Abstract
BACKGROUND
We have recently shown that women with endometriosis express an increased amount of telomerase and nucleolin, with concomitant loss of γ-H2AX in eutopic endometrium. To further examine these selected factors that regulate cell fate, in the pathogenesis of endometriosis, we studied the expression of telomerase, nucleolin, proliferating cell nuclear antigen and γ-H2AX in ectopic endometriotic deposits from women, and in matched eutopic and ectopic endometrial tissue from a baboon model of endometriosis.
METHODS
Ectopic active peritoneal endometriotic lesions were collected from seven symptomatic women. Endometriosis was induced in six baboons by intra-peritoneal autologous inoculation of menstrual endometrium. Eutopic and matched ectopic endometrial tissues were collected prior to and 6, 12 and 15 months after the induction of endometriosis as previously described. Eutopic endometrium was also obtained from eight healthy fertile control baboons. Immunohistochemistry was performed as previously described, and telomerase activity was confirmed using the telomeric repeat amplification protocol assay.
RESULTS
All active human endometriotic lesions expressed the proliferative markers but showed weak or absent staining for γ-H2AX. A similar expression pattern of these markers was seen in the ectopic lesions of the baboons with induced disease. In these baboons, the eutopic endometrium also showed intense immunoreactivity for all proliferative markers 6–12 months after induction with a parallel loss of γ-H2AX. The opposite staining pattern was seen in eutopic endometrium of healthy animals and in pre-induction endometrium of animals with induced disease.
CONCLUSIONS
Endometriotic lesions have excess proliferative potential; in baboons, these were present within 12 months of the initiation of the disease. In eutopic tissue, these changes appear to be induced by the development of endometriosis.
Keywords: telomerase, γ-H2AX, nucleolin, endometriosis, baboon-model
Introduction
Endometriosis is one of the commonest benign gynaecological conditions with huge personal and economic consequences. There are fundamental difficulties in reducing the burden of this disease due to the invasiveness of the diagnostic techniques and ineffectiveness of the currently available treatments. At present, diagnostic laparoscopy is the only useful investigative technique available. The development of novel methods of diagnosis or treatment is hampered by a lack of knowledge regarding the aetiology, pathogenesis and natural progression of endometriosis. Despite many studies, the causative mechanisms have not been identified (Bulun, 2009).
Endometriosis is defined as the presence of endometrial tissue outside the uterine cavity. The most commonly held theory is that it is caused by retrograde menstruation and trans-tubal migration of viable endometrial fragments that attach to, and invade, the peritoneal mesothelium to establish ectopic growth of endometrial tissue (Sampson, 1927). Given that most women reflux endometrial debris into the peritoneal cavity during menstruation yet endometriosis only develops in 10–15% of women, it is unclear why some women develop endometriosis, whereas others do not. It is known that endometrial cells within endometriotic deposits express steroid receptors, continue to show a cyclical response to ovarian hormones and may be resistant to apoptosis (Harada et al., 2007). Thus, investigating the mechanisms/factors involved in endometrial cell kinetics is central to an understanding of the pathogenesis of endometriosis. Our previous work identified for the first time the possible involvement of telomerase, nucleolin and γ-H2AX (factors involved in the regulation of cell fate) in the pathogenesis of endometriosis (Hapangama et al., 2008a, 2009). The expression of telomerase, nucleolin and proliferating cell nuclear antigen (PCNA) specifically correlates with cell proliferation (Yan et al. 2004; Grinstein and Wernet, 2007; Moldovan and Jentsch, 2007). We have shown that the eutopic endometrium of women with endometriosis shows persistent expression of these factors through the menstrual cycle, suggesting that endometriosis is associated with a state of sustained proliferation (Hapangama et al., 2009). Sustained proliferation depends on close regulation of cell division, including DNA replication and cell growth, and increases the risk of DNA damage. DNA damage leads to the induction of a DNA damage response (DDR). One of the earliest events in a DDR is the phosphorylation of Ser139 at the carboxyl terminus of the histone protein H2AX leading to the formation of γ-H2AX foci. γ-H2AX foci mediate the recruitment of numerous DNA damage recognition and repair factors to the immediate vicinity of the DNA lesion, which promotes repair and prevents chromosomal breaks (Fillingham et al., 2006; Franco et al., 2006). In women with endometriosis, a persistence of proliferative markers (ongoing or accelerated proliferation) was associated with a virtually complete loss of γ-H2AX in eutopic endometrial cells, during the secretory phase. This suggests that these endometrial cells not only maintain their telomeres (Hapangama et al., 2008a) and do not mount a DDR but also proliferate in the secretory phase without replication fork stalling (Hapangama et al., 2009). This state of rapid proliferation, with a potential high risk of mutation, corresponds with the increasing evidence that endometriosis can sometimes develop into gynaecological cancers (Van Gorp et al., 2004). With this observation, we suggest that hyper-efficient proliferation coupled with the loss of the DNA damage signal is an element of the phenotype of endometriosis that could contribute to a survival advantage for endometrium in the peritoneal cavity.
The significance of our earlier findings was difficult to determine because there was no conclusive information about whether endometriotic deposits have the same phenotype as eutopic endometrium sampled from women with endometriosis. The expression pattern of the factors regulating cell fate we previously described had not been assessed in endometriotic deposits. Furthermore, the relevance of our findings to the development of endometriosis was unclear because at the time of diagnosis, endometriosis was already established in women. To overcome the difficulty of longitudinal studies and to inform the design of studies of ectopic endometrium in women, we used a well-characterized baboon model of endometriosis. This model involves the autologous inoculation of menstrual endometrium into the peritoneal cavity simulating retrograde menstruation. The subsequent induction of ectopic endometrial deposits has been described in detail (D'Hooghe et al., 1995; Fazleabas et al., 2002). This model has already been utilized to broaden our current understanding of the immune-suppressant aspects (Hastings and Fazleabas, 2006), alterations in steroid receptor expression (Fazleabas et al., 2003; Jackson et al., 2007) and endometrial gene expression (Hastings and Fazleabas, 2006) in the pathogenesis of endometriosis.
In the current study, we examined the previously described (Hapangama et al., 2009) factors regulating cell fate in endometriotic deposits among women and in the baboon model of endometriosis to investigate our hypothesis that endometriosis involves an increased expression of telomerase, nucleolin and PCNA, with a concomitant loss of γ-H2AX. The baboon model allowed us to examine the time course of the expression of previously described factors regulating cell fate following the induction of endometriosis in ectopic and eutopic endometrium.
Our aims were the following:
To study the expression patterns of telomerase, nucleolin, PCNA and γ-H2AX in ectopic endometrial tissue of women with active, peritoneal endometriosis.
To study the time course of the expression patterns of telomerase, nucleolin, PCNA and γ-H2AX in ectopic endometrial tissue of baboons following the establishment of induced endometriosis.
To study the expression patterns of telomerase, nucleolin, PCNA and γ-H2AX in the eutopic endometrium of baboons with induced disease and of animals without endometriosis.
To examine whether the telomerase function assessed by telomeric repeat amplification protocol (TRAP assay) varied in parallel with the expression of telomerase detected by immunohistochemistry.
Materials and Methods
Ectopic human endometriotic lesions
Thirteen active peritoneal endometriotic red, chocolate and blue lesions were collected from seven women with symptomatic endometriosis surgically diagnosed at American Fertility Society stages I–IV. The expression of telomerase, nucleolin, PCNA and γ-H2AX in eutopic endometrial samples of these women has been reported previously (Hapangama et al., 2008a,b, 2009). All women included had regular menstrual cycles (26–30 day), were not on any hormonal therapy and were undergoing surgical excision of endometriotic tissue. Ethical approval was granted from Liverpool adult research ethics committee (04/Q1505/112) and written informed consent was obtained from all participants prior to inclusion in the study.
Induction of endometriosis in the baboons
The previously described technique of intra-pelvic autologous inoculation of menstrual endometrium was employed to induce endometriosis in six female baboons with regular menstrual cycles (Fazleabas et al., 2002, 2003). Briefly, menstrual endometrium was collected in six Unimar pipelles (Cooper Surgical Inc., Shelton, CT, USA) via a trans-cervical route and deposited into the pouch of Douglas, the left and right cul-de-sac as well as the broad ligaments near the fallopian tubes of the same baboons (autologous transplant) on two consecutive menstrual cycles under laparoscopic guidance. Following the second inoculation, tissue was harvested from the eutopic endometrium and ectopic lesions after 0 (from n = 5 animals, eutopic endometrium only), 6 (n = 6), 12–13 (n = 6) and 15–16 (n = 6) months by performing laparoscopies and laparotomies as previously described (Fazleabas et al., 2002). The extent of endometriotic deposits was assessed and counted laparoscopically prior to biopsy and changes were documented by video recording (Fazleabas et al., 2003; Gashaw et al., 2006; Hastings and Fazleabas, 2006). Endometriotic deposits were classified as red raised, blue (both reddish blue and powder burn lesions), typical blue/brown chocolate lesions and white lesions (which are white opaque endometrial explants lacking haemosiderin) according to species-specific modifications of American Fertility Society classification as previously described (D'Hooghe et al., 1995; Fazleabas et al., 2003) and one lesion per each type per animal per time point was analysed. Control endometrial tissues were obtained at a single time point from eight additional normally cycling baboons that had not been inoculated with menstrual tissue. All animals weighed between 12 and 18 kg and were aged 7–12 years, and we included tissue from previous studies (Fazleabas et al., 2002) and tissues that were collected prospectively. The induction of the disease had no effect on their menstrual cyclicity or peripheral ovarian steroid levels (Wang et al., 2009). The Animal Care Committee of the University of Illinois, Chicago, approved all experimental procedures on baboons. All tissues (ectopic and eutopic) were collected between Days 9–11 post-ovulation, which corresponds to the window of implantation (WOI) in the baboon, after determining the day of ovulation by serum E2 levels as previously described (Fazleabas et al., 2002).
After collection, human tissues were fixed in 3% paraformaldehyde for 24 h and baboon tissues were fixed in 3% (w/v) paraformaldehyde/1% (v/v) glutaraldehyde for 6 h at room temperature, dehydrated through ethanol and embedded in paraffin.
Immunohistochemistry
Immunohistochemistry was performed by standard methods as previously described using horseradish-peroxidase-conjugated secondary antibodies, and immune-reactivity was detected using the chromagen, 3,3′-diaminobenzidine (DAB) (Hapangama et al., 2008a,b, 2009). The commercially obtained primary antibodies used were against human nucleolin (Novo Castra, Newcastle-upon-Tyne, UK Mouse monoclonal), human PCNA (Dako, UK Mouse monoclonal), human telomerase (Abcam, Cambridge, UK Rabbit polyclonal) and γ-H2AX (Abcam, UK Rabbit polyclonal).
Briefly, 5-µm-thick sections of endometrial specimens were deparaffinized through a series of xylene baths, and the samples were rehydrated through ascending grades of alcohol to Tris-buffered saline (TBS). To enhance the epitope exposure, antigen retrieval was carried out by pressure cooking the slides with 0.01 M citrate buffer at pH 6 for 4 min. Endogenous peroxidase was quenched in 3% H2O2. After washing with TBS, the sections were incubated with the primary antibody in a humidifying chamber. The primary antibody was replaced by an appropriate negative controls (rabbit immunoglobulin G (IgG, Dako, USA) for telomerase and H2AX, mouse IgG (Dako, USA) for nucleolin and PCNA). After washing the sections were incubated in the labelled polymer (Envision, Dako, USA), and incubated with DAB developed brown positive staining according to the manufacturer's guidelines. The sections were lightly counterstained with Harris haematoxylin and mounted. External tissues were used as positive controls for staining.
TRAP assay for telomerase activity
To validate our immunohistochemical assay of telomerase, we carried out a photometric enzyme immunoassay for the detection of telomerase activity. The Telo TAGGG Telomerase PCR ELISA kit (Roche, GmbH, Mannheim, Germany), which utilizes a PCR-enzyme-linked immunosorbent platform, was used to measure telomerase activity as previously described (Hapangama et al., 2008a,b). Cell extracts prepared from immortalized telomerase-expressing human kidney cells (293 cells) were used as a positive control. Water and 293 cells that were pre-incubated with 1 µg/µl RNase (DNase free) and heated at 37°C for 20 min were used as negative controls.
Analysis of telomerase, nucleolin and PCNA immunostaining
All slides were coded and scored by two independent observers prior to breaking the code. Distribution and intensity of brown positive immunostaining for telomerase, nucleolin and PCNA within the different cell types in the endometrium (glandular and luminal epithelium, stroma, pericvascular cells and in the endothelial cells) were measured using a standard semi-quantitative scoring system with demonstrated high correlation between objectively measured immunoreactivity (image analysis) and subjective semi-quantitative scoring of immunostaining patterns used in previous studies (Wang et al., 1998; Hapangama et al., 2002, 2008a,b, 2009). This method employs a four point semi-quantitative scoring system based on a global assessment of all slides relating to each participant. Positive staining was classified as negative/no staining = 0, weak =+/−, strong = + and very strong = ++ as previously described (Wang et al., 1998; Hapangama et al., 2002, 2008a,b, 2009).
Analysis of γ-H2AX immunostaining
The presence or absence of distinct brown nuclear γ-H2AX immune-staining pattern against the blue haematoxylin background was assessed as a percentage of γ-H2AX antigen positive cells by employing a computer-assisted image analysis system, which was adapted for this purpose as previously described (Hapangama et al., 2009).
Statistical analysis
SPSS version 15.0 for Windows statistical programme was used for all analyses. Semi-quantitative scores and relative absorbance values from the TRAP assay of related and non-related groups were compared by non-parametric tests as appropriate (Friedman and Mann–Whitney U-test).
Results
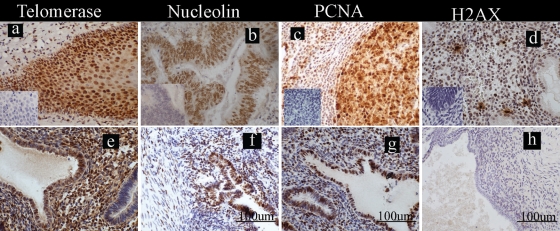
Positive brown nuclear telomerase, nucleolin, PCNA and γ-H2AX staining were seen in the external positive controls used (Fig. 1a–d).
Figure 1.
Localization of the markers of cell fate studied in the ectopic endometrial lesions from women. Photomicrographs are representatives of the immunostaining for telomerase, nucleolin, proliferating cell nuclear antigen (PCNA) and histone γ-H2AX; positive staining = brown; scale bars = 100 µm (f, g and h) applicable to all panels. (a–d) External positive control tissues with inserts illustrating the immunologically negative controls: (a) telomerase in human tonsillar cortex, (b) nucleolin in human adeno-carcinoma of the colon, (c) PCNA in human tonsil and (d) histone γ-H2AX in human late-secretory endometrium; (e–h) ectopic blue/chocolate lesions from women with endometriosis stained with (e) telomerase (f) nucleolin, (g)PCNA and (h) γ-H2AX.
Ectopic endometriotic deposits from women
We studied 13 active peritoneal endometriotic lesions (red, blue and chocolate), and they were collected at the proliferative (n = 2), mid-secretory (n = 3) and late-secretory (n = 2) phases of the menstrual cycle. They all showed strong positive immunostaining for the three proliferative markers studied in both the epithelial and stromal endometriotic cells (Fig. 1e–g). The same lesions showed either weak or absent immune-staining for γ-H2AX (0% (range 0–10%) of epithelial cells and 0% (range 0–8%) of stromal cells showed positive staining for γ-H2AX, Fig. 1h).
Baboon ectopic endometrial deposits
Expression of telomerase, nucleolin and PCNA
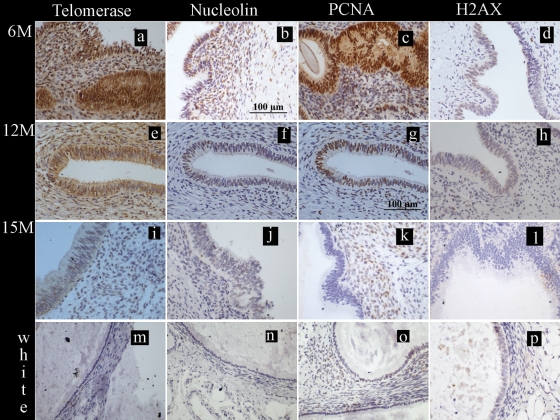
We have previously described that significantly more blue lesions were observed at 6 months after inoculation with menstrual endometrium. Following this initial phase, by 12 and 15 months an equal number of red, blue, chocolate, white and mixed lesions was observed (Hastings and Fazleabas, 2006; Kim et al., 2007a,b). We assessed ectopic endometrial tissue from 6 months, 12 months and 15 months after the inoculation procedure. All red, blue and chocolate endometriotic lesions of animals with the induced disease showed intense immune-staining for all three proliferative markers at 6 and 12 months post-inoculation. The epithelial fraction showed moderate to intense immunoreactivity for telomerase, nucleolin and PCNA at 6 months (Fig. 2a–c), which persisted at 12 months (Fig. 2e–g) yet appeared to be decreased by 15 months post-inoculation (Figs 2i–k and3). The stromal cells also showed a similar staining pattern for all three proliferative markers (Figs 2a–k and 3) in all active ectopic lesions. The effect of timing of the biopsy after induction of the disease reached statistical significance for epithelial telomerase, both epithelial and stromal nucleolin and stromal PCNA staining (P < 0.05, Friedman test and Fig. 3). White lesions or lesions described as associated with scarring in the baboon did not show any positive staining for these markers of cell proliferation (Fig. 2m–o).
Figure 2.
Localization of the markers of cell fate studied in the ectopic endometrial lesions during the window of implantation (WOI) in baboons. Photomicrographs are representatives of the immunostaining for telomerase, nucleolin, proliferating cell nuclear antigen (PCNA) and histone γ-H2AX; positive staining = brown; scale bars = 100 µm (b and g) applicable to all panels. (a–l) Ectopic blue/chocolate lesions from animals with induced disease stained for telomerase, for nucleolin, for PCNA and for γ-H2AX at 6, 12 or 15 months post-inoculation. (m–p) White lesions from induced animals did not show positive immunoreactivity for telomerase, nucleolin, PCNA or γ-H2AX.
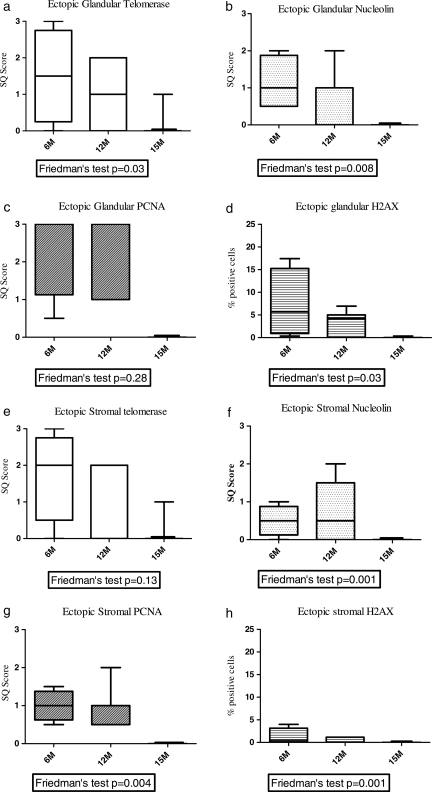
Figure 3.
Box plot showing the median semi-quantitative scores for telomerase, nucleolin and PCNA, and immune-staining and median percentage of γ-H2AX immune-staining positive cells in baboon ectopic endometrial glandular (a–d) and stromal (e–h) compartments. Ectopic blue/chocolate lesions of animals (n = 6) with induced disease at 6 months (6M, n = 6), 12 months (12M, n = 6) and 15 months (15M, n = 6) after inoculation. The P-value from Freidman's test shown under each graph. Y-axis semi-quantitative (SQ) scores or percentage (%) of positive cells.
Expression of γ-H2AX
There was also a progressive loss of γ-H2AX staining that reached statistical significance in the epithelial (P = 0.03, Friedman test and Fig. 3) cells from ectopic blue and chocolate lesions between 6 months (Fig. 2d) and 15 months (Fig. 2l) after induction of the disease. γ-H2AX immune-staining was seen in 5.7% (range 0.3–17.2%) of epithelial and 0.5% (range 0–3.9%) of stromal cells at 6 months and was reduced to 0% (range 0–0.3%) and 0% (range 0–0.03%) of the respective cells staining by 15 months after inoculation. White lesions were negative for γ-H2AX (Fig. 2p).
Baboon eutopic endometrium
Expression of telomerase, nucleolin and PCNA
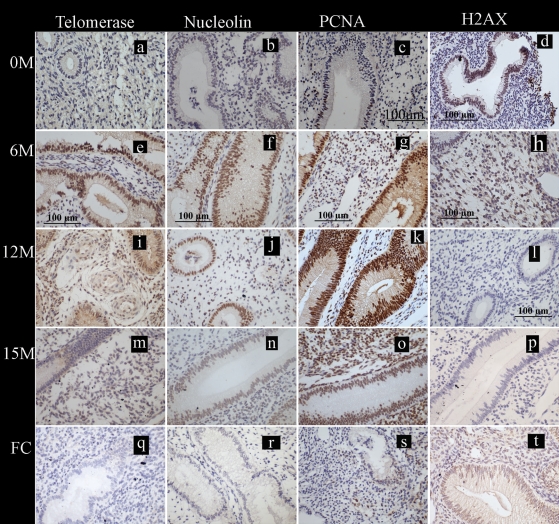
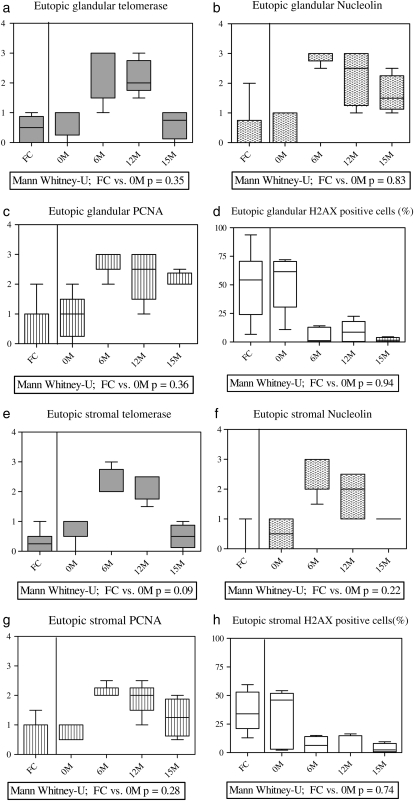
The pre-induction eutopic endometrium of animals that had intra-pelvic injection of menstrual endometrium to induce disease showed absent or weak immune-staining for all three proliferative markers (Fig. 4a–c). Following inoculation, the eutopic endometrium of these animals (n = 6) showed intense immunoreactivity for all three proliferative markers at 6 (Fig. 4e–g) and 12 (Fig. 4i–k) months. By 15 months after inoculation (Fig. 4m–o), however, telomerase staining returned to the levels of pre-induction endometrium in both epithelium and stromal cells. At 15 months, moderate staining persisted in the epithelial and stromal cells for PCNA and nucleolin (Fig. 5).
Figure 4.
Localization of the markers of cell fate studied in the eutopic endometrium collected during the WOI in baboons. Photomicrographs are representatives of the immunostaining for telomerase, nucleolin, proliferating cell nuclear antigen (PCNA) and histone γ-H2AX; positive staining = brown; scale bars = 100 µm (c–h and l) applicable to all panels. (a–d) Endometrium from baboons (n = 5) prior to inoculation at 0 M and stained for telomerase, nucleolin, PCNA and histone γ-H2AX. (e–p) Eutopic endometrium from animals with induced disease (6, 12 or 15 months post-induction tissue from six animals included at each time point) stained with telomerase, nucleolin, PCNA and γ-H2AX. (q–t) Eutopic endometrium from the control healthy fertile animals (n = 8) stained for telomerase, nucleolin, PCNA and γ-H2AX.
Figure 5.
Box plots showing the median semi-quantitative scores for nucleolin, PCNA and telomerase, and immune-staining and median percentage of γ-H2AX immune-staining positive cells in baboon eutopic endometrial glandular (a–d) and stromal (e–h) compartments. Eutopic endometrium of healthy fertile control (FC) animals (n = 8), eutopic endometrium collected prior to inoculation (n = 5) 0M, and eutopic endometrium of animals with induced disease (n = 6) at 6 months (6M), 12 months (12M) and 15 months (15M) after inoculation. The P-value from Mann–Whitney U-test comparing FC animals and experimental animals before the induction of the disease is shown under each graph. Y-axis = semi-quantitative (SQ) scores or percentage (%) of positive cells.
All control animals showed either weak or absent immune-staining for telomerase, nucleolin and PCNA (Figs 4q–s and 5) during the WOI in the functionalis layer of the endometrium similar to that in the pre-induction eutopic endometrium of the animals with induced endometriosis (Mann–Whitney U-test, P > 0.05). The weak positive telomerase staining seen in the functionalis was limited to the endometrial blood vessels (in the perivascular cells and in the endothelial cells).
Expression of γ-H2AX
The pre-induction eutopic endometrium of experimental animals showed 56% (range 29–73%) of epithelial and 43% (range 12–54%) of stromal cells staining for γ-H2AX (Figs 4d and 5d and h). All six animals with induced disease then showed a progressive decrease in γ-H2AX staining in the eutopic endometrium to: 12% (range 1–32%) of epithelial and 9.7% (range 0–15%) stromal cells at 6 months (Fig. 4h); 11% (range 0–22%) of epithelial and 6% (range 0–16%) stromal cells at 12 months (Fig. 4l); and 1% (range 0–4%) of epithelial and 1% (range 0–9%) stromal cells at 15 months (Fig. 4p) during the post-induction period.
Compared with the post-induction endometrium of animals with induced disease, the healthy animals showed high immune-reactivity for γ-H2AX in 56% (range 29–73%) of the epithelial cells and 21% (range 13–54%) of stromal cells of the eutopic endometrium (Fig. 4t), which was comparable with the pre-induction endometrium of the experimental animals.
TRAP assay
We tested telomerase activity of the eutopic endometrial samples from four baboons prior to induction of endometriosis and four baboons with induced endometriosis at 13 months. All pre-induction samples had either very low or negative telomerase activity (range 0.10–0.11). Thirteen months after the establishment of endometriosis, the eutopic samples had statistically higher values of telomerase activity (range 0.13–0.2; Mann–Whitney U-test, P = 0.013).
Discussion
This study of human ectopic endometriotic lesions and a baboon model analysing both eutopic and ectopic endometrial cells during the establishment and progression of endometriosis extends our previous work. The endometrial cells from the ectopic sites (both human and baboon) showed immune-staining for all the proliferative markers, with concomitant loss of the γ-H2AX staining. In the animals with induced disease, there was an increased expression of markers of proliferation in the eutopic endometrium after inoculation, which persisted up to 15 months. This suggests that the development of a pro-proliferative phenotype is involved in the establishment of endometriosis. We also confirmed the observed increase in telomerase immunoreactivity with telomerase activity detected by the more established TRAP method. There was some decline in the staining intensity for proliferative markers and γ-H2AX staining 12 months after inoculation in the ectopic lesions. We also observed an expression pattern of the regulators of cell fate studied in the control group of baboons without endometriosis that was similar to that found in the eutopic endometrium of the experimental animals prior to disease induction.
All active human peritoneal endometriotic lesions showed intense immune-reactivity for the three proliferative markers studied; yet, weak or absent immunostaining was observed for γ-H2AX. This is the first demonstration of telomerase, nucleolin and γ-H2AX in ectopic human endometrium. Endometrial cells in ectopic sites in baboons with induced disease also expressed the same proliferative markers as women with endometriosis. There is a plethora of evidence that ectopic endometrial tissues have pro-proliferative, invasive properties and are resistant to apoptosis (reviewed in (Garai et al., 2006; Harada et al., 2007) references therein). The pro-proliferative phenotype and the resistance to senescence and apoptosis will give these endometriotic cells a survival advantage in the more ‘hostile’ environment of the peritoneal cavity. We observed intense staining for the three proliferative markers studied in the ectopic red, blue and chocolate endometriotic lesions in the baboons with parallel loss of γ-H2AX staining at 6 and 12 months after the induction of the disease. Our findings suggest that the expression of the three proliferative markers (telomerase, nucleolin and PCNA) plays a greater role in the initial establishment of the endometriotic deposits than in the later stages, at least in the baboon model. Nucleolin expression may control endometrial cell proliferation and growth by direct involvement in DNA repair and re-modelling (Mongelard and Bouvet, 2007). PCNA levels are particularly high in proliferating cells, and PCNA can prevent cell death by becoming involved in DNA repair (Moldovan and Jentsch, 2007; Andersen et al., 2008). Our γ-H2AX data are consistent with the hypothesis that a hyper-efficient DNA damage repair mechanism prevails in the cells (Hapangama et al., 2009), but then progressive loss of γ-H2AX staining is seen in both eutopic cells and in the ectopic blue lesions with the development of endometriosis. The lack of proliferative markers seen in the white/scar lesions is in agreement with the previously suggested non-progressive, dormant nature of these lesions (Nisolle et al., 1993). Thus, ‘burnout’ of the condition may be reflected in the loss of the pro-proliferative phenotype.
Our results also suggest that the establishment of ectopic endometriotic lesions induces changes in the superficial layer of the eutopic endometrium to favour the cell kinetics of endometrial cells towards a pro-proliferative and pro-survival phenotype by expressing telomerase, nucleolin and PCNA with the parallel loss of γ-H2AX staining. The eutopic endometrium of women with endometriosis also showed a similar staining pattern to that which we observed in the baboons with induced disease at 6–12 months after inoculation (Hapangama et al., 2009). The changes in the three proliferative markers we report here are similar to changes in estrogen regulated genes and proteins together with the ultra-structural morphology of a proliferative phenotype seen in the baboon eutopic endometrial cells after induction of the disease (Hastings and Fazleabas, 2006; Gashaw et al., 2006, Jones et al., 2009). Indeed, telomerase expression is directly regulated by estrogen (Boggess et al., 2006), and it is likely that estrogen also has a role in regulating both nucleolin and PCNA.
We have previously described that in women with endometriosis there is an increased expression of these markers (particularly telomerase) in the eutopic endometrium with a concomitant loss of stromal estrogen receptor beta (ERβ) expression (Hapangama et al., 2008a). Since telomerase is inhibited by ERβ (Stettner et al., 2007), we propose that the comparable loss of ERβ staining reported in the baboon model (Hastings and Fazleabas, 2006; Jackson et al., 2007) mediates the changes seen in the proliferative markers studied in the eutopic baboon endometrium during the establishment of the disease. The ERβ/ERα expression ratio in peritoneal endometriotic lesions has been reported to be different from ovarian lesions and to be more akin to eutopic endometrial cells (Bukulmez et al., 2008), therefore the estrogen-dependent cell cycle regulation is also likely to be different in the peritoneal lesions than that in ovarian endometriotic cells (Trukhacheva et al., 2009).
The lack of proliferative markers in the presence of γ-H2AX staining seen in the control and pre-induction baboon eutopic endometrial cells may suggest cellular senescence caused by telomere dysfunction (Sedelnikova et al., 2002; Fernandez-Capetillo et al., 2004; Von Zglinicki et al., 2004; Fillingham et al., 2006). This is concordant with the observed decrease in telomerase immune-reactivity during the WOI in these animals, which would assist in embryo implantation (Hapangama et al., 2008a,b, 2009). The possible excessive proliferation and lack of apoptosis/senescence of endometrial cells in the eutopic endometrium may both play a role in the reduced fecundity, which has been reported in baboons with induced disease (D'Hooghe et al., 1996).
The primary technique in this investigation was immunohistochemistry that yields information about the localization of our markers of interest at a cellular level and is semi-quantitative. To supplement immunohistochemistry and extend the information available about telomerase expression, we used the TRAP assay that provides a functional correlate of the pro-proliferative phenotype we propose (Kim et al., 2007a,b; Hapangama et al., 2008a, 2009; Tichon et al., 2009). Although our argument would be strengthened by other parallel quantitative techniques such as western blotting for these proteins and RT–PCR for the relevant mRNA species, they would not add to the biological plausibility of our results and would not supply topographical information. Notwithstanding these potential limitations, our data are germane to the issues. Therefore, the findings presented here are consistent with our previous work in eutopic endometrium from women with regard to topography and quantitative patterns of expression (Hapangama et al., 2008a, 2009). Furthermore, our data are also consistent with other work on endometriosis using a range of techniques, including mRNA and DNA microarrays (Kim et al. 2007a,b; Aghajanova et al., 2010).
Our observations suggest that the establishment of endometriotic lesions is associated with the induction of a pro-proliferative phenotype in eutopic endometrium. A definitive demonstration of induction would require more frequent sampling in the months after inoculation. If induction is indeed occurring, it could reflect a circulating substance or the long-distance ‘paracrine’ action of a substance that can pass between the peritoneal cavity and the endometrial lumen. Furthermore, establishment of ectopic lesions induces DNA methylation and altered gene expression in the eutopic endometrium in a murine model of endometriosis (Lee et al., 2009), suggesting that epigenetic regulation may contribute to the regulation of eutopic endometrium. There is evidence that endometriosis is associated with systemic subclinical inflammation, and peritoneal fluid in women with endometriosis, in particular, has been shown to be enriched with various cytokines suggesting local inflammation (Agic et al., 2006 and references therein). There is a natural flow of peritoneal fluid through fallopian tubes from the pelvis into the uterine cavity that may ‘induce’ the eutopic endometrium and repeated retrograde menstruation of eutopic cells that have been induced provides a positive feed-back loop for the maintenance of endometriosis and the continued secretion of these cytokines by eutopic and/or ectopic endometrium. We can only speculate about the initial trigger for the establishment of ectopic endometrial lesions. It appears likely that there is a balance between seeding and not seeding at the peritoneal surface. In the baboon model of induced endometriosis, the inoculation of a relatively large amount of menstrual fluid appears to tip the balance towards implantation. There is a significant linear correlation between the numbers of lesions seen after intra-peritoneal inoculation of menstrual endometrium in baboons with the amount of tissue injected (D'Hooghe et al., 1995). The presence of the cellular component as well as the acellular menstrual exudates has a synergistic effect on the formation of the ectopic lesions (Kyama et al., 2008). Furthermore, it is interesting to note that the intra-peritoneal rather than the retro-peritoneal inoculation of the menstrual tissue yielded more ectopic lesion (D'Hooghe et al., 1995). This could represent a large number of cells or a high dose of substance(s) acting in a paracrine manner. In women, any increase in retrograde menstruation (such as obstruction to anterograde menstruation) increases the incidence of endometriosis (MacKenzie and Casey et al., 1975; D'Hooghe et al., 1991; Dick et al., 2003).
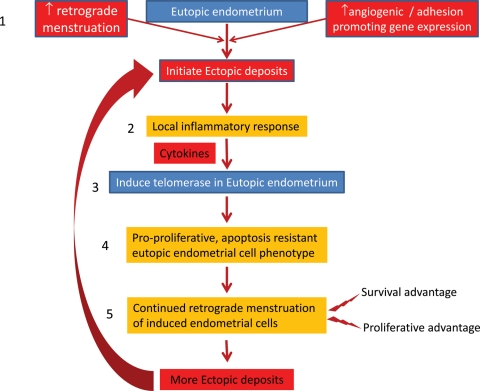
One feasible explanation for these findings in light of our observations in the baboon is the following proposed model for the pathogenesis of endometriosis (summary of Fig. 6). Firstly, ectopic endometriotic deposits are initiated by an increase in retrograde menstruation or increased activity in genes that promote angiogenesis and adhesion (Gashaw et al., 2006). Secondly, the ectopic endometriotic deposits induce a local inflammatory response and secrete various cytokines. Thirdly, cytokines, or other substances, act on the eutopic endometrium to induce the pro-proliferative markers. Fourthly, the induced eutopic endometrial cells express telomerase and adopt the pro-proliferative, apoptosis-resistant phenotype that has a survival advantage in the peritoneal cavity. Finally, retrograde menstruation of endometrium with the pro-proliferative phenotype together with other genes that also promote cell survival (Hastings and Fazleabas, 2006) gives rise to further endometriotic deposits and maintains the disease.
Figure 6.
Summary figure: our proposed model for the pathogenesis of endometriosis: (1) ectopic endometriotic deposits are initiated by an increase in retrograde menstruation or an increase activity in genes that promote angiogenesis and adhesion (Gashaw et al., 2006). (2) The ectopic endometriotic deposits induce a local inflammatory response and secrete various cytokines. (3) The cytokines (or other substances) act on the eutopic endometrium to induce the pro-proliferative markers. (4) The induced eutopic endometrial cells express telomerase and adopt the pro-proliferative, apoptosis-resistant phenotype, which has a survival advantage in the peritoneal cavity. (5) Finally, retrograde menstruation of induced eutopic endometrium with the pro-proliferative phenotype together with other genes that also promote cell survival (Hastings and Fazleabas, 2006) gives rise to further endometriotic deposits and maintains the disease.
In summary, we have shown that endometrium in endometriotic deposits in women, and matched ectopic and eutopic endometrium, in a baboon model of endometriosis shows a pro-proliferative phenotype that is similar to the phenotype we have previously described in the eutopic endometrium of women with endometriosis. We speculate that the initiation of the disease in the baboon model is associated with induction of the pro-proliferative phenotype in the eutopic endometrium and that this process of induction may be important for the maintenance of the disease.
Funding
This research was supported by Wellbeing of women (RG1073), RCOG Endometriosis millennium grant and University of Liverpool RDF to D.K.H. and Eunice Kennedy Shriver NICHD/NIH through cooperative agreement [U54 HD 40093] as part of the ‘Specialized Cooperative Centre Program in Reproduction and Infertility Research’ to ATF.
Acknowledgement
Authors are grateful for the statistical advice from Dr Anna Hart, University of Central Lancaster, UK.
References
- Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, Giudice LC. The protein kinase a pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341–1355. doi: 10.1210/en.2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agic A, Xu H, Finas D, Banz C, Diedrich K, Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest. 2006;62:139–147. doi: 10.1159/000093121. doi:10.1159/000093121. [DOI] [PubMed] [Google Scholar]
- Andersen PL, Xu F, Xiao W. Eukaryotic DNA damage tolerance and trans-lesion synthesis through covalent modifications of PCNA. Cell Res. 2008;18:162–173. doi: 10.1038/cr.2007.114. doi:10.1038/cr.2007.114. [DOI] [PubMed] [Google Scholar]
- Boggess JF, Zhou C, Bae-Jump VL, Gehrig PA, Whang YE. Estrogen-receptor-dependent regulation of telomerase activity in human endometrial cancer cell lines. Gynecol Oncol. 2006;103:417–424. doi: 10.1016/j.ygyno.2006.03.032. doi:10.1016/j.ygyno.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Bukulmez O, Hardy DB, Carr BR, Word RA, Mendelson CR. Inflammatory status influences aromatase and steroid receptor expression in endometriosis. Endocrinology. 2008;149:1190–1204. doi: 10.1210/en.2007-0665. doi:10.1210/en.2007-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun S. Endometriosis. N Engl J Med. 2009;360:268–279. doi: 10.1056/NEJMra0804690. doi:10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Bambra C, Cornillie FJ, Isahakia M, Koninckx PR. Prevalence and laparoscopic appearance of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) Biol Reprod. 1991;45:411–416. doi: 10.1095/biolreprod45.3.411. doi:10.1095/biolreprod45.3.411. [DOI] [PubMed] [Google Scholar]
- D'Hooghe T, Bambra C, Raeymaekers BM, De Jonge I, Lauweryns JM, Koninckx PR. Intrapelvic injection of menstrual endometrium causes endometriosis in baboons. Am J Obstet Gynecol. 1995;173:125–134. doi: 10.1016/0002-9378(95)90180-9. [DOI] [PubMed] [Google Scholar]
- D'Hooghe TM, Riday AM, Bambra CS, Suleman MA, Raeymaekers MS, Koninckx PR. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and III-IV disease. Fertil Steril. 1996;66:809–813. [PubMed] [Google Scholar]
- Dick EJ, Jr, Hubbard GB, Martin LJ, Leland MM. Record review of baboons with histologically confirmed endometriosis in a large established colony. J Med Primatol. 2003;32:39–47. doi: 10.1034/j.1600-0684.2003.00008.x. doi:10.1034/j.1600-0684.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci. 2002;955:308–317. doi: 10.1111/j.1749-6632.2002.tb02791.x. doi:10.1111/j.1749-6632.2002.tb02791.x. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Chai D, Langoi D, Bulun SE. Steroid receptor and aromatase expression in baboon endometriotic lesions. Fertil Steril. 2003;80:820–827. doi: 10.1016/s0015-0282(03)00982-8. doi:10.1016/S0015-0282(03)00982-8. [DOI] [PubMed] [Google Scholar]
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair. 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Fillingham J, Keogh MC, Krogan NJ. GammaH2AX and its role in DNA double-strand break repair. Biochem Cell Biol. 2006;84:568–577. doi: 10.1139/o06-072. doi:10.1139/O06-072. [DOI] [PubMed] [Google Scholar]
- Franco S, Gostissa M, Zha S, Lombard DB, Murphy MM, Zarrin AA, Yan C, Tepsuporn S, Morales JC, Adams MM, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. doi:10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Garai J, Molnar V, Varga T, Koppan M, Torok A, Bodis J. Endometriosis: harmful survival of an ectopic tissue. Front Biosci. 2006;11:595–619. doi: 10.2741/1821. doi:10.2741/1821. [DOI] [PubMed] [Google Scholar]
- Gashaw I, Hastings JM, Jackson KS, Winterhager E, Fazleabas AT. Induced endometriosis in the baboon (Papio anubis) increases the expression of the proangiogenic factor CYR61 (CCN1) in eutopic and ectopic endometria. Biol Reprod. 2006;74:1060–1066. doi: 10.1095/biolreprod.105.049320. doi:10.1095/biolreprod.105.049320. [DOI] [PubMed] [Google Scholar]
- Grinstein E, Wernet P. Cellular signaling in normal and cancerous stem cells. Cell Signal. 2007;19:2428–2433. doi: 10.1016/j.cellsig.2007.06.021. doi:10.1016/j.cellsig.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Critchley H, Henderson TA, Baird DT. Mifepristone-induced vaginal bleeding is associated with increased immunostaining for cyclooxygenase-2 and decrease in prostaglandin dehydrogenase in luteal phase endometrium. J Clin Endocrinol Metab. 2002;87:5229–5234. doi: 10.1210/jc.2002-020429. doi:10.1210/jc.2002-020429. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner M, Drury JA, Quenby S, Saretzki G, Martin-Ruiz C, Von Zglinicki T. Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Hum Reprod. 2008a;23:1511–1519. doi: 10.1093/humrep/den172. doi:10.1093/humrep/den172. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner M, Drury JA, Saretzki G, Martin-Ruiz C, Von Zglinicki T, Quenby S. Endometrial telomerase shows specific expression patterns in different types of reproductive failure. Reprod Biomed Online. 2008b;17:416–424. doi: 10.1016/s1472-6483(10)60227-1. [DOI] [PubMed] [Google Scholar]
- Hapangama DK, Turner M, Drury JA, Quenby S, Hart A, Maddick M, Martin-Ruiz C, Von Zglinicki T. Sustained replication in endometrium of women with endometriosis occurs without evoking a DNA damage response. Hum Reprod. 2009;24:687–696. doi: 10.1093/humrep/den416. doi:10.1093/humrep/den416. [DOI] [PubMed] [Google Scholar]
- Harada T, Taniguchi F, Izawa M, Ohama Y, Takenaka Y, Tagashira Y, Ikeda A, Watanabe A, Iwabe T, Terakawa N. Apoptosis and endometriosis. Front Biosci. 2007;12:3140–3151. doi: 10.2741/2302. doi:10.2741/2302. [DOI] [PubMed] [Google Scholar]
- Hastings JM, Fazleabas AT. A baboon model for endometriosis: implications for fertility. Reprod Biol Endocrinol. 2006;4:S7. doi: 10.1186/1477-7827-4-S1-S7. doi:10.1186/1477-7827-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KS, Brudney A, Hastings JM, Mavrogianis PA, Kim JJ, Fazleabas AT. The altered distribution of the steroid hormone receptors and the chaperone immunophilin FKBP52 in a baboon model of endometriosis is associated with progesterone resistance during the window of uterine receptivity. Reprod Sci. 2007;14:137–150. doi: 10.1177/1933719106298409. doi:10.1177/1933719106298409. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Nardo LG, Litta P, Fazleabas AT. Ultrastructure of ectopic peritoneal lesions from women with endometriosis, including observations on the contribution of coelomic mesothelium. Reprod Sci. 2009;16:43–55. doi: 10.1177/1933719108324891. doi:10.1177/1933719108324891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, Wu Y, Guo SW, Fazleabas AT. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007a;13:323–332. doi: 10.1093/molehr/gam005. doi:10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- Kim CM, Oh YJ, Cho SH, Chung DJ, Hwang JY, Park KH, Cho DJ, Choi YM, Lee BS. Increased telomerase activity and human telomerase reverse transcriptase mRNA expression in the endometrium of patients with endometriosis. Hum Reprod. 2007b;22:843–849. doi: 10.1093/humrep/del425. doi:10.1093/humrep/del425. [DOI] [PubMed] [Google Scholar]
- Kyama CM, Falconer H, Chai D, Cuneo S, Mihalyi A, Mwenda JM, D'Hooghe TM. Acellular menstrual fluid can induce endometriosis in baboons. Hum Reprod. 2008;23:i65. [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80:79–85. doi: 10.1095/biolreprod.108.070391. doi:10.1095/biolreprod.108.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie WF, Casey HW. Animal model of human disease: endometriosis, animal model: endometriosis in rhesus monkeys. Am J Pathol. 1975;80:341–344. [PMC free article] [PubMed] [Google Scholar]
- Moldovan GL, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. doi:10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Mongelard F, Bouvet P. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 2007;17:80–86. doi: 10.1016/j.tcb.2006.11.010. doi:10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Nisolle M, Casanas-Roux F, Anaf V, Mine J, Donnez J. Morphometric study of the stromal vascularisation in peritoneal endometriosis. Fertil Steril. 1993;59:681–684. [PubMed] [Google Scholar]
- Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:422–425. [Google Scholar]
- Sedelnikova OA, Rogakou EP, Panyutin IG, Bonner WM. Quantitative detection of (125) IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158:486–492. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. doi:10.1667/0033-7587(2002)158[0486:QDOIID]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Stettner M, Kaulfuss S, Burfeind P, Schweyer S, Strauss A, Ringert RH, Thelen P. The relevance of estrogen receptor-beta expression to the antiproliferative effects observed with histone deacetylase inhibitors and phytoestrogens in prostate cancer treatment. Mol Cancer Ther. 2007;6:2626–2633. doi: 10.1158/1535-7163.MCT-07-0197. doi:10.1158/1535-7163.MCT-07-0197. [DOI] [PubMed] [Google Scholar]
- Tichon A, Gowda BK, Slavin S, Gazit A, Priel E. Telomerase activity and expression in adult human mesenchymal stem cells derived from amyotrophic lateral sclerosis individuals. Cytotherapy. 2009;11:837–848. doi: 10.3109/14653240903136979. doi:10.3109/14653240903136979. [DOI] [PubMed] [Google Scholar]
- Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, Bulun SE. Estrogen receptor (ER) {beta} regulates ER{alpha} expression in stromal cells derived from ovarian endometriosis. J Clin Endocrinol Metab. 2009;94:615–622. doi: 10.1210/jc.2008-1466. doi:10.1210/jc.2008-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–371. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Von Zglinicki T, Saretzki G, Ladhoff J, d'Adda di Fagana F, Jackson SP. Human cell senescence as a DNA damage response. Mech Ageing Dev. 2004;126:111–117. doi: 10.1016/j.mad.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4:407–412. doi: 10.1093/molehr/4.4.407. doi:10.1093/molehr/4.4.407. [DOI] [PubMed] [Google Scholar]
- Wang C, Mavrogianis PA, Fazleabas AT. Endometriosis is associated with progesterone resistance in the baboon (Papio anubis) oviduct: evidence based on the localization of oviductal glycoprotein 1 (OVGP1) Biol Reprod. 2009;80:272–278. doi: 10.1095/biolreprod.108.072496. doi:10.1095/biolreprod.108.072496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P, Benhattar J, Seelentag W, Stehle JC, Bosman FT. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004;121:391–397. doi: 10.1007/s00418-004-0645-5. [DOI] [PubMed] [Google Scholar]