Abstract
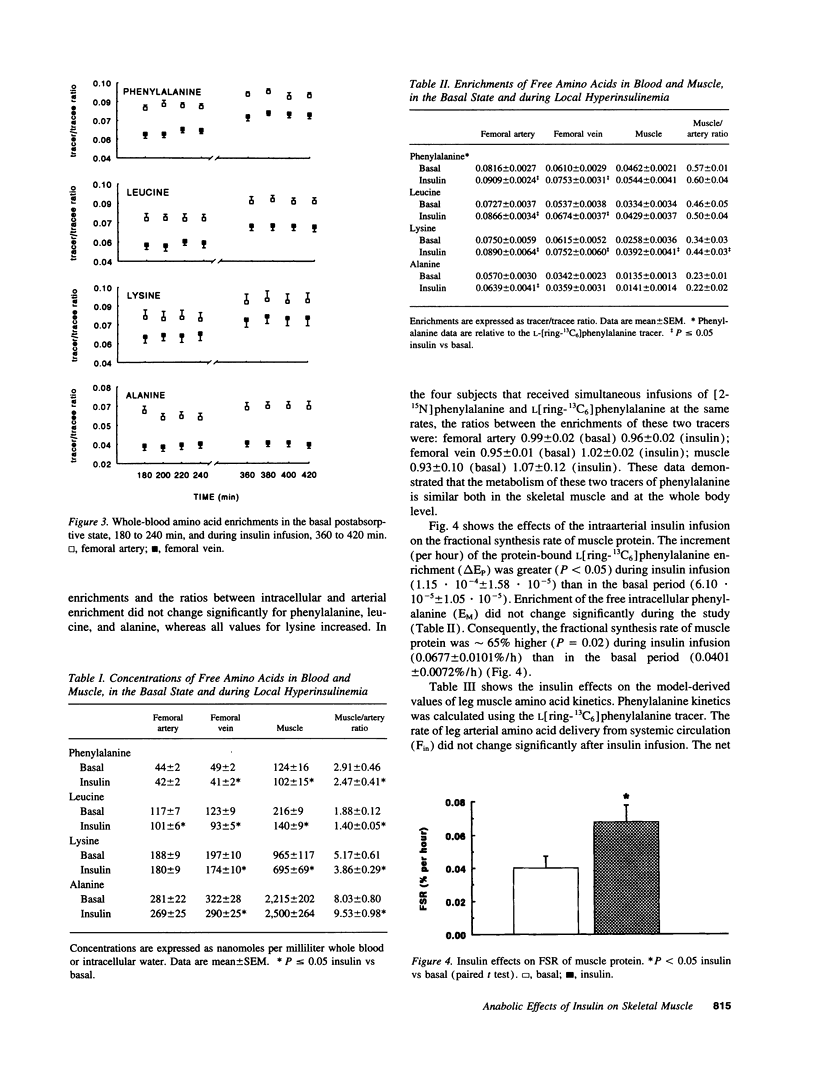
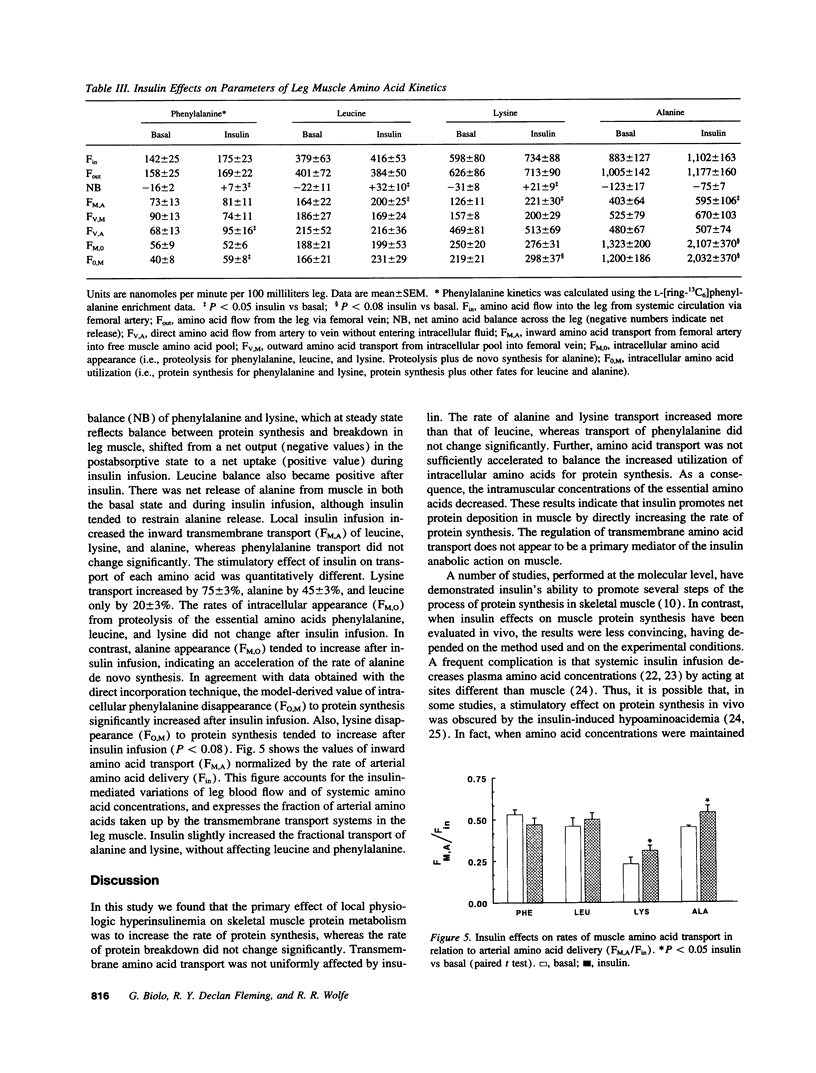
We have investigated the mechanisms of the anabolic effect of insulin on muscle protein metabolism in healthy volunteers, using stable isotopic tracers of amino acids. Calculations of muscle protein synthesis, breakdown, and amino acid transport were based on data obtained with the leg arteriovenous catheterization and muscle biopsy. Insulin was infused (0.15 mU/min per 100 ml leg) into the femoral artery to increase femoral venous insulin concentration (from 10 +/- 2 to 77 +/- 9 microU/ml) with minimal systemic perturbations. Tissue concentrations of free essential amino acids decreased (P < 0.05) after insulin. The fractional synthesis rate of muscle protein (precursor-product approach) increased (P < 0.01) after insulin from 0.0401 +/- 0.0072 to 0.0677 +/- 0.0101%/h. Consistent with this observation, rates of utilization for protein synthesis of intracellular phenylalanine and lysine (arteriovenous balance approach) also increased from 40 +/- 8 to 59 +/- 8 (P < 0.05) and from 219 +/- 21 to 298 +/- 37 (P < 0.08) nmol/min per 100 ml leg, respectively. Release from protein breakdown of phenylalanine, leucine, and lysine was not significantly modified by insulin. Local hyperinsulinemia increased (P < 0.05) the rates of inward transport of leucine, lysine, and alanine, from 164 +/- 22 to 200 +/- 25, from 126 +/- 11 to 221 +/- 30, and from 403 +/- 64 to 595 +/- 106 nmol/min per 100 ml leg, respectively. Transport of phenylalanine did not change significantly. We conclude that insulin promoted muscle anabolism, primarily by stimulating protein synthesis independently of any effect on transmembrane transport.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airhart J., Arnold J. A., Stirewalt W. S., Low R. B. Insulin stimulation of protein synthesis in cultured skeletal and cardiac muscle cells. Am J Physiol. 1982 Jul;243(1):C81–C86. doi: 10.1152/ajpcell.1982.243.1.C81. [DOI] [PubMed] [Google Scholar]
- Arfvidsson B., Zachrisson H., Möller-Loswick A. C., Hyltander A., Sandström R., Lundholm K. Effect of systemic hyperinsulinemia on amino acid flux across human legs in postabsorptive state. Am J Physiol. 1991 Jan;260(1 Pt 1):E46–E52. doi: 10.1152/ajpendo.1991.260.1.E46. [DOI] [PubMed] [Google Scholar]
- Baumann P. Q., Stirewalt W. S., O'Rourke B. D., Howard D., Nair K. S. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol. 1994 Aug;267(2 Pt 1):E203–E209. doi: 10.1152/ajpendo.1994.267.2.E203. [DOI] [PubMed] [Google Scholar]
- Bennet W. M., Connacher A. A., Scrimgeour C. M., Jung R. T., Rennie M. J. Euglycemic hyperinsulinemia augments amino acid uptake by human leg tissues during hyperaminoacidemia. Am J Physiol. 1990 Aug;259(2 Pt 1):E185–E194. doi: 10.1152/ajpendo.1990.259.2.E185. [DOI] [PubMed] [Google Scholar]
- Bergström J., Fürst P., Norée L. O., Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974 Jun;36(6):693–697. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- Biolo G., Chinkes D., Zhang X. J., Wolfe R. R. Harry M. Vars Research Award. A new model to determine in vivo the relationship between amino acid transmembrane transport and protein kinetics in muscle. JPEN J Parenter Enteral Nutr. 1992 Jul-Aug;16(4):305–315. doi: 10.1177/0148607192016004305. [DOI] [PubMed] [Google Scholar]
- Biolo G., Gastaldelli A., Zhang X. J., Wolfe R. R. Protein synthesis and breakdown in skin and muscle: a leg model of amino acid kinetics. Am J Physiol. 1994 Sep;267(3 Pt 1):E467–E474. doi: 10.1152/ajpendo.1994.267.3.E467. [DOI] [PubMed] [Google Scholar]
- Biolo G., Wolfe R. R. Insulin action on protein metabolism. Baillieres Clin Endocrinol Metab. 1993 Oct;7(4):989–1005. doi: 10.1016/s0950-351x(05)80242-3. [DOI] [PubMed] [Google Scholar]
- Bonadonna R. C., Saccomani M. P., Cobelli C., DeFronzo R. A. Effect of insulin on system A amino acid transport in human skeletal muscle. J Clin Invest. 1993 Feb;91(2):514–521. doi: 10.1172/JCI116230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolati O., Laris P. C., Nucci F. A., Dall'Asta V., Longo N., Guidotti G. G., Gazzola G. C. Dependence of L-arginine accumulation on membrane potential in cultured human fibroblasts. Am J Physiol. 1987 Sep;253(3 Pt 1):C391–C397. doi: 10.1152/ajpcell.1987.253.3.C391. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990 Jan;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Denne S. C., Liechty E. A., Liu Y. M., Brechtel G., Baron A. D. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991 Dec;261(6 Pt 1):E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J. 1987 Apr 15;243(2):335–343. doi: 10.1042/bj2430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukagawa N. K., Minaker K. L., Young V. R., Rowe J. W. Insulin dose-dependent reductions in plasma amino acids in man. Am J Physiol. 1986 Jan;250(1 Pt 1):E13–E17. doi: 10.1152/ajpendo.1986.250.1.E13. [DOI] [PubMed] [Google Scholar]
- Fulks R. M., Li J. B., Goldberg A. L. Effects of insulin, glucose, and amino acids on protein turnover in rat diaphragm. J Biol Chem. 1975 Jan 10;250(1):290–298. [PubMed] [Google Scholar]
- Furuno K., Goldberg A. L. The activation of protein degradation in muscle by Ca2+ or muscle injury does not involve a lysosomal mechanism. Biochem J. 1986 Aug 1;237(3):859–864. doi: 10.1042/bj2370859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. J., Grant I. Amino acid infusion increases the sensitivity of muscle protein synthesis in vivo to insulin. Effect of branched-chain amino acids. Biochem J. 1988 Sep 1;254(2):579–584. doi: 10.1042/bj2540579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Glickman M. G., Castellino P., Louard R. J., DeFronzo R. A. Measurement of L-[1-14C]leucine kinetics in splanchnic and leg tissues in humans. Effect of amino acid infusion. Diabetes. 1988 Oct;37(10):1365–1372. doi: 10.2337/diab.37.10.1365. [DOI] [PubMed] [Google Scholar]
- Guidotti G. G., Borghetti A. F., Gazzola G. C. The regulation of amino acid transport in animal cells. Biochim Biophys Acta. 1978 Dec 15;515(4):329–366. doi: 10.1016/0304-4157(78)90009-6. [DOI] [PubMed] [Google Scholar]
- Heslin M. J., Newman E., Wolf R. F., Pisters P. W., Brennan M. F. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol. 1992 Jun;262(6 Pt 1):E911–E918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- Hundal H. S., Rennie M. J., Watt P. W. Characteristics of L-glutamine transport in perfused rat skeletal muscle. J Physiol. 1987 Dec;393:283–305. doi: 10.1113/jphysiol.1987.sp016824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundal H. S., Rennie M. J., Watt P. W. Characteristics of acidic, basic and neutral amino acid transport in the perfused rat hindlimb. J Physiol. 1989 Jan;408:93–114. doi: 10.1113/jphysiol.1989.sp017449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone S. T., Li K. X., Sperelakis N., Lathrop D. A. Insulin-induced hyperpolarization in mammalian skeletal muscle. Am J Physiol. 1989 Feb;256(2 Pt 1):C368–C374. doi: 10.1152/ajpcell.1989.256.2.C368. [DOI] [PubMed] [Google Scholar]
- Jahoor F., Herndon D. N., Wolfe R. R. Role of insulin and glucagon in the response of glucose and alanine kinetics in burn-injured patients. J Clin Invest. 1986 Sep;78(3):807–814. doi: 10.1172/JCI112644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson L. S., Koehler J. O., Morgan H. E. Effect of insulin on protein synthesis in skeletal muscle of an isolated perfused preparation of rat hemicorpus. Proc Natl Acad Sci U S A. 1972 Apr;69(4):816–820. doi: 10.1073/pnas.69.4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorfeldt L., Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978 Jan;27(1):97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L., Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971 Nov;41(5):459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Katch V., Weltman A. Predictability of body segment volumes in living subjects. Hum Biol. 1975 May;47(2):203–218. [PubMed] [Google Scholar]
- Kettelhut I. C., Wing S. S., Goldberg A. L. Endocrine regulation of protein breakdown in skeletal muscle. Diabetes Metab Rev. 1988 Dec;4(8):751–772. doi: 10.1002/dmr.5610040805. [DOI] [PubMed] [Google Scholar]
- Kimball S. R., Jefferson L. S. Cellular mechanisms involved in the action of insulin on protein synthesis. Diabetes Metab Rev. 1988 Dec;4(8):773–787. doi: 10.1002/dmr.5610040806. [DOI] [PubMed] [Google Scholar]
- Lowell B. B., Ruderman N. B., Goodman M. N. Evidence that lysosomes are not involved in the degradation of myofibrillar proteins in rat skeletal muscle. Biochem J. 1986 Feb 15;234(1):237–240. doi: 10.1042/bj2340237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCHESTER K. L., YOUNG F. G. The effect of insulin on incorporation of amino acids into protein of normal rat diaphragm in vitro. Biochem J. 1958 Nov;70(3):353–358. doi: 10.1042/bj0700353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller-Loswick A. C., Zachrisson H., Hyltander A., Körner U., Matthews D. E., Lundholm K. Insulin selectively attenuates breakdown of nonmyofibrillar proteins in peripheral tissues of normal men. Am J Physiol. 1994 Apr;266(4 Pt 1):E645–E652. doi: 10.1152/ajpendo.1994.266.4.E645. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Albertse E. C., Garlick P. J. Protein metabolism in skeletal muscle, diaphragm, and heart of diabetic rats. Am J Physiol. 1983 Dec;245(6):E604–E610. doi: 10.1152/ajpendo.1983.245.6.E604. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Royle G. T., Molnar J. A., Wolfe M. H., Wolfe R. R., Burke J. F. Urea, glucose and alanine kinetics in man: effects of glucose infusion. Clin Sci (Lond) 1982 May;62(5):553–556. doi: 10.1042/cs0620553. [DOI] [PubMed] [Google Scholar]
- Shotwell M. A., Kilberg M. S., Oxender D. L. The regulation of neutral amino acid transport in mammalian cells. Biochim Biophys Acta. 1983 May 24;737(2):267–284. doi: 10.1016/0304-4157(83)90003-5. [DOI] [PubMed] [Google Scholar]
- Tessari P., Inchiostro S., Biolo G., Vincenti E., Sabadin L. Effects of acute systemic hyperinsulinemia on forearm muscle proteolysis in healthy man. J Clin Invest. 1991 Jul;88(1):27–33. doi: 10.1172/JCI115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari P., Trevisan R., Inchiostro S., Biolo G., Nosadini R., De Kreutzenberg S. V., Duner E., Tiengo A., Crepaldi G. Dose-response curves of effects of insulin on leucine kinetics in humans. Am J Physiol. 1986 Sep;251(3 Pt 1):E334–E342. doi: 10.1152/ajpendo.1986.251.3.E334. [DOI] [PubMed] [Google Scholar]