Abstract
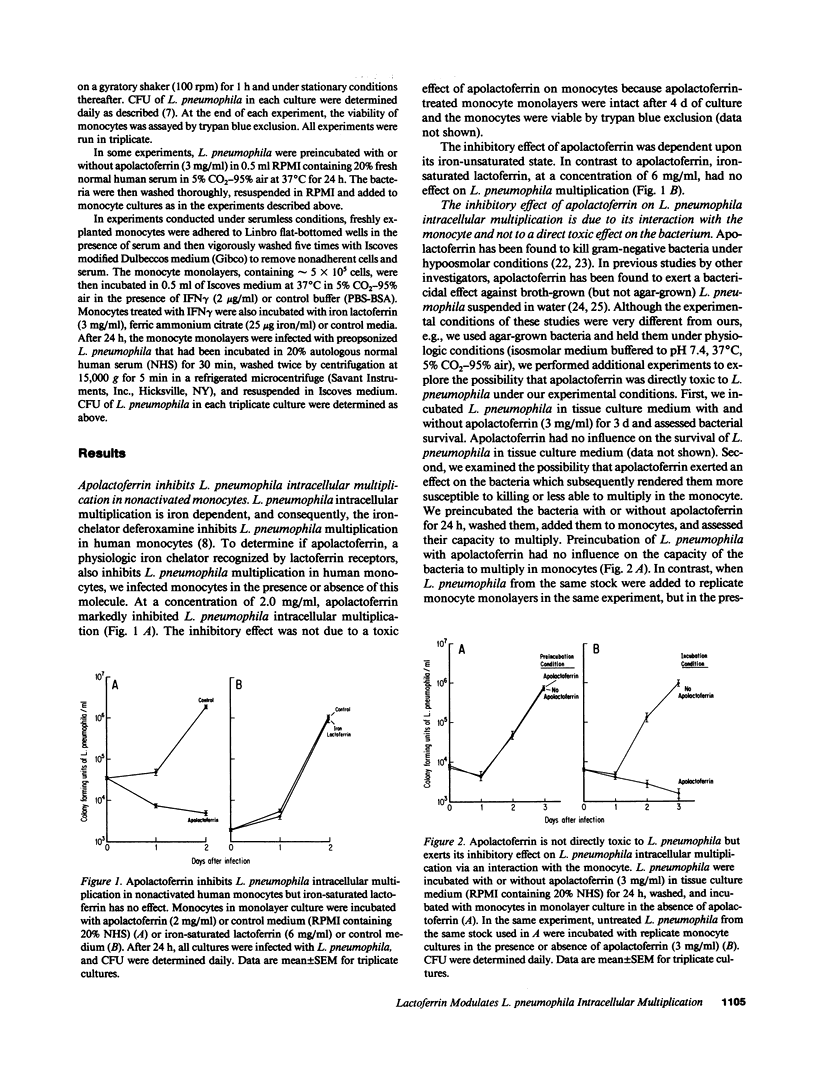
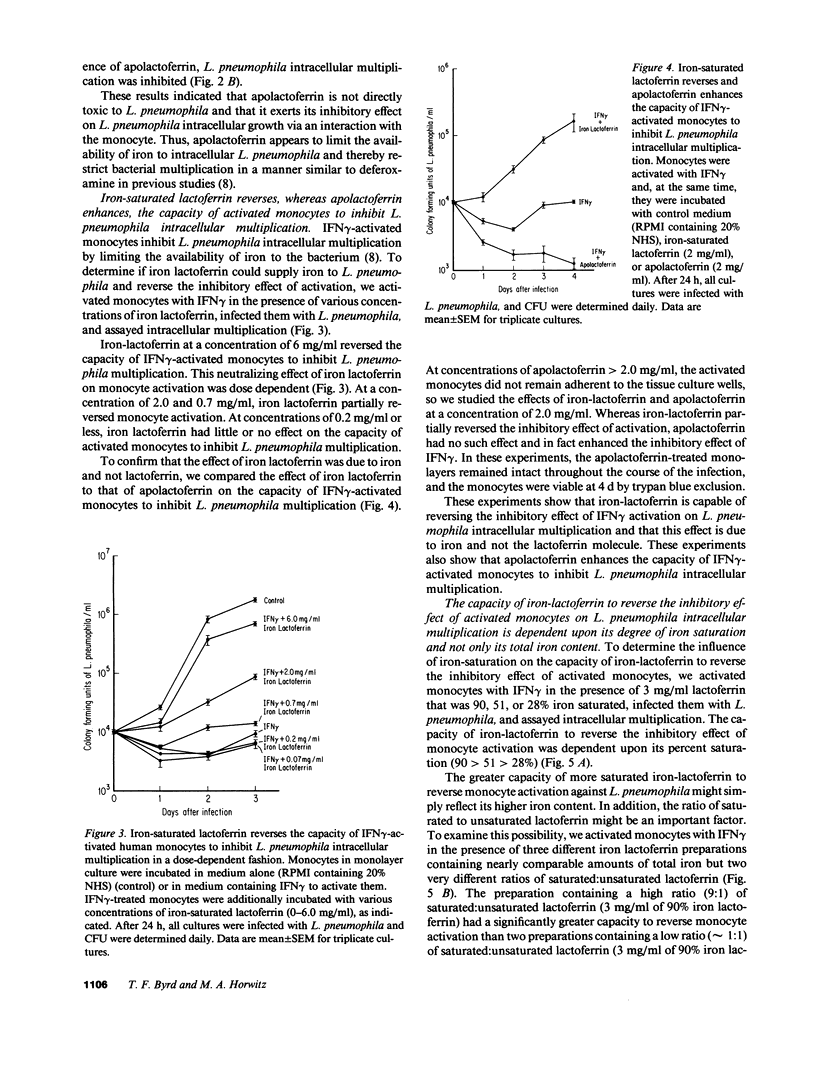
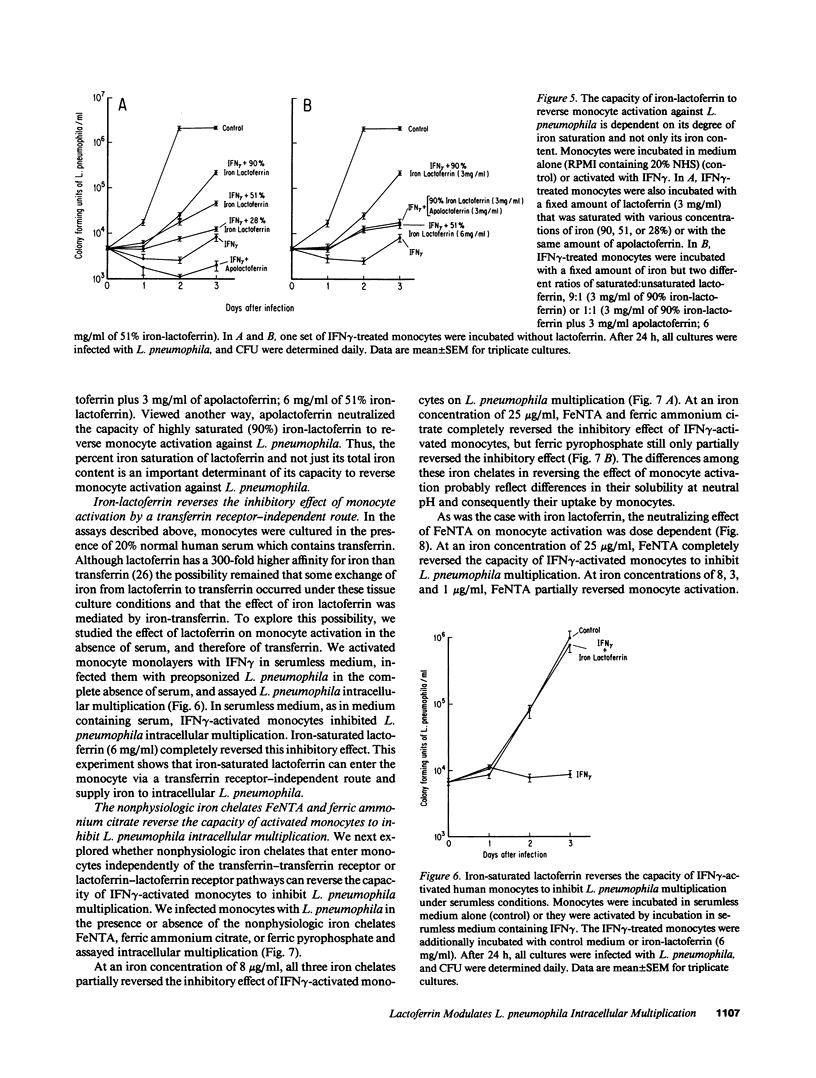
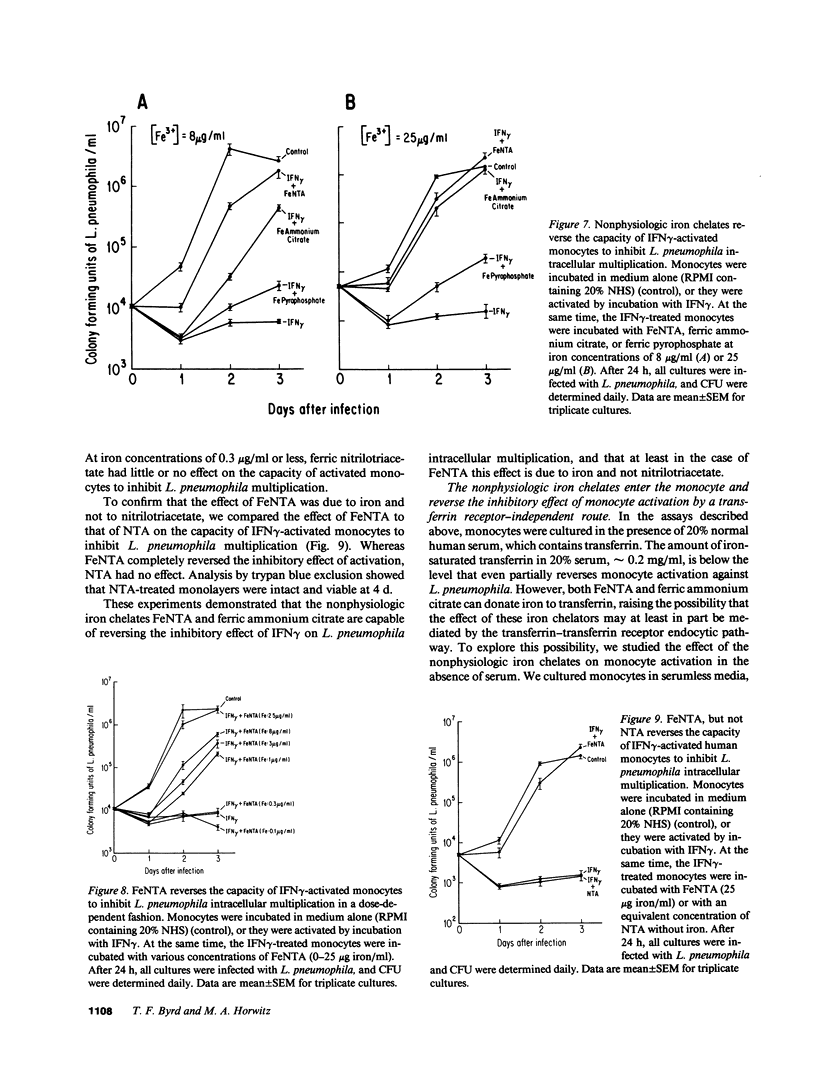
We have been exploring the role of iron in the pathogenesis of the intracellular bacterial pathogen Legionella pneumophila. In previous studies, we have demonstrated that L. pneumophila intracellular multiplication in human monocytes is iron dependent and that IFN gamma-activated monocytes inhibit L. pneumophila intracellular multiplication by limiting the availability of iron. In this study, we have investigated the effect on L. pneumophila intracellular multiplication of lactoferrin, an iron-binding protein which is internalized via specific receptors on monocytes, and of nonphysiologic iron chelates which enter monocytes by a receptor-independent route. Apolactoferrin completely inhibited L. pneumophila multiplication in nonactivated monocytes, and enhanced the capacity of IFN gamma-activated monocytes to inhibit L. pneumophila intracellular multiplication. In contrast, iron-saturated lactoferrin had no effect on the already rapid rate of L. pneumophila multiplication in nonactivated monocytes. Moreover, it reversed the capacity of activated monocytes to inhibit L. pneumophila intracellular multiplication, demonstrating that L. pneumophila can utilize iron from the lactoferrin-lactoferrin receptor pathway. The capacity of iron-lactoferrin to reverse monocyte activation was dependent upon its percent iron saturation and not just its total iron content. Similarly, the nonphysiologic iron chelates ferric nitrilotriacetate and ferric ammonium citrate completely reverse and ferric pyrophosphate partially reversed the capacity of IFN gamma-activated monocytes to inhibit L. pneumophila intracellular multiplication, demonstrating that L. pneumophila can utilize iron derived from nonphysiologic iron chelates internalized by monocytes independently of the transferrin and lactoferrin endocytic pathways. This study suggests that at sites of inflammation, lactoferrin may inhibit or promote L. pneumophila intracellular multiplication in mononuclear phagocytes depending upon its degree of iron saturation. In addition, this study suggests a potential role for PMN in host defense against L. pneumophila--providing apolactoferrin to infected monocytes--and it supports the concept that PMN and monocytes may cooperate in host defense against intracellular parasites and other pathogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P., Leibman A. Lactoferrin and transferrin: a comparative study. Biochim Biophys Acta. 1972 Feb 29;257(2):314–323. doi: 10.1016/0005-2795(72)90283-8. [DOI] [PubMed] [Google Scholar]
- Arnold R. R., Cole M. F., McGhee J. R. A bactericidal effect for human lactoferrin. Science. 1977 Jul 15;197(4300):263–265. doi: 10.1126/science.327545. [DOI] [PubMed] [Google Scholar]
- Bates G. W., Wernicke J. The kinetics and mechanism of iron(3) exchange between chelates and transferrin. IV. The reaction of transferrin with iron(3) nitrilotriacetate. J Biol Chem. 1971 Jun 10;246(11):3679–3685. [PubMed] [Google Scholar]
- Bennett R. M., Kokocinski T. Lactoferrin content of peripheral blood cells. Br J Haematol. 1978 Aug;39(4):509–521. doi: 10.1111/j.1365-2141.1978.tb03620.x. [DOI] [PubMed] [Google Scholar]
- Bhardwaj N., Nash T. W., Horwitz M. A. Interferon-gamma-activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986 Oct 15;137(8):2662–2669. [PubMed] [Google Scholar]
- Birgens H. S., Hansen N. E., Karle H., Kristensen L. O. Receptor binding of lactoferrin by human monocytes. Br J Haematol. 1983 Jul;54(3):383–391. doi: 10.1111/j.1365-2141.1983.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Birgens H. S., Kristensen L. O., Borregaard N., Karle H., Hansen N. E. Lactoferrin-mediated transfer of iron to intracellular ferritin in human monocytes. Eur J Haematol. 1988 Jul;41(1):52–57. doi: 10.1111/j.1600-0609.1988.tb00868.x. [DOI] [PubMed] [Google Scholar]
- Bortner C. A., Arnold R. R., Miller R. D. Bactericidal effect of lactoferrin on Legionella pneumophila: effect of the physiological state of the organism. Can J Microbiol. 1989 Nov;35(11):1048–1051. doi: 10.1139/m89-174. [DOI] [PubMed] [Google Scholar]
- Bortner C. A., Miller R. D., Arnold R. R. Bactericidal effect of lactoferrin on Legionella pneumophila. Infect Immun. 1986 Feb;51(2):373–377. doi: 10.1128/iai.51.2.373-377.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. H., Rankin M. C. Transferrin binding and iron uptake by mouse lymph node cells during transformation in response to concanavalin A. Immunology. 1981 Jun;43(2):393–398. [PMC free article] [PubMed] [Google Scholar]
- Brogan T. D., Ryley H. C., Neale L., Yassa J. Soluble proteins of bronchopulmonary secretions from patients with cystic fibrosis, asthma, and bronchitis. Thorax. 1975 Feb;30(1):72–79. doi: 10.1136/thx.30.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonrod J. D. The role of extracellular bactericidal factors in pulmonary host defense. Semin Respir Infect. 1986 Jun;1(2):118–129. [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison R. T., 3rd, Giehl T. J., LaForce F. M. Damage of the outer membrane of enteric gram-negative bacteria by lactoferrin and transferrin. Infect Immun. 1988 Nov;56(11):2774–2781. doi: 10.1128/iai.56.11.2774-2781.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein R. A., Sciortino C. V., McIntosh M. A. Role of iron in microbe-host interactions. Rev Infect Dis. 1983 Sep-Oct;5 (Suppl 4):S759–S777. doi: 10.1093/clinids/5.supplement_4.s759. [DOI] [PubMed] [Google Scholar]
- Gutteberg T. J., Haneberg B., Jørgensen T. The latency of serum acute phase proteins in meningococcal septicemia, with special emphasis on lactoferrin. Clin Chim Acta. 1984 Jan 31;136(2-3):173–178. doi: 10.1016/0009-8981(84)90289-4. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A. Cell-mediated immunity in Legionnaires' disease. J Clin Invest. 1983 Jun;71(6):1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Interaction of the Legionnaires' disease bacterium (Legionella pneumophila) with human phagocytes. I. L. pneumophila resists killing by polymorphonuclear leukocytes, antibody, and complement. J Exp Med. 1981 Feb 1;153(2):386–397. doi: 10.1084/jem.153.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Legionnaires' disease bacterium (Legionella pneumophila) multiples intracellularly in human monocytes. J Clin Invest. 1980 Sep;66(3):441–450. doi: 10.1172/JCI109874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- LaForce F. M., Boose D. S., Ellison R. T., 3rd Effect of aerosolized Escherichia coli and Staphylococcus aureus on iron and iron-binding proteins in lung lavage fluid. J Infect Dis. 1986 Dec;154(6):959–965. doi: 10.1093/infdis/154.6.959. [DOI] [PubMed] [Google Scholar]
- LaForce F. M., Boose D. S. Release of lactoferrin by polymorphonuclear leukocytes after aerosol challenge with Escherichia coli. Infect Immun. 1987 Sep;55(9):2293–2295. doi: 10.1128/iai.55.9.2293-2295.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W., Ekblom P. Iron delivery during proliferation and differentiation of kidney tubules. J Biol Chem. 1985 Dec 15;260(29):15580–15584. [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locksley R. M., Wilson C. B., Klebanoff S. J. Role for endogenous and acquired peroxidase in the toxoplasmacidal activity of murine and human mononuclear phagocytes. J Clin Invest. 1982 May;69(5):1099–1111. doi: 10.1172/JCI110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. J., Wauters G. Immunohistochemical localization and bacteriostatic properties of an iron-binding protein from bronchial mucus. Thorax. 1966 Nov;21(6):538–544. doi: 10.1136/thx.21.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Prignot J. Studies on the proteins of human bronchial secretions. Biochim Biophys Acta. 1965 Dec 16;111(2):466–478. doi: 10.1016/0304-4165(65)90056-5. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurier J., Legrand D., Hu W. L., Montreuil J., Spik G. Expression of human lactotransferrin receptors in phytohemagglutinin-stimulated human peripheral blood lymphocytes. Isolation of the receptors by antiligand-affinity chromatography. Eur J Biochem. 1989 Feb 1;179(2):481–487. doi: 10.1111/j.1432-1033.1989.tb14578.x. [DOI] [PubMed] [Google Scholar]
- Mazurier J., Spik G. Comparative study of the iron-binding properties of human transferrins. I. Complete and sequential iron saturation and desaturation of the lactotransferrin. Biochim Biophys Acta. 1980 May 7;629(2):399–408. doi: 10.1016/0304-4165(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Moguilevsky N., Masson P. L., Courtoy P. J. Lactoferrin uptake and iron processing into macrophages: a study in familial haemochromatosis. Br J Haematol. 1987 May;66(1):129–136. [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Nogueira N. M., Klebanoff S. J., Cohn Z. A. T. cruzi: sensitization to macrophage killing by eosinophil peroxidase. J Immunol. 1982 Apr;128(4):1705–1708. [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the human lactoferrin-binding protein from Neisseria meningitidis. Infect Immun. 1988 May;56(5):1144–1149. doi: 10.1128/iai.56.5.1144-1149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Morris L. J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988 Mar;2(2):281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- Silva M. T., Silva M. N., Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989 May;6(5):369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- Stein B. S., Bensch K. G., Sussman H. H. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984 Dec 10;259(23):14762–14772. [PubMed] [Google Scholar]
- Thompson A. B., Bohling T., Payvandi F., Rennard S. I. Lower respiratory tract lactoferrin and lysozyme arise primarily in the airways and are elevated in association with chronic bronchitis. J Lab Clin Med. 1990 Feb;115(2):148–158. [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- White G. P., Jacobs A. Iron uptake by Chang cells from transferrin, nitriloacetate and citrate complexes: the effects of iron-loading and chelation with desferrioxamine. Biochim Biophys Acta. 1978 Oct 3;543(2):217–225. doi: 10.1016/0304-4165(78)90066-1. [DOI] [PubMed] [Google Scholar]
- Winn W. C., Jr, Myerowitz R. L. The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol. 1981 May;12(5):401–422. doi: 10.1016/s0046-8177(81)80021-4. [DOI] [PubMed] [Google Scholar]
- Wright T. L., Fitz J. G., Weisiger R. A. Non-transferrin-bound iron uptake by rat liver. Role of membrane potential difference. J Biol Chem. 1988 Feb 5;263(4):1842–1847. [PubMed] [Google Scholar]
- Yamashiro D. J., Tycko B., Fluss S. R., Maxfield F. R. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984 Jul;37(3):789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- van Snick J. L., Markowetz B., Masson P. L. The ingestion and digestion of human lactoferrin by mouse peritoneal macrophages and the transfer of its iron into ferritin. J Exp Med. 1977 Sep 1;146(3):817–827. doi: 10.1084/jem.146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]


