Abstract
BACKGROUND
Animal studies have shown that in utero exposure to chemicals in tobacco smoke reduces female fertility, but epidemiological findings have been inconsistent.
METHODS
We examined the association between in utero exposure to tobacco smoke and female fertility among women in the Norwegian Mother and Child Cohort Study, enrolled from 1999 to 2007. Around the 17th week of pregnancy, participants reported how long they took to conceive (time to pregnancy), and whether their mother smoked while pregnant with the participant. This analysis included 48 319 planned pregnancies among women aged 15–44 years. We estimated fecundability odds ratios (FORs) using a discrete-time survival analysis, adjusting for age, education and adult tobacco smoking.
RESULTS
The adjusted FOR for in utero exposure to tobacco smoke among all subjects was 0.96 [95% confidence interval (CI): 0.93, 0.98], among subjects reporting no adult tobacco smoking or passive exposure it was 0.96 (95% CI: 0.93, 0.99) and among subjects reporting adult tobacco smoking or passive exposure it was 0.95 (95% CI: 0.91, 0.99). We performed a probabilistic sensitivity analysis to estimate the effect of exposure and outcome misclassification on the results, and, as expected, the association became more pronounced after taking misclassification into account.
CONCLUSIONS
This large cohort study supports a small-to-modest association between in utero exposure to tobacco smoke and reduced fertility.
Keywords: tobacco smoking, in utero exposure, fertility
Introduction
Smoking is a well-established risk factor for many human health conditions, including impaired reproduction (CDC, 2004a). Women who smoke have an increased risk of subfertility, infertility, pregnancy loss, preterm delivery and of giving birth to an infant who is small-for-gestational age (Augood et al., 1998; Kharrazi et al., 2004; Wilks and Hay, 2004; Triche and Hossain, 2007). About 10–30% of women in western countries smoke during pregnancy (CDC, 2004b; Egebjerg Jensen et al., 2008). The effects of passive smoking on reproductive outcomes are less clear (CDC, 2006). Animal studies, however, have shown that in utero exposure to smoking-related chemicals reduces female fertility. For example, mice exposed in utero to polycyclic aromatic hydrocarbons had fewer and smaller litters (MacKenzie and Angevine, 1981). In rats, in utero exposure to nicotine caused decreased ovarian function and increased time to pregnancy (TTP) (Holloway et al., 2007). Epidemiological studies on the association between a mother's smoking during pregnancy and her daughter's fertility have, however, yielded inconsistent findings, which may in part be related to limited sample size (Baird and Wilcox, 1986; Weinberg et al., 1989; Jensen et al., 1998, 2006; Joffe and Barnes, 2000). To examine the association between a mother's smoking during pregnancy and a daughter's fertility in a large study, we analyzed data from a pregnancy cohort in Norway.
Materials and Methods
The Norwegian Mother and Child Cohort Study
This study is based on the Norwegian Mother and Child Cohort Study (MoBa), conducted by the Norwegian Institute of Public Health (Magnus et al., 2006). Enrollment was from 1999 to 2008 and about 107 000 pregnancies among 90 000 women were included. The present study is based on the 90 190 pregnancies recruited from 1999 to 2007 whose data appeared in the MoBa version 4.201 data set. The majority of all pregnant women in Norway were invited to participate, and the response rate was about 44%. During weeks 17–18 of gestation, participants were asked to complete a questionnaire about demographic characteristics, reproductive health, disease and medication history, lifestyle and socioeconomic status. The Regional Committee for Medical Research and the Norwegian Data Inspectorate approved the study, and informed consent was obtained from each participant.
In utero exposure to tobacco smoking
Women were asked: ‘Did your mother smoke when she was pregnant with you?’ Women could answer ‘yes’, ‘no’ or ‘don't know’. Those who answered ‘yes’ to this question were classified as having been exposed to tobacco smoke while in utero; those who responded ‘no’ were considered unexposed.
Time to pregnancy
TTP is a measure of a couple's ability to conceive with regular intercourse and no use of birth control (Baird et al., 1986). To ascertain TTP, women were first asked: ‘Was this pregnancy planned?’ Those who planned their pregnancy were further asked: ‘How many months did you have regular intercourse without contraception before you became pregnant?’ Women could choose one of three response options: ‘less than 1 month’, ‘1–2 months’ and ‘3 months or more’. Women choosing the ‘3 months or more’ option were further asked to report the actual number of months. Women were also asked if they had received any infertility treatment in connection with this pregnancy.
Exclusions
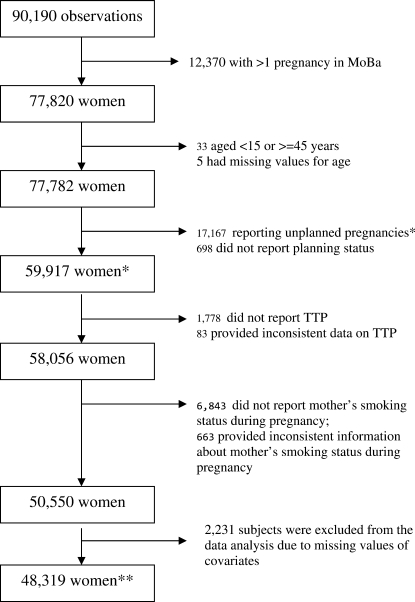
As noted above, data for 90 190 pregnancies were available. The selection of the analysis sample with the exclusion criteria is shown in Fig. 1. We restricted our analysis to the woman's first MoBa pregnancy. Women who met at least one of the following criteria were excluded from the primary analysis: (i) age <15 or >44 years old; (ii) reported an unplanned pregnancy (including those who reported contraceptive failures) or did not report this information; (iii) did not report TTP or reported TTP inconsistently (TTP was considered as inconsistent if, for example, a woman reported that she became pregnant within ‘less than 1 month’ or ‘1–2 months’ but then answered ≥3 to the next question [‘number of month if more than 3’]); and (iv) did not report her mother's smoking when pregnant (question not answered or answered ‘don't know’) or reported inconsistent information. The reported information on the mother's smoking was considered inconsistent if a woman was enrolled for more than one pregnancy and gave a different answer on two questionnaires. Women with missing values for covariates (n = 2231) were excluded from the final data analysis as described below. Overall, 48 319 women met the criteria for inclusion in the primary analysis.
Figure 1.
Flowchart for subject selection in the study of the association between in utero exposure to tobacco smoke and female fertility among women in the Norwegian Mother and Child Cohort Study (MoBa), enrolled from 1999 to 2007. *Characteristics of these subjects are shown in Table I. **Characteristics of these women are shown in Table II.
Data analysis
To analyze the association between in utero exposure to tobacco smoke and TTP, the fecundability odds ratio (FOR) was estimated using the logistic regression analog of discrete-time survival analysis (Abbott, 1985) in Stata/SE (Stata Statistical Software, release 10.0; Statacorp, College Station, TX, USA). An FOR < 1 indicates reduced fecundity. Those who reported ‘less than 1 month’ were assigned a TTP of 1 (to indicate that they conceived in the first cycle at risk) and those who reported ‘1–2 months’ were assigned a TTP of 2. Those with a TTP of ≥3 months were assigned the value corresponding to the reported number of months. Among couples who had been trying to conceive for >12 months, TTP was censored at 13 months because the prevalence of infertility treatment is high in such groups (Boivin et al., 2007), and was 45% in these data. Overall, 11% were censored at 13 months. Women who received infertility treatment for the current pregnancy and became pregnant after fewer than 13 months had their TTP censored at TTP − 1 (n = 980).
In the adjusted analysis, a priori we included subject's age, education level and adult tobacco smoking status before pregnancy as categorical variables, as shown in Table I. In addition, we evaluated as potential adjustment factors her partner's smoking status (yes versus no), history of sexually transmitted diseases (yes versus no) and chronic diseases before pregnancy (yes versus no). Starting with a model including all a priori and potential adjustment factors, we used a backward stepwise elimination algorithm to identify those whose omission changed the FOR for in utero smoking by 10% or more (Atashili and Ta, 2007). Elimination of the potential adjustment factors had essentially no effect on the adjusted FOR, and the final model (primary analysis) included only the variables selected a priori. We also examined effect modification by the subject's adult tobacco smoking exposure before pregnancy, using likelihood ratio statistics. Effect modification was considered potentially important if the P-value of the likelihood ratio test was <0.1.
Table I.
Characteristics of pregnancy planners and non-planners in the study of the association between in utero exposure to tobacco smoke and female fertility among women in the Norwegian Mother and Child Cohort Study, enrolled from 1999 to 2007.a
| Variable | Planners (n = 59 917) | Non-planners (n = 17 167) |
|---|---|---|
| Mother smoked when she was pregnant with the subject (%)b | ||
| No | 64.5 | 59.7 |
| Yes | 24.0 | 27.6 |
| Don't know | 10.0 | 10.8 |
| Missing | 1.8 | 1.9 |
| Subject's age at enrollment [mean (SD), years] | 29.8 (4.3) | 28.4 (5.6) |
| Subject's age at enrollment (%) | ||
| <20 | 0.6 | 5.2 |
| 20–24 | 10.0 | 21.3 |
| 25–29 | 37.2 | 30.7 |
| 30–34 | 38.1 | 27.4 |
| 35–39 | 12.9 | 12.9 |
| ≥40 | 1.3 | 2.5 |
| Subject's completed education (%) | ||
| <High school | 1.8 | 6.1 |
| High school | 26.7 | 38.9 |
| College or university | 62.1 | 41.1 |
| Other education | 6.4 | 7.6 |
| Missing | 3.1 | 6.2 |
| Subject's adult tobacco smoking before pregnancy (%) | ||
| None | 64.7 | 48.9 |
| Active smoking only | 22.4 | 30.0 |
| Passive smoking only | 5.0 | 6.4 |
| Both | 5.3 | 11.2 |
| Missing | 2.7 | 3.4 |
aAll differences between pregnancy planners and non-planners shown in the table were statistically significant (χ2 or t-test) at the P < 0.001 level. b663 women who reported inconsistent information on this item were excluded for this comparison.
We performed a probabilistic sensitivity analysis to evaluate and attempt to correct for bias owing to non-differential misclassification of exposure and outcome (Fox et al., 2005). A description of the specific technical details employed in this sensitivity analysis is given in the Supplementary data. The median and 95% simulation (confidence) interval of the corrected distributions are presented.
We conducted sensitivity analyses that have been recommended to assess the potential effect of biases that can influence the results of retrospective studies of TTP (Weinberg et al., 1994; Olsen et al., 1998; Joffe et al., 2005). We fit a logistic model to evaluate whether contraceptive failure was associated with in utero exposure to tobacco smoke (Joffe et al., 2005). We repeated the primary analysis after (i) including non-planners with imputed TTPs of 1, 2, 3 or 4; (ii) excluding all subjects with a TTP of 1 or 2; (iii) censoring TTP at different thresholds (6, 9, 12 and 14 months); (iv) recalculating TTP in terms of menstrual cycles instead of months (restricted to those reporting regular cycles); and (v) after assigning those who were missing data or who reported ‘do not know’ for in utero smoking (13% of the study population) as either exposed or unexposed.
In other sensitivity analyses, we repeated the primary analysis after (i) adjusting for the use of hormonal contraceptives in the past year (in the subset of women with a TTP < 13 months; (ii) adjusting for parity; (iii) adjusting for amount smoked by the mother before pregnancy (non-smoker, <10, 10–20, 20+ cigarettes/day); and (iv) restricting the analysis to the first MoBa pregnancy that was also the woman's first pregnancy.
Finally, beginning with the 59 917 women with planned pregnancies (Fig. 1), we used multiple imputation with chained equations to impute values that were missing for TTP, in utero smoking, education and prepregnancy exposure to tobacco (Raghunathan et al., 2007). Adjusted FORs for in utero exposure to tobacco smoke based on five imputed data sets were calculated, and the entire procedure was repeated to assess its reliability.
Results
Compared with planners, a higher proportion of non-planners reported in utero exposure to tobacco smoke (Table I). Planners tended to be older than those who had not planned their pregnancies. Sixty-two percent of planners had completed college or university, versus 41% among non-planners. A lower proportion of planners (27%) were exposed to active or passive smoking before the pregnancy than non-planners (36%). The two groups did not differ substantially on other variables (data not shown).
Among planners, the proportion with TTP > 12 months was higher among women with in utero exposure to tobacco smoke (12%) than in those without (11%) (Table II). The average age in the two groups was similar. Compared with unexposed subjects, women exposed prenatally to tobacco smoke were less likely to have completed college or university, and a higher proportion of them were exposed to active or passive smoking.
Table II.
Characteristics of planners with and without in utero exposure to tobacco smoking.
| Variable |
In utero exposure to tobacco smoking |
|
|---|---|---|
| No (n = 35 195) | Yes (n = 13 124) | |
| Time to pregnancy 13 months or more (%) | 10.8 | 12.0 |
| Age at enrollment [mean (SD), years] | 30.0 (4.2) | 29.6 (4.3) |
| Age at enrollment (%) | ||
| <20 | 0.4 | 0.8 |
| 20–24 | 8.9 | 11.0 |
| 25–29 | 36.8 | 37.3 |
| 30–34 | 38.9 | 38.1 |
| 35–39 | 13.5 | 11.9 |
| ≥40 | 1.5 | 0.9 |
| Completed education (%) | ||
| <High school | 1.3 | 2.8 |
| High school | 23.5 | 35.4 |
| College or university | 69.3 | 54.4 |
| Other education | 5.9 | 7.5 |
| Adult tobacco smoking before pregnancy (%) | ||
| None | 71.3 | 56.1 |
| Active smoking only | 20.1 | 28.9 |
| Passive smoking only | 4.6 | 5.9 |
| Both | 3.7 | 9.1 |
The unadjusted FOR for exposure to in utero tobacco smoke among all subjects was 0.93 [95% confidence interval (CI): 0.91, 0.96), and the adjusted FOR was 0.96 (95% CI: 0.93, 0.98) (Table III). We saw no effect modification between a subject's adult tobacco smoking history before pregnancy and in utero tobacco smoke exposure (likelihood ratio test, P = 0.65, 3 degrees of freedom). Regardless of the non-significance of the effect modification test, we stratified the analysis by the subject's adult tobacco smoking before pregnancy (two categories: no adult tobacco exposure, with adult tobacco exposure including passive smoking) because subjects exposed in utero had higher proportions of exposure to adult tobacco smoking. The association among those exposed in utero but without any adult exposure would be less likely to be biased by residual confounding by adult smoking. The adjusted FORs for those who had no adult tobacco smoking exposure was 0.96 (95% CI: 0.93, 0.99) and for those who had adult tobacco smoking exposure it was 0.95 (95% CI: 0.91, 0.99).
Table III.
Fecundability odds ratios (FORs) associated with in utero exposure to tobacco smoking (yes versus no) before and after probabilistic sensitivity analysis.
| Model | Conventional analysis |
After analysis accounting for |
||||
|---|---|---|---|---|---|---|
| Exposure misclassification |
Outcome misclassification |
|||||
| FORsc | 95% CIc | FORs | 95% CI | FORs | 95% CI | |
| Unadjusted model (all subjects, n = 48 319) | 0.93 | 0.91, 0.96 | 0.90 | 0.87, 0.93 | 0.89 | 0.82, 0.95 |
| Adjusted modela (all subjects, n = 48 319) | 0.96 | 0.93, 0.98 | 0.92 | 0.88, 0.95 | 0.89 | 0.80, 0.94 |
| Adjusted model,b stratified by subject's adult tobacco smoking history before pregnancy | ||||||
| No | 0.96 | 0.93, 0.99 | 0.94 | 0.89, 0.98 | 0.92 | 0.86, 0.97 |
| Yes | 0.95 | 0.91, 0.99 | 0.91 | 0.86, 0.95 | 0.87 | 0.74, 0.94 |
aAdjusted for woman's age, completed education level and adult tobacco smoking before pregnancy. bAdjusted for woman's age and completed education level. cFORs, fecundability odds ratios; CI, confidence interval.
After taking into account exposure misclassification, the FOR for all subjects was 0.92 (95% CI: 0.88, 0.95), for subjects without adult tobacco smoking exposure it was 0.94 (95% CI: 0.89, 0.98) and for subjects who had a history of such exposure it was 0.91 (95% CI: 0.86, 0.95) (Table III). The association also became more pronounced after taking into account outcome (TTP) misclassification.
The odds of contraceptive failure were unrelated to in utero exposure to tobacco smoke (data not shown). The association between TTP and in utero exposure to tobacco smoking was slightly attenuated when we included non-planners in the analysis [e.g. adjusted FOR = 0.97 (95% CI: 0.95, 0.99), after imputation of non-planners' TTP as 1 month]. The other sensitivity analyses also indicated that the results were robust. For example, the estimated association was virtually the same when we restricted the analyses to first pregnancies (not shown), and when we assigned the 7506 women who provided an uncertain answer about in utero exposure to tobacco smoke as exposed or unexposed (data not shown). Using different censoring thresholds or adjusting for additional variables did not change the association (not shown).
Discussion
In the present study, we found a small-to-modest association between in utero exposure to tobacco smoke and reduced fertility. As noted earlier, the findings of previous epidemiological studies on the association between in utero exposure to tobacco smoke and fertility have been inconsistent (Table IV) (Baird and Wilcox, 1986; Weinberg et al., 1989; Jensen et al., 1998, 2006; Joffe and Barnes, 2000). The reason for the inconsistency remains unclear, although in both of the prospective studies a reduced fecundability was found (Weinberg et al., 1989; Jensen et al., 1998). Although we also found reduced fecundability, the magnitude of the association was small enough that residual confounding could account for it. Once the effect of misclassification was considered, however, the possibility of a true effect was more evident.
Table IV.
A summary of epidemiological studies on in utero exposure to tobacco smoke and female fertility.
| Author | Location | Sample size | Smoking status reporter | TTP data collected | FORsa,b | 95% CIb |
|---|---|---|---|---|---|---|
| Baird and Wilcox (1986) | USA/Minnesota | 663 | Daughter | Rc, in months | 1.0 | 0.9, 1.2 |
| Weinberg et al. (1989) | USA/North Carolina | 221 | Daughter | Pc, in cycles | 0.5 | 0.4, 0.8 |
| Jensen et al. (1998) | Denmark | 423 | Daughter | P, in cycles | 0.64d | 0.47, 0.87d |
| Joffe and Barnes (2000) | UK | 2587 | Mother (at the time of delivery) | R, in months | 1.02 | 0.92, 1.13 |
| Jensen et al. (2006) | Denmark | 1653e | Daughter (20%) and mother (80%) | R, in months | 0.81 | 0.65, 1.02 |
| Present study | Norway | 48 319 | Daughter | R, in months | 0.96 | 0.93 0.98 |
aFor early life smoking exposure: yes versus no. bFORs, fecundability odds ratios; CI, confidence interval. cR, retrospectively; P, prospectively. dWeighted average of stratified FORs (0.70 and 0.53) given in their Table 2. eTwins.
As noted earlier, animal studies provide some evidence for mechanisms through which in utero exposure to tobacco smoke could cause reduced fertility in females. Prenatal exposure to cigarette smoke or polycyclic aromatic hydrocarbons (important toxicants in cigarette smoke) induced fetal ovarian germ cell loss, resulting in a significant loss of primordial follicles in mice (Vahakangas et al., 1985; Matikainen et al., 2002). Prenatal exposure to nicotine resulted in subsequent ovarian dysfunction and increased TTP in adult female rat offspring (Holloway et al., 2006). Furthermore, other toxic components in cigarette smoke may have similar effects (Miller et al., 2004; Rogers, 2008). In utero tobacco smoke exposure has recently been associated with earlier age at menopause (Strohsnitter et al., 2008). This finding provides additional support for an adverse effect of in utero smoke exposure on female reproduction.
A daughter's report about her mother's smoking status during pregnancy has been previously shown to be reasonably reliable and valid (Sandler and Shore, 1986; Coultas et al., 1989; Sanderson et al., 1998; Simard et al., 2008). Retrospective TTP data have also been shown to be fairly accurate (Zielhuis et al., 1992). The data on the validity of the measures employed suggested that the effect of non-differential misclassification on our results would be modest. The results of the probabilistic sensitivity analysis verified this.
Our additional sensitivity analyses to assess other potential sources of bias (Weinberg et al., 1994; Olsen et al., 1998; Joffe et al., 2005) did not suggest any substantial differences in the associations between in utero exposure to smoke and TTP when planning status, infertility treatment history, TTP cutoffs or unknown reports for in utero exposure were accounted for, suggesting that the effect of these biases was probably minor in the present study.
Nonetheless, our questionnaire-based study had several limitations. A daughter's reports about her mother's smoking status during pregnancy have not been validated with biomarkers of the mother's tobacco exposure. Some evidence from biomarker-based studies suggests underreporting of smoking during pregnancy (Shipton et al., 2009), and this would attenuate observed associations. We did not have data on the number of cigarettes smoked per day by any subject's mother or information about the subjects' childhood exposure. Studies have shown that most mothers who smoked during pregnancy continued smoking after pregnancy (Weinberg et al., 1989; Simard et al., 2008), suggesting that adjusting for childhood exposure may lessen the association of in utero smoke exposure with fertility. On the other hand, the production of oocytes occurs only during fetal life and this may be a critical window of susceptibility (Pryor et al., 2000). Although retrospective reports of TTP are good (Zielhuis et al., 1992), they are not perfect. Furthermore, we cannot rule out the possibility that differential misclassification affected our findings. Finally, selection bias may have occurred owing to the exclusion of sterile women and pregnancies ending before participation (about the 17th week of gestation), possibly causing an underestimation of effect (Weinberg and Wilcox, 2008).
In summary, we observed a small-to-modest association between in utero exposure to tobacco smoke and reduced fertility in this large cohort study, and the association was more pronounced after accounting for exposure and outcome misclassification.
Authors' roles
X.Y. participated in the planning of the analysis, analysis and interpretation of the data and drafted and revised the manuscript. O.B. took part in the conception, planning and interpretation of the analysis, and writing of the manuscript. D.B. took part in the interpretation of the analysis and writing of the manuscript. R.S. and K.H. are investigators with the MoBa, and participated in the planning of and conducting this cohort study, and writing of the manuscript. M.E. and L.A.C.U. participated in the interpretation of results and writing of the manuscript. M.P.L. participated in the conception, design and planning of the study, and supervised the data analysis, interpretation of results and preparation and revision of the manuscript. All authors saw and approved the final version of the manuscript.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Funding
This study was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, NIH/NIEHS (grant no. N01-ES-85433), NIH/NINDS (grant no. 1 UO1 NS 047537-01) and the Norwegian Research Council/FUGE (grant no. 151918/S10).
Supplementary Material
Acknowledgements
We thank Dr Tim Lash from the University of Boston for providing his probabilistic bias analysis SAS program. Government Department: National Institutes of Health, Department of Health and Human Services.
References
- Abbott RD. Logistic regression in survival analysis. Am J Epidemiol. 1985;121:465–471. doi: 10.1093/oxfordjournals.aje.a114019. [DOI] [PubMed] [Google Scholar]
- Atashili J, Ta M. A SAS macro for automating the ‘change-in-estimate’ strategy for assessing confounding. 2007. SAS Global Forum, Coder's Corner, paper 032-2007http://www.2.sas.com/proceedings/forum2007/032-2007.pdf. (22 February 2010, date last accessed) [Google Scholar]
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–1539. doi: 10.1093/humrep/13.6.1532. doi:10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ. Future fertility after prenatal exposure to cigarette smoke. Fertil Steril. 1986;46:368–372. [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Hum Reprod. 2007;22:1506–1512. doi: 10.1093/humrep/dem046. doi:10.1093/humrep/dem046. [DOI] [PubMed] [Google Scholar]
- CDC. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: Centers for Diseases Control and Prevention, Department of Human and Health Services; 2004a. http://www.surgeongeneral.gov/library/smokingconsequences/ (3 November 2008, date last accessed) [PubMed] [Google Scholar]
- CDC. Smoking during pregnancy–United States, 1990–2002. MMWR. 2004b;53:911–915. [PubMed] [Google Scholar]
- CDC. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Centers for Diseases Control and Prevention, Department of Human and Health Services; 2006. http://www.surgeongeneral.gov/library/secondhandsmoke/ (3 November 2008, date last accessed) [PubMed] [Google Scholar]
- Coultas DB, Peake GT, Samet JM. Questionnaire assessment of lifetime and recent exposure to environmental tobacco smoke. Am J Epidemiol. 1989;130:338–347. doi: 10.1093/oxfordjournals.aje.a115340. [DOI] [PubMed] [Google Scholar]
- Egebjerg Jensen K, Jensen A, Nøhr B, Kruger Kjaer S. Do pregnant women still smoke? A study of smoking patterns among 261,029 primiparous women in Denmark 1997–2005. Acta Obstet Gynecol Scand. 2008;87:760–767. doi: 10.1080/00016340802179814. doi:10.1080/00016340802179814. [DOI] [PubMed] [Google Scholar]
- Fox MP, Lash TL, Greenland S. A method to automate probabilistic sensitivity analyses of misclassified binary variables. Int J Epidemiol. 2005;34:1370–1376. doi: 10.1093/ije/dyi184. doi:10.1093/ije/dyi184. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Kellenberger LD, Petrik JJ. Fetal and neonatal exposure to nicotine disrupts ovarian function and fertility in adult female rats. Endocrine. 2006;30:213–216. doi: 10.1385/ENDO:30:2:213. doi:10.1385/ENDO:30:2:213. [DOI] [PubMed] [Google Scholar]
- Holloway AC, Cuu DQ, Morrison KM, Gerstein HC, Tarnopolsky MA. Transgenerational effects of fetal and neonatal exposure to nicotine. Endocrine. 2007;31:254–259. doi: 10.1007/s12020-007-0043-6. doi:10.1007/s12020-007-0043-6. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Henriksen TB, Hjollund NH, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Adult and prenatal exposures to tobacco smoke as risk indicators of fertility among 430 Danish couples. Am J Epidemiol. 1998;148:992–997. doi: 10.1093/oxfordjournals.aje.a009576. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Joffe M, Scheike T, Skytthe A, Gaist D, Petersen I, Christensen K. Early exposure to smoking and future fecundity among Danish twins. Int J Androl. 2006;29:603–613. doi: 10.1111/j.1365-2605.2006.00701.x. doi:10.1111/j.1365-2605.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Joffe M, Barnes I. Do parental factors affect male and female fertility? Epidemiology. 2000;11:700–705. doi: 10.1097/00001648-200011000-00015. doi:10.1097/00001648-200011000-00015. [DOI] [PubMed] [Google Scholar]
- Joffe M, Key J, Best N, Keiding N, Scheike T, Jensen TK. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;62:115–124. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]
- Kharrazi M, DeLorenze GN, Kaufman FL, Eskenazi B, Bernert JT, Jr, Graham S, Pearl M, Pirkle J. Environmental tobacco smoke and pregnancy outcome. Epidemiology. 2004;15:660–670. doi: 10.1097/01.ede.0000142137.39619.60. doi:10.1097/01.ede.0000142137.39619.60. [DOI] [PubMed] [Google Scholar]
- MacKenzie KM, Angevine DM. Infertility in mice exposed in utero to benzo(a)pyrene. Biol Reprod. 1981;24:183–191. doi: 10.1095/biolreprod24.1.183. doi:10.1095/biolreprod24.1.183. [DOI] [PubMed] [Google Scholar]
- Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C MoBa Study Group. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35:1146–1150. doi: 10.1093/ije/dyl170. doi:10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. doi:10.1210/en.143.2.615. [DOI] [PubMed] [Google Scholar]
- Miller KP, Borgeest C, Greenfeld C, Tomic D, Flaws JA. In utero effects of chemicals on reproductive tissues in females. Toxicol Appl Pharmacol. 2004;198:111–131. doi: 10.1016/j.taap.2003.07.016. doi:10.1016/j.taap.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Olsen J, Juul S, Basso O. Measuring time to pregnancy. Methodological issues to consider. Hum Reprod. 1998;13:1751–1753. doi: 10.1093/humrep/13.7.1751. doi:10.1093/humrep/13.7.1751. [DOI] [PubMed] [Google Scholar]
- Pryor JL, Hughes C, Foster W, Hales BF, Robaire B. Critical windows of exposure for children's health: the reproductive system in animals and humans. Environ Health Perspect. 2000;108(Suppl. 3):491–503. doi: 10.1289/ehp.00108s3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghunathan TE, Solenberger PW, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software. Ann Arbor, MI: Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan; 2007. http://www.isr.umich.edu/src/smp/ive/ (22 February 2010, date last accessed) [Google Scholar]
- Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defects Res C. 2008;84:1–15. doi: 10.1002/bdrc.20119. doi:10.1002/bdrc.20119. [DOI] [PubMed] [Google Scholar]
- Sanderson M, Williams M, White E, Daling JR, Holt VL, Malone KE, Self SG, Moore DE. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- Sandler DP, Shore DL. Quality of data on parents' smoking and drinking provided by adult offspring. Am J Epidemiol. 1986;124:768–778. doi: 10.1093/oxfordjournals.aje.a114453. [DOI] [PubMed] [Google Scholar]
- Simard J, Rosner B, Michels K. Exposure to cigarette smoke in utero: comparison of reports from mother and daughter. Epidemiology. 2008;19:628–633. doi: 10.1097/EDE.0b013e3181761cbd. doi:10.1097/EDE.0b013e3181761cbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. doi:10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohsnitter WC, Hatch EE, Hyer M, Troisi R, Kaufman RH, Robboy SJ, Palmer JR, Titus-Ernstoff L, Anderson D, Hoover RN, et al. The association between in utero cigarette smoke exposure and age at menopause. Am J Epidemiol. 2008;167:727–733. doi: 10.1093/aje/kwm351. doi:10.1093/aje/kwm351. [DOI] [PubMed] [Google Scholar]
- Triche EW, Hossain N. Environmental factors implicated in the causation of adverse pregnancy outcome. Semin Perinatol. 2007;31:240–242. doi: 10.1053/j.semperi.2007.07.013. doi:10.1053/j.semperi.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahakangas K, Rajaniemi H, Pelkonen O. Ovarian toxicity of cigarette smoke exposure during pregnancy in mice. Toxicol Lett. 1985;25:75–80. doi: 10.1016/0378-4274(85)90103-1. doi:10.1016/0378-4274(85)90103-1. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ. Methodologic issues in reproductive epidemiology. In: Rothman K, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd edn. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–1078. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Baird DD, Wilcox AJ. Sources of bias in studies of time to pregnancy. Stat Med. 1994;13:671–681. doi: 10.1002/sim.4780130528. doi:10.1002/sim.4780130528. [DOI] [PubMed] [Google Scholar]
- Wilks DJ, Hay AW. Smoking and female fecundity: the effect and importance of study design. Eur J Obstet Gynecol Reprod Biol. 2004;112:127–135. doi: 10.1016/s0301-2115(03)00332-4. doi:10.1016/S0301-2115(03)00332-4. [DOI] [PubMed] [Google Scholar]
- Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol. 1992;21:1151–1156. doi: 10.1093/ije/21.6.1151. doi:10.1093/ije/21.6.1151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.