Abstract
BACKGROUND
The birth of a boy is significantly more common than a girl prior to secondary recurrent miscarriage (SRM) and is associated with a poorer chance of a subsequent live birth. Children born after SRM are more likely to be girls. High-titer antisera specific for male antigens (H-Y) have been shown to arrest development of male bovine embryos efficiently. We consequently questioned the role of H-Y antibodies in women with SRM.
METHODS
Serum samples from patients with unexplained SRM (n = 84), unexplained primary recurrent miscarriage (PRM) (n = 12) and healthy women (n = 37) were obtained. The samples were taken during pregnancy (gestational weeks 4–5) for 77 (80%) of the patients. Enzyme-linked immunosorbent assay was used to detect immunoglobulin G antibodies that specifically recognized any of the five recombinant H-Y proteins (EIF1AY, RPS4Y1, ZFY, DDX3Y and UTY) and their H-X homologs.
RESULTS
H-Y-specific antibodies were more frequent in SRM patients (46%) compared with female controls (19%, P = 0.004) and PRM patients (8%, P = 0.01). The presence of H-Y antibodies in early pregnancy was associated with a low male: female birth ratio among the subsequent live births, as only 12% of children born to H-Y antibody-positive patients were boys compared with 44% boys born to H-Y antibody negative patients (P = 0.03).
CONCLUSIONS
The high frequency of H-Y antibody-positive SRM patients and the association between the presence of these antibodies in early pregnancy and the low number of male offspring, suggest that maternal immune responses against H-Y antigens can cause pregnancy losses. Further exploring these mechanisms may increase our understanding of unexplained SRM.
Keywords: Secondary recurrent miscarriage, H-Y antibodies, pregnancy outcome, sex ratio, H-Y proteins
Introduction
Secondary recurrent miscarriage (SRM) is defined as three or more consecutive miscarriages after the birth of a child and applies to ∼40% of patients suffering recurrent miscarriages (Jivraj et al., 2001). A possible explanation for the miscarriages is identified in less than half of the cases following standard investigation (Quenby and Farquharson, 1993). Some of these unexplained miscarriages are the result of chromosomal abnormalities that are incompatible with survival (Morales et al., 2008). Supposedly, more SRMs than primary recurrent miscarriages (PRMs) are caused by non-chromosomal causes since the frequency of abnormal embryonic karyotypes is significantly lower in patients with SRM compared with PRM (Coulam et al., 1996).
Based on a 20-year cohort of unexplained SRM patients seen in our tertiary center for recurrent pregnancy losses, we have observed an excess of boys born prior to SRM, whereas an excess of girls has been born after the diagnosis (sex ratio 1.49 versus 0.76; Nielsen et al., 2010b). Furthermore, the chance of a subsequent live birth is significantly reduced in patients with a firstborn boy compared with a girl (Christiansen et al., 2004; Nielsen et al., 2008a), but this association applies only to patients carrying HLA class II alleles, restricting immune responses to male-specific H-Y antigens (Nielsen et al., 2009).
H-Y antigens are encoded by a number of genes on the Y-chromosome and are ubiquitously expressed by male cells, including fetal and trophoblast cells (Warren et al., 2000). Maternal immune recognition of H-Y antigens can be demonstrated in women following pregnancies with boys (Verdijk et al., 2004). In the non-physiological situation of hematopoietic stem cell (SC) transplantation, recipients of parous female SC grafts are more prone to develop graft-versus-host disease (GvHD) than recipients of either nulliparous female SC grafts or male SC grafts (Kollman et al., 2001; Loren et al., 2006). This increased risk of GvHD is believed to be due to the frequent development of a cytotoxic immune response against H-Y antigens (Gratwohl et al., 2001). H-Y antibodies also develop significantly more often in male recipients with female SC donors compared with male recipients of male donors (Miklos et al., 2004, 2005), and the presence of the H-Y antibodies correlates with chronic GvHD. In addition, H-Y antisera have been shown to be potent in arresting the development of bovine male embryos in contrast to female embryos (Utsumi et al., 1993; Ramalho et al., 2004).
The main objective of this study is to investigate, for the first time, the quantitative and qualitative presence of H-Y antibodies in patients with SRM using an established sensitive enzyme-linked immunosorbent assay (ELISA; Miklos et al., 2005).
Materials and Methods
SRM patients
Patients with unexplained SRM, referred to the Danish recurrent miscarriage clinic from July 2004 to March 2008, were invited to participate in this study. SRM was defined as three or more consecutive losses of intrauterine pregnancies before the 22nd gestational week subsequent to at least one pregnancy of minimum 22 weeks gestation. The miscarriages were considered to be unexplained if the woman had normal uterine anatomy evaluated by hysterosalpingography, hysteroscopy or saline hydrosonography, their menstrual cycles were regular with 21–35-day intervals, they were negative for the lupus anticoagulant, and the couples had normal karyotypes. All pregnancies in the patient's history were confirmed by a positive hCG test of urine or serum, ultrasonic examination and/or histology of aspirated tissue from the uterus, data which were documented in the hospital records or those of the practitioner. Only four of the prior miscarriages had been karyotyped; one 46 XX and three 46 XY. Sixty-eight SRM patients with a boy prior to the series of miscarriages and 16 SRM patients with a firstborn girl were included in the study.
PRM patients
This patient group consisted of 12 patients with unexplained (as specified for the SRM patient group) PRM, defined as 3 or more consecutive losses of intrauterine pregnancies before the 22nd gestational week and no history of live births or stillbirths.
Healthy female controls
The control group consisting of 37 women was recruited by advertising for women without known diseases, who never experienced miscarriages and who had given birth to two (n = 26) or three liveborn boys (n = 5) after uncomplicated pregnancies and births (n = 31), and women who had never been pregnant (n = 6). Among the never pregnant women, two never had sexual intercourse with a man, two had used oral contraceptives since sexual debut and two had a history with years of infertility.
Patients and controls could only participate if they had never received a transplant or a blood transfusion. Table I provides information on all included patients and controls.
Table I.
Characteristics of patients and healthy female controls analyzed in this study of the role of H-Y antibodies in women with SRM.
| SRM patients |
Non-SRM women |
||||
|---|---|---|---|---|---|
| Boy born prior to SRM | Girl born prior to SRM | PRM | Controls two or three boys | Never pregnant controls | |
| n = 68 | n = 16 | n = 12 | n = 31 | n = 6 | |
| Age, years | |||||
| Median | 34 | 34 | 29 | 35 | 25 |
| Range | 22–42 | 25–40 | 24–36 | 26–44 | 16–50 |
| No. births (range) | 1 (1–2) | 1 (1–2) | 0 | 2 or 3 | 0 |
| No. miscarriages median (range) | 4 (3–9) | 4 (3–9) | 4 (3–7) | 0 | 0 |
PRM, primary recurrent miscarriage.
Blood samples from patients and controls
Serum was obtained after peripheral blood sampling in the first pregnancy after referral to the Danish recurrent miscarriage clinic at gestational weeks 4–5 for 77 (80%) patients before i.v. immune globulin (IvIg) treatment was started. Samples from the remaining patients and the controls were obtained when they were not pregnant but no later than 2 years after their last pregnancy. Serum samples were cryopreserved and stored at −80°C until use.
Treatment
Thirty-nine of the pregnant SRM patients were treated with IvIg as soon as pregnancy was noted in week 4. A dose of 24–30 g per treatment was infused weekly 4–5 times, followed by fortnightly infusions until gestational weeks 16–20. No other medical treatment was administered. Patients not treated with IvIg were followed closely with weekly ultrasound scans throughout the first trimester followed by individual follow-up plans.
Pregnancy outcome
Among the 77 pregnant patients in the study, 7 (9%) conceived after assisted reproduction treatment (ART). Information regarding pregnancy outcome for patients who were pregnant at the time of blood sampling was retrieved from our database of April 2009. The database contains information on whether the patient miscarried again or whether the pregnancy ended with a birth. Database information is based on the clinical registrations if the patient miscarried, or questionnaires given to the patients with ongoing pregnancies covering obstetric complications, sex of the newborn and its birth weight.
Detection of H-Y and H-X antibodies by ELISA
Using H-Y antigen expression methods previously published (Miklos et al., 2004), five H-Y genes [EIF1AY (Entrez gene ID:9086); RPS4Y1 (Entrez gene ID:6192); ZFY (Entrez gene ID:7544); DDX3Y (Entrez gene ID:8653) and UTY (Entrez gene ID:7404, 75 kDa fragment encoded by nucleotides 1365-3127)] and their corresponding 86–93% identical X homologs [EIF1AX (Entrez gene ID:1964); RPS4X1 (Entrez gene ID:6191); ZFX (Entrez gene ID:7543); DDX3X (Entrez gene ID:1654) and UTX (Entrez gene ID:7403, 75 kDa fragment encoded by nucleotides 1480-3290)] were reverse-transcribed from male peripheral blood mononuclear cells, PCR amplified with primers derived from GenBank sequences, and complementary DNA sequences were confirmed. Each H-Y and H-X clone was expressed with a C-terminal V5 epitope tag and six histidine residues in Escherichia coli and purified by histidine affinity chromatography. Human immunodeficiency virus (HIV) p24 was expressed and purified in a similar fashion to serve as a negative control for background non-specific binding. Purified H-Y, H-X and HIVp24 proteins were individually diluted to 5.0 µg/ml in carbonate binding buffer before coating 96-well ELISA plates (NUNC Scientific, Rochester, NY, USA) with 50 µl (0.25 µg antigen) per well. Serum samples (diluted 1:50) were tested for the presence of immunoglobulin (Ig) G antibodies reactive with each of the recombinant proteins. The quantity of IgG specific for each protein was measured in optical density (OD) units by absorption at 550–450 nm. Antibody testing was performed in duplicate and the experiments were repeated three times to ensure reproducibility. All testing was undertaken in a blinded setup. Previously established positive serum samples from transplantation studies were included as technical controls (Miklos et al., 2005).
Positivity for H-Y antibodies
Positivity was defined as OD ≥0.1 based on previously tested donors as described in details elsewhere (Miklos et al., 2004, 2005). Non-specific responses were defined as positive responses against both the H-Y and the corresponding H-X protein in contrast to specific responses solely directed at the H-Y protein. All results presented in this article relate to specific H-Y responses.
Detection of H-Y and H-X antibodies by western blotting
Purified H-Y (EIF1AY, RPS4Y, UTY) and H-X (EIF1AX, RPS4X, UTX) proteins (2 µg/lane) were separated by sodium-dodecyl sulphate-polyacrylamide gel electrophoresis and were electrophoretically transferred onto Hybond C+ membranes (Amersham Biosciences, NJ, USA). Proteins were detected with anti-V5 (Invitrogen, CA, USA), or patient sera (1:500). After washing, the membranes were incubated with secondary antibody goat anti-human IgG conjugated to horseradish peroxidase (Jackson Immuno Research Laboratories, West Grove, PA, USA), and visualized by enhanced chemiluminescence (Amersham Biosciences). Fifteen samples were tested by western blot to confirm both specific and non-specific ELISA results.
Statistical methods
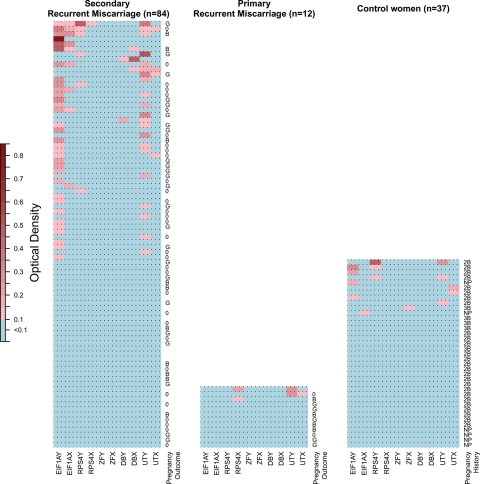
For 2 × 2 table analysis, either Fisher's exact test or χ2 test was performed based on the expected and observed distribution of results. Heatmaps visualizing the antibody response in OD to each H-Y and H-X protein of each participating individual were produced using the statistical programming language R (www.R-project.org) and individuals in each category are sorted on the basis of average OD. Statistical significance was set at P < 0.05.
Ethics
The Danish Central National Committee on Biomedical Research Ethics approved the study including the recruitment of healthy controls and transport of blood samples out of the country (approval no. 2004-7041-12). Oral and written informed consent was obtained from all subjects participating in the study.
Results
The presence of IgG antibodies to five different H-Y proteins and their H-X homologs was investigated in serum samples from 133 women: 84 SRM patients (66 were early pregnant at time of serum sampling); 12 PRM patients (11 were early pregnant at the time of serum sampling) and 31 non-pregnant control women with a history of uncomplicated pregnancies with boys and six women without pregnancy history. Figure 1 depicts the ODs of the IgG responses against each of the H-Y and H-X proteins for each individual included in this study. None of the controls but 12.5% of the SRM patients had antibody responses directed at both the H-Y and the H-X homolog (non-specific responses), P = 0.02. Table II summarizes the frequency of specific H-Y antibody-positive individuals according to patient or control category.
Figure 1.
H-Y and H-X antibody responses in serum samples from patients with unexplained recurrent miscarriage and healthy women. Heat maps visualizing the antibody response in OD units to each H-Y and H-X protein of each participating individual, grouped according to patient or control status and sorted with the highest mean OD at the top. Positivity is defined as OD ≥0.1. H-Y-specific responses are responses directed at the H-Y protein and not the corresponding H-X protein. For patients who were pregnant at blood sampling, pregnancy outcome is given. G, girl; B, boy; 0, miscarriage. For control women pregnancy history is given. 2B: given birth to only two boys, 3B: given birth to only three boys, NP, never pregnant.
Table II.
Frequencies of H-Y antibody-positive eSRM patients, PRM patients and control women.
| SRM patients |
Non-SRM women |
|||||||
|---|---|---|---|---|---|---|---|---|
| Boy prior to SRM (n = 68) | Girl prior to SRM (n = 16) | All SRM (n = 84) | Never pregnant controls (n = 6) | Controls two boys (n = 26) | Controls three boys (n = 5) | All Control women (n = 37) | PRM (n = 12) | |
| H-Y antigens | n (%) | |||||||
| EIF1AY | 19 (28) | 6 (38) | 25 (30) | 1 (17) | 3 (12) | 0 | 4 (11) | 0 |
| RPS4Y1 | 5 (7) | 0 | 5 (6) | 0 | 3 (12) | 0 | 3 (8) | 0 |
| ZFY | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DDX3Y | 1 (2) | 0 | 1 (1) | 0 | 0 | 0 | 0 | 0 |
| UTY | 16 (24) | 2 (13) | 18 (21) | 0 | 3 (12) | 0 | 3 (8) | 1 (8) |
| ≥1 H-Y | 33 (49)a | 6 (38)a | 39 (46)b,c | 1 (17)d | 6 (23) | 0 | 7 (19)b | 1 (8)c |
aDifference in frequencies of H-Y antibody-positive SRM patients according to sex of child born prior to SRM: 11% (−15%; 33%), P = 0.33.
bDifference in frequencies of H-Y antibody-positive SRM patients and control women: 28% (9%; 42%), P = 0.004.
cDifference in frequencies of H-Y antibody-positive SRM and PRM patients: 38% (4–52%), P = 0.01.
dThis patient had a history of years of infertility.
eAntibodies with specificity against the H-Y antigen but not the corresponding H-X homolog.
Analyses and confirmation of H-Y and H-X responses by western blotting
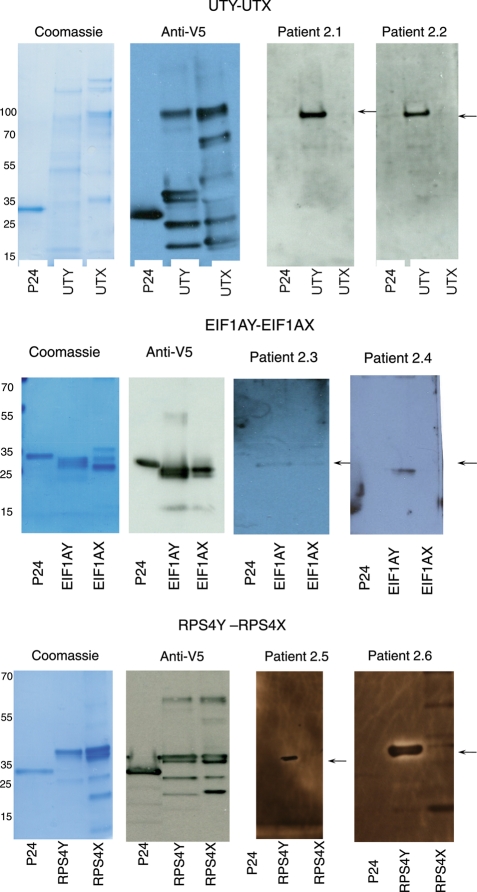
Western blot analyses confirmed the ELISA results in 14 of 15 samples tested. The one sample that was not confirmed was UTY for one patient whereby the ELISA OD was 0.11 and the western blot was negative. Five of the tested samples had a low H-Y antibody titer by ELISA (OD <0.2). Figure 2 shows two representative samples for each protein. We found almost full agreement between the results obtained by ELISA and western blot.
Figure 2.
Representative western blots confirming the ELISA results. Recombinant p24, H-Y and H-X-specific proteins produced in Escherichia coli were probed in western blots with antibodies specific for the V5 epitope tag and serum obtained from patients, as indicated below. The arrow indicates the location of the full-length recombinant H-Y protein. Corresponding ELISA results (in parenthesis) are reported as OD units, determined by absorption at 550–450 nm. 2.1 SRM pregnant patient who gave birth to a girl (UTY 0.55, UTX negative). 2.2 SRM pregnant patient who miscarried (UTY 0.27, UTX negative). 2.3 SRM pregnant patient who miscarried (EIF1AY 0.22, EIF1AX 0.17). 2.4 SRM pregnant patient who gave birth to a girl (EIF1AY 0.22, EIF1AX negative). 2.5 SRM pregnant patient miscarried (RPS4Y 0.13, RPS4X negative). 2.6 Control woman, 2 prior births (boys) (RPS4Y 0.48, RPS4X negative).
Frequencies of H-Y-specific antibodies in patients with recurrent miscarriage
Table II summarizes the frequency of H-Y antibodies in SRM patients and in non-SRM women.
The frequencies of H-Y-specific antibodies were significantly higher in SRM patients compared with controls as 39 (46%) of patients had H-Y-specific IgG antibodies compared with 7 (19%) in controls (P = 0.004). H-Y antibodies were also significantly more frequent in women with SRM compared with PRM (8%, P = 0.01). H-Y-specific antibodies were detected in 33 (49%) of SRM patients with a boy and in 6 (38%) of those women who delivered a girl prior to the miscarriages (P = 0.33). The frequency of H-Y-specific antibody responses directed against two H-Y proteins did not differ between SRM patients (13%) and controls (8%, P = 0.55).
In non-SRM women, H-Y specific antibody responses were detected in 7 (19%) of the 37 control women (Table II). The frequency of antibody responses did not differ between parous controls and women who had never been pregnant; 6 (19%) of the women with prior uncomplicated pregnancies with boys had H-Y-specific antibodies compared with 1(17% ) of those who never had been pregnant (P = 1.0). Also the frequency of H-Y antibody response did not differ in healthy women with two, compared with three, boys (P = 0.55). Accordingly, these groups are combined and subsequently referred to as controls. The one woman who had never been pregnant and who was H-Y antibody-positive had a history of years of unsuccessful attempts to become pregnant.
Age of all subjects, number of miscarriages, frequency of pregnant status at the time of blood sampling and whether pregnancy in the patients was spontaneous or after ART did not differ between H-Y antibody-positive and -negative women (P = 0.57, P = 0.11, P = 0.63 and P = 0.45 respectively; data not shown).
In summary, H-Y antibody responses were significantly more frequent in SRM patients compared with healthy controls and with PRM patients.
H-Y-specific antibodies and pregnancy outcome
The presence of H-Y specific antibodies in early pregnancy may influence its outcome. This proposition was analyzed in the 77 patients who were in early pregnancy at the time of blood sampling; 43 (56%) of these pregnancies ended with a live birth, whereas 34 pregnancies ended as a miscarriage; gestation of the miscarriages varied from 5 to 16 weeks. Fetal heart was initially detected in 26 of these cases, whereas no fetal heart was visualized in the remaining 8 (24%) patients who miscarried in week 5 or 6. Eight of the 34 miscarriages were karyotyped; one of these was found to be a trisomy 21, 5 were 46 XX and 2 were 46 XY.
Table III compares the presence or absence of H-Y-specific antibodies early in pregnancy with pregnancy outcome and with the sex of the liveborn offspring. Live birth rates were not significantly different in H-Y antibody-positive patients (48%) compared with H-Y antibody negative patients (61%) (P = 0.26). As shown in Table III, only 2 (12%) of the children delivered by H-Y antibody-positive patients were boys, which is significantly lower than the 12 (44%) boys delivered by H-Y antibody negative patients (P = 0.03) and the 51% boys among newborns in the general population (Khashan et al., 2009) (P = 0.002). Thus, the presence of H-Y-specific IgG antibodies in early pregnancy is associated with a low male: female ratio among the offspring.
Table III.
Association of H-Y-specific antibodies in early pregnancy, with pregnancy outcome and sex of surviving pregnancies in 77 patients with recurrent miscarriage.
| Pregnancy outcome n (%) |
P-value | Sex of surviving pregnancies n (%) |
P-value | |||
|---|---|---|---|---|---|---|
| Miscarriage | Birth | Girls | Boys | |||
| No H-Y antibodies, n = 44 | 17 (39) | 27 (61) | 0.26 | 15 (56) | 12 (44) | 0.03** |
| ≥1 H-Y antibodies, n = 33 | 17 (52) | 16 (48) | 14 (88) | 2 (12)* | ||
| Total (n) | 34 | 43 | 29 | 14 | ||
*P = 0.002 compared with the 51% boys among newborns in the general population (Khashan et al., 2009).
**P = 0.03 comparing boys delivered by H-Y antibody negative mothers with boys delivered by H-Y antibody-positive mothers.
H-Y antibodies and IvIg treatment
Twenty-one (54%) of SRM patients treated with IvIg had H-Y antibodies compared with 12 (44%) of the patients not receiving IvIg. Among the H-Y antibody negative patients 18 received IvIg while 15 did not receive this treatment. Live birth rates did not differ among IvIg-treated and non-treated H-Y antibody-positive SRM patients [8/21 (38%) versus 8/12 (67%), respectively; P = 0.11] or H-Y antibody negative patients [11/18 (61%) versus 7/15 (47%), respectively; P = 0.41].
Discussion
In this study we demonstrate that IgG antibodies to male chromosome-encoded H-Y proteins are more frequent in SRM patients than in controls. Additionally, the presence of H-Y antibodies in early pregnancy correlates with a low frequency of male offspring among patients with recurrent miscarriage.
This is the first study on H-Y antibodies in SRM patients; therefore we had no a priori knowledge of the frequency of H-Y antibodies according to obstetric history, preventing the usual sample size calculation. Larger studies are needed to confirm the herewith-postulated detrimental effect of H-Y antibodies on the outcome of pregnancies in SRM patients. Also it is challenging to study the role of HY antibodies in healthy pregnancy, or whether there is any role at all. None of the controls were pregnant at the time of blood sampling, which precludes drawing any conclusions from the present study about the impact of H-Y antibodies in healthy pregnant women. We found one woman to be H-Y antibody positive, who to the best of her knowledge had never been pregnant and she had a history of years of infertility. Whether unprotected sexual intercourse or unrecognized early pregnancy losses were the causes of H-Y antibody formation remains unanswered. The live birth rate reported here for unexplained SRM patients are lower than reported by other centers (Kaandorp et al., 2010). One explanation for this discrepancy may be the strict criterion for referral to our clinic: patients were to have a minimum of three previous consecutive miscarriages verified in medical records. Another explanation for this discrepancy could be the very close monitoring of the subsequent pregnancies of our patients: all patients were advised to call the clinic as soon as the next pregnancy test was positive in very early pregnancy. The strength of this study is the large cohort of otherwise unexplained SRM patients and the use of obstetrically well-described controls. Patients who were in early pregnancy when blood was sampled for H-Y antibody analysis were all followed up in our clinical database until subsequent birth or miscarriage. In accordance with previous publications, we found almost full agreement between results obtained by ELISA and western blot (Tan et al., 2008). Transplantation and blood transfusion as possible reasons for H-Y antibody formation were prevented by the exclusion criteria.
We found H-Y-specific antibodies significantly more frequently in SRM patients compared with PRM patients and healthy control women. SRM patients also showed higher non-specific responses (against both H-Y and the corresponding H-X proteins) compared with the female controls, pointing to a generally higher humoral immune responses in these patients. Although limited by size, the low frequency of H-Y antibody-positive PRM patients indicates that only recurrent miscarriages and no prior live birth is unlikely to induce anti-H-Y immunization. Most patients, but none of the controls were very early pregnant (gestational weeks 4–5) at the time of sampling, which could explain the higher frequency of H-Y-specific antibody responses in SRM patients. However, there are two indications that the state of early pregnancy per se does not induce H-Y antibodies. First, the frequency of H-Y antibodies did not differ between the 77 pregnant and 19 non-pregnant recurrent miscarriage patients (P = 0.6). Second, 92% of the PRM patients were early pregnant at the time of blood sampling, and their frequency of H-Y antibodies was much lower than in SRM patients.
We explored the impact of H-Y-specific antibodies in early pregnancy in the 77 recurrent miscarriage patients who were early pregnant (gestational weeks 4–5) at blood sampling. The live birth rate in H-Y antibody-positive and -negative pregnant patients was not significantly different, indicating that these antibodies do not increase the risk of clinically recognized miscarriage. Strikingly, however, patients with circulating H-Y antibodies in early pregnancy almost exclusively gave birth to girls (Table III) compared with patients who were negative for the H-Y antibodies, suggesting a strong and selective response directed against male conceptuses. The low male: female birth ratio in H-Y antibody-positive patients and a live birth rate that seemed to be similar to that of H-Y antibody negative recurrent miscarriage patients suggest that there is an increased risk of male-specific preclinical losses before pregnancy is confirmed. Such losses are reported to affect ∼ 60% of human embryos either as implantation failure or very early miscarriage before detection of hCG in the serum is possible (Macklon et al., 2002). Interestingly, the apparent potency of H-Y antisera has been shown in studies aimed at developing noninvasive techniques for sex selection of pre-implantation cattle embryos in order to increase the profitability of dairy and beef cattle production (Ramalho et al., 2004). Between 80 and 87% of murine and bovine male embryos that are cultured in high-titer rat H-Y antisera (OD >1 at 1/256 dilution) arrest their development at the morula stage in contrast to female embryos (Utsumi et al., 1993; Ramalho et al., 2004). Although our study provides no data on the H-Y antibody concentration in the uterus, the animal studies on sex selection would suggest male-specific preclinical losses as an explanation for our findings. None of our control women with repeated male births were pregnant at the time of sampling and, consequently, no information exists on the role of H-Y-specific antibodies in early pregnancy in women without a history of recurrent miscarriage.
The causes of the high frequency of H-Y antibodies in SRM patients are as yet unclear. Unlike PRM, SRM is preceded by a pregnancy that has lasted to the third trimester, where traffic of potentially allogeneic fetal cells into the mother's circulation is peaking (Huppertz et al., 2006; Adams et al., 2007). Furthermore, and in contrast to the parous controls with uncomplicated pregnancies with boys, the preceding pregnancies in SRM patients are often complicated with stillbirth, antepartum hemorrhage, preeclampsia, small for gestational age and low birthweight.
(Weintraub et al., 2005; Yang et al., 2006; Nielsen et al., 2010b), characterized by a large transfer of fetal antigens into the maternal circulation as well as abundant release of pro-inflammatory cytokines into the maternal circulation and locally in the uterus (Gerber et al., 2005; Girardi et al., 2006; Germain et al., 2007). Accordingly, the circumstances for sensitization of the adaptive immune system against fetal or trophoblast antigens are clearly present in SRM patients.
A series of recent studies supports the involvement of male-specific immunological factors in pregnancy outcome: a poorer prognosis of male fetuses of asthmatic mothers (Clifton et al., 2009; Clifton, 2010), reduced lifetime reproductive success of siblings either having a twin brother or preceded by older brothers (Lummaa et al., 2007; Rickard et al., 2007) and indications that preceding brothers increase the risk of stillbirth (Nielsen et al., 2010a) and reduce the birthweight (Nielsen et al., 2008b) in subsequent siblings.
We included patients with SRM subsequent to the birth of a girl, hypothesizing that only a few of them would be H-Y antibody positive; that is namely, priming against H-Y antigens most likely happens in the pregnancy prior to the miscarriages that progress to the third trimester where the transfer of fetal cells to maternal circulation is the highest. Although the frequency of H-Y antibodies was 11% lower in SRM patients with a firstborn girl compared with those with a firstborn boy, the difference between the two groups was not significant. Early miscarriage of male fetuses as an H-Y sensitization source may explain the high frequency of H-Y antibodies in patients with firstborn girls. Since early miscarriages can induce formation of antibodies against paternally derived allo-antigens, such as rhesus antigens (Murray and Barron, 1971). However, in our study H-Y-specific antibodies were rarely detected in patients with only early miscarriages and no prior birth. An explanation for the findings could be that SRM patients with a firstborn girl may be sensitized against autosomal minor histocompatibility antigens in the first ongoing pregnancy and H-Y antibodies may result from further immunization during subsequent unsuccessful pregnancies as a consequence of determinant-spreading processes (Lehmann et al., 1993; Ott et al., 2004).
Finally, SRM patients have been treated in some centers with IvIg. Our data do not indicate that IvIg treatment increases live birth rates in H-Y antibody-positive SRM patients. On the contrary, our data show a trend toward lower live birth rates in SRM-positive women who did receive IvIg. However, precautions need to be taken when evaluating these results, as the numbers are small and the a priori chance of a successful pregnancy is considerably lower for those allocated for IvIg treatment compared with those who do not receive treatment. In our center, this expensive treatment is only offered to patients with a high number of miscarriages and/or other negative prognostic factors. Larger randomized trials with sequential H-Y antibody testing throughout pregnancy are needed to evaluate the impact of IvIg on pregnancy outcome in anti-H-Y antibody-positive patients.
In summary, we report high frequencies of H-Y-specific antibody-positive patients with SRM. These responses in early pregnancy are associated with the sex of the liveborn offspring, suggesting a specific response against male embryos in patients who are positive for H-Y antibodies. Our results are the first of their kind and may shed light on the as yet unknown immunological causes of SRM. Further investigation may provide more knowledge on the hitherto unanswered questions regarding maternal–fetal interactions in normal and pathological pregnancies.
Authors' roles
H.S.N. planned and designed the study, collected the sera, performed experiments, analyzed data and drafted the manuscript. F.W., Z.A. and R.S. participated in the laboratory work and data analysis. A.G.H. and E.S. were involved in the study planning, design and data interpretation. O.B.C. collected sera and O.B.C., D.M. and E.G. were involved in the study design, interpretation of the results and writing of the final report.
Funding
NHLBI R21 funded the H-Y antibody detection and supported D.M. and F.W.; The Netherlands Organization for Scientific Research (NWO) rewarded a travel grant for H.S.N.; and The University Hospital Copenhagen Rigshospitalet rewarded a PhD grant for H.S.N.
References
- Adams KM, Yan Z, Stevens AM, Nelson JL. The changing maternal ‘self’ hypothesis: a mechanism for maternal tolerance of the fetus. Placenta. 2007;28:378–382. doi: 10.1016/j.placenta.2006.07.003. doi:10.1016/j.placenta.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Christiansen OB, Pedersen B, Nielsen HS, Nybo Andersen AM. Impact of the sex of first child on the prognosis in secondary recurrent miscarriage. Hum Reprod. 2004;19:2946–2951. doi: 10.1093/humrep/deh516. doi:10.1093/humrep/deh516. [DOI] [PubMed] [Google Scholar]
- Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31:S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Engel P, Smith R, Gibson P, Brinsmead M, Giles WB. Maternal and neonatal outcomes of pregnancies complicated by asthma in an Australian population. Aust N Z J Obstet Gynaecol. 2009;49:619–626. doi: 10.1111/j.1479-828X.2009.01077.x. doi:10.1111/j.1479-828X.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Stephenson M, Stern JJ, Clark DA. Immunotherapy for recurrent pregnancy loss: analysis of results from clinical trials. Am J Reprod Immunol. 1996;35:352–359. doi: 10.1111/j.1600-0897.1996.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Gerber S, Vardhana S, Meagher-Villemure K, Vial Y, Hohlfeld P, Witkin SS. Association between fetal interleukin-1 receptor antagonist gene polymorphism and unexplained fetal death. Am J Obstet Gynecol. 2005;193:1472–1477. doi: 10.1016/j.ajog.2005.02.112. doi:10.1016/j.ajog.2005.02.112. [DOI] [PubMed] [Google Scholar]
- Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. doi:10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwohl A, Hermans J, Niederwieser D, van BA, van Houwelingen HC, Apperley J. Female donors influence transplant-related mortality and relapse incidence in male recipients of sibling blood and marrow transplants. Hematol J. 2001;2:363–370. doi: 10.1038/sj.thj.6200117. doi:10.1038/sj.thj.6200117. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Kadyrov M, Kingdom JC. Apoptosis and its role in the trophoblast. Am J Obstet Gynecol. 2006;195:29–39. doi: 10.1016/j.ajog.2005.07.039. doi:10.1016/j.ajog.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Jivraj S, Anstie B, Cheong YC, Fairlie FM, Laird SM, Li TC. Obstetric and neonatal outcome in women with a history of recurrent miscarriage: a cohort study. Hum Reprod. 2001;16:102–106. doi: 10.1093/humrep/16.1.102. doi:10.1093/humrep/16.1.102. [DOI] [PubMed] [Google Scholar]
- Kaandorp SP, Goddijn M, van der Post JA, Hutten BA, Verhoeve HR, Hamulyak K, Mol BW, Folkeringa N, Nahuis M, Papatsonis DN, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med. 2010;362:1586–1596. doi: 10.1056/NEJMoa1000641. doi:10.1056/NEJMoa1000641. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Mortensen PB, McNamee R, Baker PN, Abel KM. Sex ratio at birth following prenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24:1754–1757. doi: 10.1093/humrep/dep082. doi:10.1093/humrep/dep082. [DOI] [PubMed] [Google Scholar]
- Kollman C, Howe CW, Anasetti C, Antin JH, Davies SM, Filipovich AH, Hegland J, Kamani N, Kernan NA, King R, et al. Donor characteristics as risk factors in recipients after transplantation of bone marrow from unrelated donors: the effect of donor age. Blood. 2001;98:2043–2051. doi: 10.1182/blood.v98.7.2043. doi:10.1182/blood.V98.7.2043. [DOI] [PubMed] [Google Scholar]
- Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. doi: 10.1016/0167-5699(93)90163-F. doi:10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- Loren AW, Bunin GR, Boudreau C, Champlin RE, Cnaan A, Horowitz MM, Loberiza FR, Porter DL. Impact of donor and recipient sex and parity on outcomes of HLA-identical sibling allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:758–769. doi: 10.1016/j.bbmt.2006.03.015. doi:10.1016/j.bbmt.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Lummaa V, Pettay JE, Russell AF. Male twins reduce fitness of female co-twins in humans. Proc Natl Acad Sci USA. 2007;104:10915–10920. doi: 10.1073/pnas.0605875104. doi:10.1073/pnas.0605875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002;8:333–343. doi: 10.1093/humupd/8.4.333. doi:10.1093/humupd/8.4.333. [DOI] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. doi:10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos DB, Kim HT, Zorn E, Hochberg EP, Guo L, Mattes-Ritz A, Viatte S, Soiffer RJ, Antin JH, Ritz J. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. doi:10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales C, Sanchez A, Bruguera J, Margarit E, Borrell A, Borobio V, Soler A. Cytogenetic study of spontaneous abortions using semi-direct analysis of chorionic villi samples detects the broadest spectrum of chromosome abnormalities. Am J Med Genet. 2008;146A:66–70. doi: 10.1002/ajmg.a.32058. doi:10.1002/ajmg.a.32058. [DOI] [PubMed] [Google Scholar]
- Murray S, Barron SL. Rhesus isoimmunization after abortion. Br Med J. 1971;3:90–92. doi: 10.1136/bmj.3.5766.90. doi:10.1136/bmj.3.5766.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen HS, Andersen AM, Kolte AM, Christiansen OB. A firstborn boy is suggestive of a strong prognostic factor in secondary recurrent miscarriage: a confirmatory study. Fertil Steril. 2008a;89:907–911. doi: 10.1016/j.fertnstert.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Mortensen L, Nygaard U, Schnor O, Christiansen OB, Andersen AM. Brothers and reduction of the birth weight of later-born siblings. Am J Epidemiol. 2008b;167:480–484. doi: 10.1093/aje/kwm330. doi:10.1093/aje/kwm330. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Steffensen R, Varming K, van Halteren AG, Spierings E, Ryder LP, Goulmy E, Christiansen OB. Association of HY-restricting HLA class II alleles with pregnancy outcome in patients with recurrent miscarriage subsequent to a firstborn boy. Hum Mol Genet. 2009;18:1684–1691. doi: 10.1093/hmg/ddp077. doi:10.1093/hmg/ddp077. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Mortensen LH, Nygaard U, Schnor O, Christiansen OB, Andersen AM. Sex of prior children and risk of stillbirth in subsequent pregnancies. Epidemiology. 2010a;21:114–117. doi: 10.1097/EDE.0b013e3181c04dcf. doi:10.1097/EDE.0b013e3181c04dcf. [DOI] [PubMed] [Google Scholar]
- Nielsen HS, Steffensen R, Lund M, Egestad L, Mortensen LH, Andersen AM, Lidegaard O, Christiansen OB. Frequency and impact of obstetric complications prior and subsequent to unexplained secondary recurrent miscarriage. Hum Reprod. 2010b;25:1543–1552. doi: 10.1093/humrep/deq091. doi:10.1093/humrep/deq091. [DOI] [PubMed] [Google Scholar]
- Ott PA, Dittrich MT, Herzog BA, Guerkov R, Gottlieb PA, Putnam AL, Durinovic-Bello I, Boehm BO, Tary-Lehmann M, Lehmann PV. T cells recognize multiple GAD65 and proinsulin epitopes in human type 1 diabetes, suggesting determinant spreading. J Clin Immunol. 2004;24:327–339. doi: 10.1023/B:JOCI.0000029120.77824.41. doi:10.1023/B:JOCI.0000029120.77824.41. [DOI] [PubMed] [Google Scholar]
- Quenby SM, Farquharson RG. Predicting recurring miscarriage: what is important? Obstet Gynecol. 1993;82:132–138. [PubMed] [Google Scholar]
- Ramalho MF, Garcia JM, Esper CR, Vantini R, Alves BC, meida Junior IL, Hossepian dLV, Moreira-Filho CA. Sexing of murine and bovine embryos by developmental arrest induced by high-titer H-Y antisera. Theriogenology. 2004;62:1569–1576. doi: 10.1016/j.theriogenology.2004.03.014. doi:10.1016/j.theriogenology.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Rickard IJ, Russell AF, Lummaa V. Producing sons reduces lifetime reproductive success of subsequent offspring in pre-industrial Finns. Proc Biol Sci. 2007;274:2981–2988. doi: 10.1098/rspb.2007.1051. doi:10.1098/rspb.2007.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JC, Wadia PP, Coram M, Grumet FC, Kambham N, Miller K, Pereira S, Vayntrub T, Miklos DB. H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation. 2008;86:75–81. doi: 10.1097/TP.0b013e31817352b9. doi:10.1097/TP.0b013e31817352b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi K, Hayashi M, Takakura R, Utaka K, Iritani A. Embryo sex selection by a rat male-specific antibody and the cytogenetic and developmental confirmation in cattle embryos. Mol Reprod Dev. 1993;34:25–32. doi: 10.1002/mrd.1080340105. doi:10.1002/mrd.1080340105. [DOI] [PubMed] [Google Scholar]
- Verdijk RM, Kloosterman A, Pool J, van de KM, Naipal AM, van Halteren AG, Brand A, Mutis T, Goulmy E. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–1964. doi: 10.1182/blood-2003-05-1625. doi:10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- Warren EH, Gavin MA, Simpson E, Chandler P, Page DC, Disteche C, Stankey KA, Greenberg PD, Riddell SR. The human UTY gene encodes a novel HLA-B8-restricted H-Y antigen. J Immunol. 2000;164:2807–2814. doi: 10.4049/jimmunol.164.5.2807. [DOI] [PubMed] [Google Scholar]
- Weintraub AY, Sheiner E, Bashiri A, Shoham-Vardi I, Mazor M. Is there a higher prevalence of pregnancy complications in a live-birth preceding the appearance of recurrent abortions? Arch Gynecol Obstet. 2005;271:350–354. doi: 10.1007/s00404-004-0640-z. doi:10.1007/s00404-004-0640-z. [DOI] [PubMed] [Google Scholar]
- Yang CJ, Stone P, Stewart AW. The epidemiology of recurrent miscarriage: a descriptive study of 1214 prepregnant women with recurrent miscarriage. Aust N Z J Obstet Gynaecol. 2006;46:316–322. doi: 10.1111/j.1479-828X.2006.00599.x. doi:10.1111/j.1479-828X.2006.00599.x. [DOI] [PubMed] [Google Scholar]