred superior transfection efficiency to the corresponding lipoplexes. By raising the SS14 content in DOPC/DOPE/SS14 liposome series up to 50% (mol/mol), transfection efficiency increased, overreaching by almost three-fold the transfection efficiency observed with the commercially available Lipofectamine 2000 (33.4±0.9% vs. 12.4±1.4% for Lipofectamine 2000, p<0.05). Moreover, we demonstrated that physiological concentration of GSH could mediate DOPC/DOPE/SS14 (16.7∶33.3∶50 molar ratio) lipoplex disruption and DNA release induced by SS14 reduction in batch. Finally, the major finding of this work regards the linear correlation between GSH content and transfection efficiency in cells transfected with these lipoplexes, underscoring the fundamental role of GSH levels in modulating transfection effectiveness of bioreducible lipoplexes.
Overall this work underlines for the first time the pivotal role of high intracellular GSH content in gene delivery efficiency of bioreducible carriers, opening the door towards an improved development of these and other bioreducible transfectants as potential therapeutics of the future in particular disease contexts, as increased levels of intracellular GSH have been associated with anticancer drug resistance [63]–[65] and xenobiotic liver detoxification [66]. Since new bioreducible SS14-based transfectants seem interesting for evaluating the effect of innate glutathione levels on transfection efficiency of bioreducible transfectants, in vivo effectiveness of DOPC/DOPE/SS14 lipoplexes will be investigated in the future.
Materials and Methods
Materials
Plasmid DNA encoding for the Enhanced Green Fluorescent Protein (pEGFP-N1) or for the secreted Gaussia Luciferase (pCMV-GLuc) were purchased from Clontech Laboratories (Mountain View, CA, USA) and from New England BioLabs (Hitchin, UK), respectively. DOPC, DMPC, DMPE, and DOPE were from Avanti Polar Lipids (Alabaster, AL, USA). Lipofectamine 2000 was from Invitrogen Life Technologies (San Giuliano Milanese, Italy). All chemicals were from Sigma-Aldrich (Milan, Italy) if not differently specified. SS14 gemini surfactant was previously synthesized by our group [27]. U87-MG (human glioblastoma-astrocytoma, epithelial-like cell line, HTB-14), MG63 (human osteosarcoma cell line, CRL-1427) and COS-7 (African green monkey kidney fibroblast-like cell line, CRL-1651) were purchased from the European Collection of Cell Cultures (ECACC, Salisbury, UK).
Liposome preparation
Stock solutions of liposomes were prepared from binary mixtures of DOPC/SS14 and DMPC/SS14 at 75∶25 molar ratio each and from ternary mixtures of DMPC/DMPE/SS14 at 25∶50∶25 molar ratio, and DOPC/DOPE/SS14 at 29.2∶58.3∶12.5, 25∶50∶25, and 16.7∶33.3∶50 molar ratios, with a final concentration of helper lipids of 14 mM. Mixtures of dry lipid powders were dissolved in chloroform and after solvent evaporation the lipid film was swollen at room temperature (r.t.) with deionized water. Multilamellar vesicles obtained upon vortexing were then submitted to eight freeze/thaw cycles and extruded through 100 nm-pore polycarbonate membranes (27 passages; LiposoFast apparatus, Avestin, Ottawa, Canada) to obtain monodisperse small monolamellar vesicles. Samples were stored at 4°C.
Lipoplex formation and disruption
Each lipoplex sample was prepared at r.t. by adding a solution of nucleic acids (pEGFP-N1) to a liposome suspension, at the desired lipid concentration, yielding different CR. The DNA binding ability of each liposome formulation was monitored by a fluorophore-exclusion titration assay. For each condition, 0.12 µg of pEGFP-N1 in 2.4 µl of SYBR Green I (λex = 497 nm; λem = 520 nm) were added to 3.6 µl of liposome suspension at different concentrations in order to achieve the desired CR. The fluorescence of the intercalated dye was measured using GENios Plus reader (Tecan, Segrate, Italy) in black 384-well microplates. The effect of reduction on lipoplex stability was examined on DOPC/DOPE/SS14 (16.6∶33.3∶50 molar ratio) complexed at CR5 by measuring the ability of GSH to restore the fluorescence of DNA/SYBR Green I. Five µl of lipoplexes containing 0.1 µg of pEGFP-N1 in SYBR Green I solution were diluted 1∶20 in 10 mM aqueous solution of either GSH or GSSG. Lipoplex reduction was monitored at 37°C by measuring the fluorescence emitted as described above.
Liposome and lipoplex dimensions and overall charge
The size of liposomes and the corresponding lipoplexes were determined by Dynamic Light Scattering (DLS) with a Malvern ZS ZEN3500 particle sizer. In this apparatus the light scattered by a laser of 532 nm wavelength, is detected at 173° with respect to the incoming ray (Back Scattering technique), amplified and then analyzed by a correlator. The obtained correlation function, whose shape depends on the size of the scattering objects, was analyzed by a procedure based on the algorithm CONTIN, which gives mean diameter and polydispersity index of the liposome size distribution. The ζ-potential was obtained by Laser Doppler Velocimetry in the same Malvern ZS ZEN3500 apparatus. In this case, the electrophoretic mobility was measured with Phase Analysis Light Scattering (PALS) technique, from which the ζ-potential is extracted by using the Smoluchowsky equation. All liposome and lipoplex suspensions were diluted 1∶20 for both size and ζ-potential measurements, in order to meet the best sensitivity requirements.
Transfection experiments
U87-MG, MG63, and COS-7 cell lines were cultured at 37°C in a humidified atmosphere of 5% CO2, with complete medium consisting in Dulbecco's Modified Eagle Medium (D-MEM) with 10% (v/v) FBS, 1 mM sodium pyruvate, 10 mM Hepes buffer, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2 mM glutamine. Before experiments, 1×104 cells/cm2 were plated in 12-well cell culture plates and allowed to adhere overnight. The day of transfection, cells were washed once in phosphate-buffered saline (PBS) and the culture medium was replaced with 800 µl/well of complete medium containing lipoplexes (80 ng/cm2 of pDNA) at the desired CR and transfected for 48 h. Transfection experiments with Lipofectamine 2000 were carried in Opti-MEM according to the manufacturer's procedures, utilizing 80 ng/cm2 of pEGFP-N1 to easily compare results among transfectants. The cells were trypsinized, fixed in 4% (w/v) paraformaldehyde (PFA) in PBS and stored at 4°C. Transfection efficiency was measured evaluating the percentage of EGFP-positive cells in each sample by means of a flow cytometer (FACS-Calibur, Becton Dickinson, Buccinasco, Italy); a minimum of 1×104 cells was analyzed for each sample. EGFP was excited at 488 nm and emitted light was collected at 520 nm (green fluorescence) and 575 nm (orange fluorescence) to enable correction for autofluorescence by diagonal gating [11]. Background fluorescence and autofluorescence were determined using mock-transfected cells (pCMV-GLuc) and subtracted to EGFP-positive cells. Cellular debris showing reduced side scattering (SSD) and forward scattering (FSD) were excluded from analysis. Data were analyzed by WinMDI2.9 software program and transfection efficiency was expressed as the percentage of EGFP-positive cells over the total cell number. Cytotoxicity of lipoplexes was tested using AlamarBlue cell viability assay (Invitrogen Life Technologies, San Giuliano Milanese, Italy) according to manufacturer's guidelines. Viability of untreated control cells was assigned as 100%.
GSH depletion/repletion
For GSH depletion/repletion study, MG63 cells were plated in T25 flasks at a density of 1×104 cells/cm2 in complete medium. Eight hours after plating, cell culture medium was supplemented with 0.05 mM BSO. After 20 h incubation, cells were washed in PBS and the medium was replaced with fresh complete medium supplemented either with 0.05 mM BSO, 1 mM NAC, or 0.2 mM Vit-C for further 20 h (t0). Finally, the medium was replaced with complete medium containing either DOPC/DOPE/SS14 (16.6∶33.3∶50 molar ratio) liposomes corresponding to CR5 or 0.16 µl of Lipofectamine 2000 complexed with 80 ng/cm2 of pEGFP-N1 and cells were transfected for 48 h (tfinal). Mock-transfected cells were done in parallel. At t0 and tfinal cells were trypsinized, harvested and each sample was splitted into two aliquots. In one-half aliquots, cell rupture was achieved by five pulses (1 min each, 20 kHz, 100 W) on Labsonic L sonicator (B Braun, Melsungen, Germany), alternated by 1 min intervals on ice and the cell debris were removed by centrifugation (1×104 rpm for 10 min). Protein content in cell lysate was determined by BCA protein assay kit (Pierce, Rockford, IL, USA). After the addition of 5% (w/v) sulfosalicylic acid (SSA), GSH levels were determined with Glutathione assay kit (Sigma-Aldrich, Milan, Italy) according to the manufacturer's instructions, except that Glutathione Reductase and NADPH were not added to samples. In the remaining half-aliquots, cells were counted (trypan blue staining), fixed, and transfection efficiency was evaluated by means of a flow cytometer. Oxidative stress was monitored by 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay [67]. Briefly, at the desired time point, cells were washed in PBS, incubated for 15 min at 37°C with 10 µM DCFH-DA in PBS and washed twice with PBS and lysed with 50 mM Tris-HCl buffer pH 7.5, containing 0.5% (v/v) Tween 20. Finally, cells were detached and centrifuged for 5 min at 1×104 rpm to remove cell debris. Intracellular de-esterification and oxidation of DCFH-DA produce the highly fluorescent 2′,7′-dichlorofluorescein (DCF). DCF fluorescence (λex = 485 nm; λem = 530 nm) was measured using GENios Plus reader. Fluorescence results were normalized over protein content of each sample.
Statistical analysis
Statistical analysis was carried out by GraphPad version 5.0 (GraphPad software, La Jolla, CA, USA). Comparisons among groups were performed by one-way ANOVA, with Bonferroni's Multiple Comparison Test and correlations were analyzed by Pearson Test. Significance was retained when p<0.05.
Acknowledgments
We wish to thank KemoTech s.r.l. for providing SS14 gemini surfactant. The authors would like to acknowledge the staff of the Laboratory of Biocompatibility and Cell Culture - BioCell, Politecnico di Milano, for their technical support, Dr A. Kajaste-Rudnitski for critical reading and Dr. T. Marcelli for providing graphical model of SS14 molecule.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: We wish to thank Politecnico di Milano (Grant: 5 per Mille Junior) and the Italian Institute of Technology (IIT) for economic support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gao X, Kim KS, Liu D. Nonviral gene delivery: what we know and what is next. AAPS J. 2007;9:E92–104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev. 2009;109:259–302. doi: 10.1021/cr800409e. [DOI] [PubMed] [Google Scholar]
- 3.Eliyahu H, Barenholz Y, Domb AJ. Polymers for DNA delivery. Molecules. 2005;10:34–64. doi: 10.3390/10010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giordano C, Causa F, Candiani G. Gene therapy: The state of the art and future directions. Journal of Applied Biomaterials & Biomechanics. 2006;4:73–79. [PubMed] [Google Scholar]
- 5.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Guo X, Szoka FC., Jr Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 7.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 8.Blessing T, Remy JS, Behr JP. Monomolecular collapse of plasmid DNA into stable virus-like particles. Proc Natl Acad Sci U S A. 1998;95:1427–1431. doi: 10.1073/pnas.95.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang F, Hughes JA. Use of dithiodiglycolic acid as a tether for cationic lipids decreases the cytotoxicity and increases transgene expression of plasmid DNA in vitro. Bioconjugate chemistry. 1999;10:791–796. doi: 10.1021/bc990016i. [DOI] [PubMed] [Google Scholar]
- 10.Wetzer B, Byk G, Frederic M, Airiau M, Blanche F, et al. Reducible cationic lipids for gene transfer. Biochem J. 2001;356:747–756. doi: 10.1042/0264-6021:3560747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Read ML, Bremner KH, Oupicky D, Green NK, Searle PF, et al. Vectors based on reducible polycations facilitate intracellular release of nucleic acids. J Gene Med. 2003;5:232–245. doi: 10.1002/jgm.331. [DOI] [PubMed] [Google Scholar]
- 12.Ciani L, Candiani G, Frati A, Ristori S. DNA induced dimerization of a sulfhydryl surfactant in transfection agents studied by ESR spectroscopy. Biophysical Chemistry. 2010;151:81–85. doi: 10.1016/j.bpc.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Bauhuber S, Hozsa C, Breunig M, Gopferich A. Delivery of Nucleic Acids via Disulfide-Based Carrier Systems. Advanced Materials. 2009;21:3286–3306. doi: 10.1002/adma.200802453. [DOI] [PubMed] [Google Scholar]
- 14.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui SW, Langner M, Zhao YL, Ross P, Hurley E, et al. The role of helper lipids in cationic liposome-mediated gene transfer. Biophys J. 1996;71:590–599. doi: 10.1016/S0006-3495(96)79309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thierry AR, Rabinovich P, Peng B, Mahan LC, Bryant JL, et al. Characterization of liposome-mediated gene delivery: expression, stability and pharmacokinetics of plasmid DNA. Gene Ther. 1997;4:226–237. doi: 10.1038/sj.gt.3300350. [DOI] [PubMed] [Google Scholar]
- 17.Simberg D, Danino D, Talmon Y, Minsky A, Ferrari ME, et al. Phase behavior, DNA ordering, and size instability of cationic lipoplexes. Relevance to optimal transfection activity. J Biol Chem. 2001;276:47453–47459. doi: 10.1074/jbc.M105588200. [DOI] [PubMed] [Google Scholar]
- 18.Ewert K, Ahmad A, Evans HM, Schmidt HW, Safinya CR. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J Med Chem. 2002;45:5023–5029. doi: 10.1021/jm020233w. [DOI] [PubMed] [Google Scholar]
- 19.Koynova R, MacDonald RC. Lipid transfer between cationic vesicles and lipid-DNA lipoplexes: effect of serum. Biochim Biophys Acta. 2005;1714:63–70. doi: 10.1016/j.bbamem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Zelphati O, Szoka FC., Jr Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci U S A. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciani L, Ristori S, Salvati A, Calamai L, Martini G. DOTAP/DOPE and DC-Chol/DOPE lipoplexes for gene delivery: zeta potential measurements and electron spin resonance spectra. Biochim Biophys Acta. 2004;1664:70–79. doi: 10.1016/j.bbamem.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Bajaj A, Kondiah P, Bhattacharya S. Design, synthesis, and in vitro gene delivery efficacies of novel cholesterol-based gemini cationic lipids and their serum compatibility: a structure-activity investigation. J Med Chem. 2007;50:2432–2442. doi: 10.1021/jm0611253. [DOI] [PubMed] [Google Scholar]
- 23.Kirby AJ, Camilleri P, Engberts JB, Feiters MC, Nolte RJ, et al. Gemini surfactants: new synthetic vectors for gene transfection. Angew Chem Int Ed Engl. 2003;42:1448–1457. doi: 10.1002/anie.200201597. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya S, De S, George SK. Synthesis and vesicle formation from novel pseudoglyceryl dimeric lipids. Evidence of formation of widely different membrane organizations with exceptional thermotropic properties. Chemical Communications. 1997:2287–2288. [Google Scholar]
- 25.Bhattacharya S, De S. Synthesis and vesicle formation from dimeric pseudoglyceryl lipids with (CH2)(m) spacers: pronounced m-value dependence of thermal properties, vesicle fusion, and cholesterol complexation. Chemistry-a European Journal. 1999;5:2335–2347. [Google Scholar]
- 26.McGregor C, Perrin C, Monck M, Camilleri P, Kirby AJ. Rational approaches to the design of cationic gemini surfactants for gene delivery. J Am Chem Soc. 2001;123:6215–6220. doi: 10.1021/ja005681c. [DOI] [PubMed] [Google Scholar]
- 27.Candiani G, Frigerio M, Viani F, Verpelli C, Sala C, et al. Dimerizable redox-sensitive triazine-based cationic lipids for in vitro gene delivery. ChemMedChem. 2007;2:292–296. doi: 10.1002/cmdc.200600267. [DOI] [PubMed] [Google Scholar]
- 28.Bombelli C, Faggioli F, Luciani P, Mancini G, Sacco MG. Efficient transfection of DNA by liposomes formulated with cationic gemini amphiphiles. J Med Chem. 2005;48:5378–5382. doi: 10.1021/jm050477r. [DOI] [PubMed] [Google Scholar]
- 29.Candiani G, Pezzoli D, Cabras M, Ristori S, Pellegrini C, et al. A dimerizable cationic lipid with potential for gene delivery. J Gene Med. 2008;10:637–645. doi: 10.1002/jgm.1186. [DOI] [PubMed] [Google Scholar]
- 30.Huynh GH, Deen DF, Szoka FC. Barriers to carrier mediated drug and gene delivery to brain tumors. Journal of Controlled Release. 2006;110:236–259. doi: 10.1016/j.jconrel.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 31.MacKay JA, Li W, Huang Z, Dy EE, Huynh G, et al. HIV TAT peptide modifies the distribution of DNA nanolipoparticles following convection-enhanced delivery. Molecular Therapy. 2008;16:893–900. doi: 10.1038/mt.2008.36. [DOI] [PubMed] [Google Scholar]
- 32.Congiu A, Pozzi D, Esposito C, Castellano C, Mossa G. Correlation between structure and transfection efficiency: a study of DC-Chol--DOPE/DNA complexes. Colloids Surf B Biointerfaces. 2004;36:43–48. doi: 10.1016/j.colsurfb.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Yang JP, Huang L. Overcoming the inhibitory effect of serum on lipofection by increasing the charge ratio of cationic liposome to DNA. Gene Ther. 1997;4:950–960. doi: 10.1038/sj.gt.3300485. [DOI] [PubMed] [Google Scholar]
- 34.Boomer JA, Thompson DH, Sullivan SM. Formation of plasmid-based transfection complexes with an acid-labile cationic lipid: characterization of in vitro and in vivo gene transfer. Pharm Res. 2002;19:1292–1301. doi: 10.1023/a:1020342523694. [DOI] [PubMed] [Google Scholar]
- 35.Audouy S, Molema G, de Leij L, Hoekstra D. Serum as a modulator of lipoplex-mediated gene transfection: dependence of amphiphile, cell type and complex stability. J Gene Med. 2000;2:465–476. doi: 10.1002/1521-2254(200011/12)2:6<465::AID-JGM141>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 36.Bagnacani V, Sansone F, Donofrio G, Baldini L, Casnati A, et al. Macrocyclic nonviral vectors: high cell transfection efficiency and low toxicity in a lower rim guanidinium calix[4]arene. Org Lett. 2008;10:3953–3956. doi: 10.1021/ol801326d. [DOI] [PubMed] [Google Scholar]
- 37.Kim A, Lee EH, Choi SH, Kim CK. In vitro and in vivo transfection efficiency of a novel ultradeformable cationic liposome. Biomaterials. 2004;25:305–313. doi: 10.1016/s0142-9612(03)00534-9. [DOI] [PubMed] [Google Scholar]
- 38.Walker WE, Porteous DJ, Boyd AC. The effects of plasmid copy number and sequence context upon transfection efficiency. J Control Release. 2004;94:245–252. doi: 10.1016/j.jconrel.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 40.Caracciolo G, Pozzi D, Caminiti R, Marchini C, Montani M, et al. Transfection efficiency boost by designer multicomponent lipoplexes. Biochim Biophys Acta. 2007;1768:2280–2292. doi: 10.1016/j.bbamem.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 41.Caracciolo G, Pozzi D, Caminiti R, Marchini C, Montani M, et al. Enhanced transfection efficiency of multicomponent lipoplexes in the regime of optimal membrane charge density. J Phys Chem B. 2008;112:11298–11304. doi: 10.1021/jp803077n. [DOI] [PubMed] [Google Scholar]
- 42.Litzinger DC, Huang L. Phosphatidylethanolamine liposomes: drug delivery, gene transfer and immunodiagnostic applications. Biochim Biophys Acta. 1992;1113:201–227. doi: 10.1016/0304-4157(92)90039-d. [DOI] [PubMed] [Google Scholar]
- 43.Shangguan T, Cabral-Lilly D, Purandare U, Godin N, Ahl P, et al. A novel N-acyl phosphatidylethanolamine-containing delivery vehicle for spermine-condensed plasmid DNA. Gene Ther. 2000;7:769–783. doi: 10.1038/sj.gt.3301156. [DOI] [PubMed] [Google Scholar]
- 44.Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- 45.Pinnaduwage P, Schmitt L, Huang L. Use of a quaternary ammonium detergent in liposome mediated DNA transfection of mouse L-cells. Biochim Biophys Acta. 1989;985:33–37. doi: 10.1016/0005-2736(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 46.Manickam DS, Hirata A, Putt DA, Lash LH, Hirata F, et al. Overexpression of Bcl-2 as a proxy redox stimulus to enhance activity of non-viral redox-responsive delivery vectors. Biomaterials. 2008;29:2680–2688. doi: 10.1016/j.biomaterials.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 48.Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- 49.Manickam DS, Li J, Putt DA, Zhou QH, Wu C, et al. Effect of innate glutathione levels on activity of redox-responsive gene delivery vectors. J Control Release. 2010;141:77–84. doi: 10.1016/j.jconrel.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeong JH, Kim SH, Christensen LV, Feijen J, Kim SW. Reducible poly(amido ethylenimine)-based gene delivery system for improved nucleus trafficking of plasmid DNA. Bioconjug Chem. 2010;21:296–301. doi: 10.1021/bc9003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turanov AA, Kehr S, Marino SM, Yoo MH, Carlson BA, et al. Mammalian thioredoxin reductase 1: roles in redox homeostasis and charcterization of cellular targets. Biochem J. 2010 doi: 10.1042/BJ20091378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Limon-Pacheco JH, Hernandez NA, Fanjul-Moles ML, Gonsebatt ME. Glutathione depletion activates mitogen-activated protein kinase (MAPK) pathways that display organ-specific responses and brain protection in mice. Free Radic Biol Med. 2007;43:1335–1347. doi: 10.1016/j.freeradbiomed.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Gilge JL, Fisher M, Chai YC. The effect of oxidant and the non-oxidant alteration of cellular thiol concentration on the formation of protein mixed-disulfides in HEK 293 cells. PLoS One. 2008;3:e4015. doi: 10.1371/journal.pone.0004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Small-Howard A, Yin A, Berry MJ. The responses of Ht22 cells to oxidative stress induced by buthionine sulfoximine (BSO). BMC Neurosci. 2005;6:10. doi: 10.1186/1471-2202-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adamy C, Mulder P, Khouzami L, Andrieu-abadie N, Defer N, et al. Neutral sphingomyelinase inhibition participates to the benefits of N-acetylcysteine treatment in post-myocardial infarction failing heart rats. J Mol Cell Cardiol. 2007;43:344–353. doi: 10.1016/j.yjmcc.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 57.Adamy C, Le Corvoisier P, Candiani G, Kirsch M, Pavoine C, et al. Tumor necrosis factor alpha and glutathione interplay in chronic heart failure. Arch Mal Coeur Vaiss. 2005;98:906–912. [PubMed] [Google Scholar]
- 58.Shang F, Lu M, Dudek E, Reddan J, Taylor A. Vitamin C and vitamin E restore the resistance of GSH-depleted lens cells to H2O2. Free Radic Biol Med. 2003;34:521–530. doi: 10.1016/s0891-5849(02)01304-7. [DOI] [PubMed] [Google Scholar]
- 59.Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 60.Damy T, Kirsch M, Khouzami L, Caramelle P, Le Corvoisier P, et al. Glutathione deficiency in cardiac patients is related to the functional status and structural cardiac abnormalities. PLoS One. 2009;4:e4871. doi: 10.1371/journal.pone.0004871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao JK, Dougherty GG, Jr, Reddy RD, Keshavan MS, Montrose DM, et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naive patients with schizophrenia. PLoS One. 2010;5:e9508. doi: 10.1371/journal.pone.0009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, Neil J, Reis I, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002;8:3658–3668. [PubMed] [Google Scholar]
- 63.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 64.Ray G, Batra S, Shukla NK, Deo S, Raina V, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 2000;59:163–170. doi: 10.1023/a:1006357330486. [DOI] [PubMed] [Google Scholar]
- 65.Skrzydlewska E, Kozuszko B, Sulkowska M, Bogdan Z, Kozlowski M, et al. Antioxidant potential in esophageal, stomach and colorectal cancers. Hepatogastroenterology. 2003;50:126–131. [PubMed] [Google Scholar]
- 66.Kretzschmar M. Regulation of hepatic glutathione metabolism and its role in hepatotoxicity. Exp Toxicol Pathol. 1996;48:439–446. doi: 10.1016/S0940-2993(96)80054-6. [DOI] [PubMed] [Google Scholar]
- 67.Ohashi T, Mizutani A, Murakami A, Kojo S, Ishii T, et al. Rapid oxidation of dichlorodihydrofluorescin with heme and hemoproteins: formation of the fluorescein is independent of the generation of reactive oxygen species. FEBS Lett. 2002;511:21–27. doi: 10.1016/s0014-5793(01)03262-8. [DOI] [PubMed] [Google Scholar]