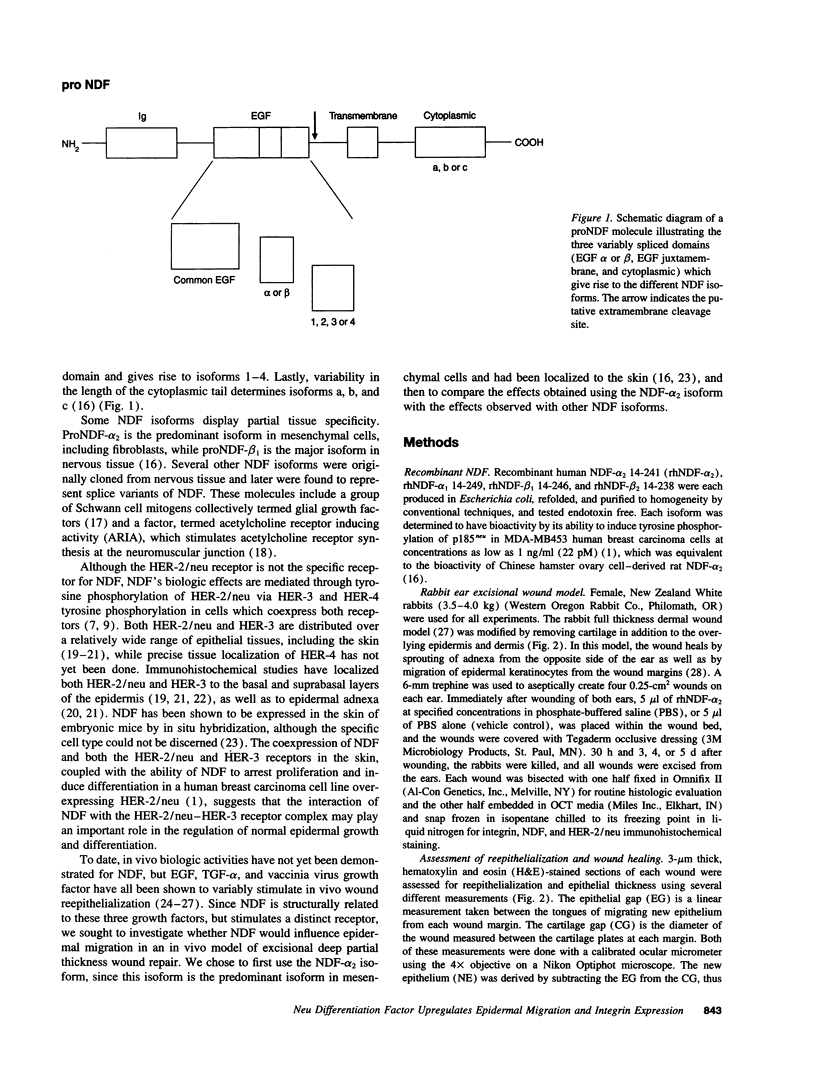
Abstract
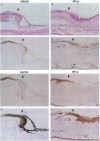
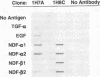
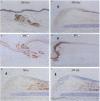
Neu differentiation factor (NDF) is a 44-kD glycoprotein which was isolated from ras-transformed rat fibroblasts and indirectly induces tyrosine phosphorylation of the HER-2/neu receptor via binding to either the HER-3 or HER-4 receptor. NDF contains a receptor binding epidermal growth factor (EGF)-like domain and is a member of the EGF family. There are multiple different isoforms of NDF which arise by alternative splicing of a single gene. To date, in vivo biologic activities have not been demonstrated for any NDF isoform. Since NDF, HER-2/neu, and HER-3 are present in skin, and other EGF family members can influence wound keratinocytes in vivo, we investigated whether NDF would stimulate epidermal migration and proliferation in a rabbit ear model of excisional wound repair. In this model, recombinant human NDF-alpha 2 (rhNDF-alpha 2), applied once at the time of wounding, induced a highly significant increase in both epidermal migration and epidermal thickness at doses ranging from 4 to 40 micrograms/cm2. In contrast, rhNDF-alpha 1, rhNDF-beta 1, and rhNDF-beta 2 had no apparent biologic effects in this model. rhNDF-alpha 2 also induced increased neoepidermal expression of alpha 5 and alpha 6 integrins, two of the earliest integrins to appear during epidermal migration. In addition, rhNDF-alpha 2-treated wounds exhibited increased neoepidermal expression of cytokeratin 10 and filaggrin, both epidermal differentiation markers. NDF alpha isoforms were expressed in dermal fibroblasts of wounded and unwounded skin, while both HER-2/neu and HER-3 were expressed in unwounded epidermis and dermal adnexa. In wounds, HER-2/neu expression was markedly decreased in the wound neoepidermis while neoepidermal HER-3 expression was markedly upregulated. Taken together, these results suggest that endogenous NDF-alpha 2 may function as a paracrine mediator directing initial epidermal migration during cutaneous tissue repair.
Full text
PDF

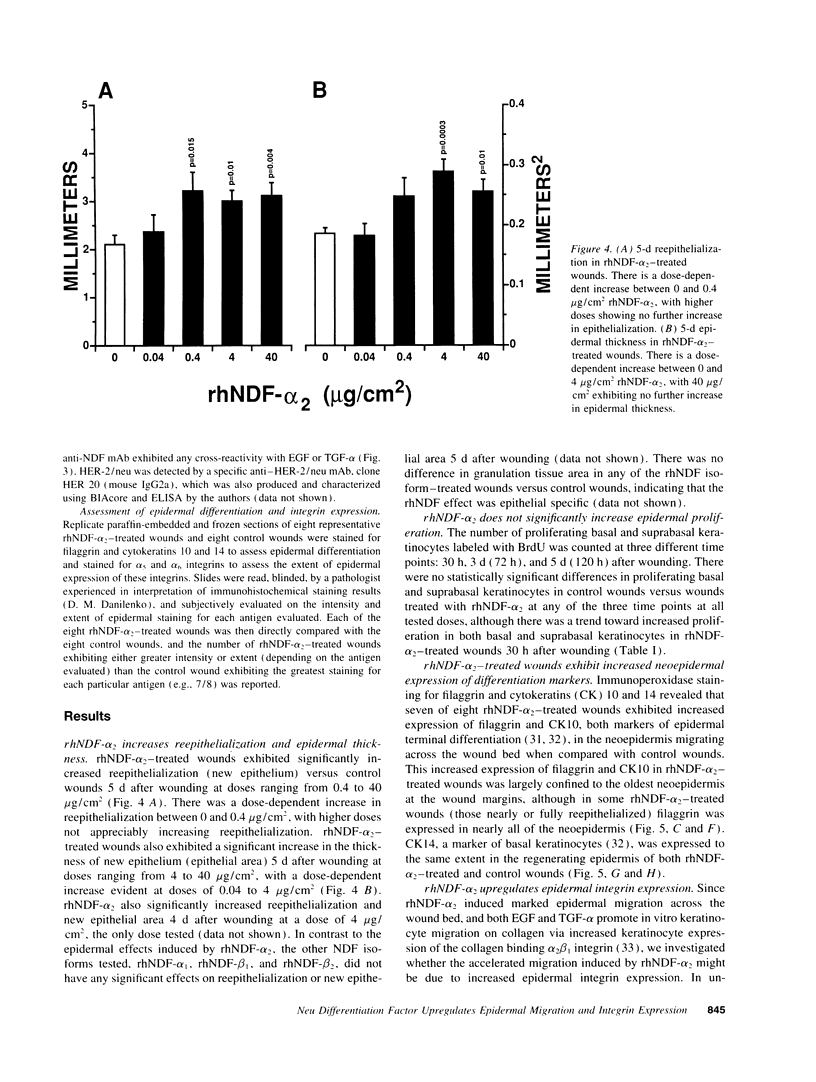

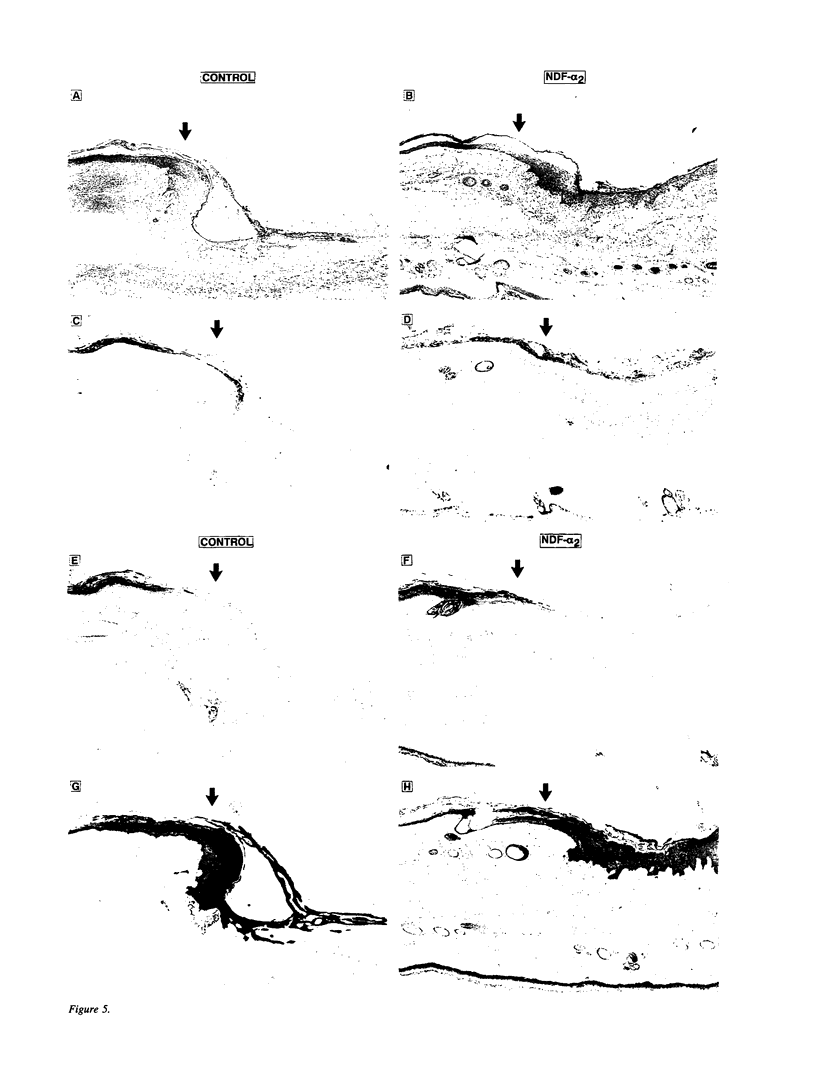




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Watt F. M. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes alpha 5 beta 1 integrin loss from the cell surface. Cell. 1990 Oct 19;63(2):425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Blecher S. R., Kapalanga J., Lalonde D. Induction of sweat glands by epidermal growth factor in murine X-linked anhidrotic ectodermal dysplasia. Nature. 1990 Jun 7;345(6275):542–544. doi: 10.1038/345542a0. [DOI] [PubMed] [Google Scholar]
- Brigham-Burke M., Edwards J. R., O'Shannessy D. J. Detection of receptor-ligand interactions using surface plasmon resonance: model studies employing the HIV-1 gp120/CD4 interaction. Anal Biochem. 1992 Aug 15;205(1):125–131. doi: 10.1016/0003-2697(92)90588-x. [DOI] [PubMed] [Google Scholar]
- Brown G. L., Nanney L. B., Griffen J., Cramer A. B., Yancey J. M., Curtsinger L. J., 3rd, Holtzin L., Schultz G. S., Jurkiewicz M. J., Lynch J. B. Enhancement of wound healing by topical treatment with epidermal growth factor. N Engl J Med. 1989 Jul 13;321(2):76–79. doi: 10.1056/NEJM198907133210203. [DOI] [PubMed] [Google Scholar]
- Carraway K. L., 3rd, Sliwkowski M. X., Akita R., Platko J. V., Guy P. M., Nuijens A., Diamonti A. J., Vandlen R. L., Cantley L. C., Cerione R. A. The erbB3 gene product is a receptor for heregulin. J Biol Chem. 1994 May 13;269(19):14303–14306. [PubMed] [Google Scholar]
- Carter W. G., Ryan M. C., Gahr P. J. Epiligrin, a new cell adhesion ligand for integrin alpha 3 beta 1 in epithelial basement membranes. Cell. 1991 May 17;65(4):599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. D., Kim J. P., Zhang K., Sarret Y., Wynn K. C., Kramer R. H., Woodley D. T. Epidermal growth factor (EGF) promotes human keratinocyte locomotion on collagen by increasing the alpha 2 integrin subunit. Exp Cell Res. 1993 Dec;209(2):216–223. doi: 10.1006/excr.1993.1304. [DOI] [PubMed] [Google Scholar]
- Choi Y., Fuchs E. TGF-beta and retinoic acid: regulators of growth and modifiers of differentiation in human epidermal cells. Cell Regul. 1990 Oct;1(11):791–809. doi: 10.1091/mbc.1.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Lanigan J. M., DellaPelle P., Manseau E., Dvorak H. F., Colvin R. B. Fibronectin and fibrin provide a provisional matrix for epidermal cell migration during wound reepithelialization. J Invest Dermatol. 1982 Nov;79(5):264–269. doi: 10.1111/1523-1747.ep12500075. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Dale B. A., Holbrook K. A., Steinert P. M. Assembly of stratum corneum basic protein and keratin filaments in macrofibrils. Nature. 1978 Dec 14;276(5689):729–731. doi: 10.1038/276729a0. [DOI] [PubMed] [Google Scholar]
- Deuel T. F. Polypeptide growth factors: roles in normal and abnormal cell growth. Annu Rev Cell Biol. 1987;3:443–492. doi: 10.1146/annurev.cb.03.110187.002303. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993 Mar 12;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Gailit J., Welch M. P., Clark R. A. TGF-beta 1 stimulates expression of keratinocyte integrins during re-epithelialization of cutaneous wounds. J Invest Dermatol. 1994 Aug;103(2):221–227. doi: 10.1111/1523-1747.ep12393176. [DOI] [PubMed] [Google Scholar]
- Galvin S., Loomis C., Manabe M., Dhouailly D., Sun T. T. The major pathways of keratinocyte differentiation as defined by keratin expression: an overview. Adv Dermatol. 1989;4:277–300. [PubMed] [Google Scholar]
- Guo M., Kim L. T., Akiyama S. K., Gralnick H. R., Yamada K. M., Grinnell F. Altered processing of integrin receptors during keratinocyte activation. Exp Cell Res. 1991 Aug;195(2):315–322. doi: 10.1016/0014-4827(91)90379-9. [DOI] [PubMed] [Google Scholar]
- Hertle M. D., Kubler M. D., Leigh I. M., Watt F. M. Aberrant integrin expression during epidermal wound healing and in psoriatic epidermis. J Clin Invest. 1992 Jun;89(6):1892–1901. doi: 10.1172/JCI115794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama S., Abraham J. A., Miller J., Fiddes J. C., Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991 Feb 22;251(4996):936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D. Identification of heregulin, a specific activator of p185erbB2. Science. 1992 May 22;256(5060):1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- Jiang C. K., Magnaldo T., Ohtsuki M., Freedberg I. M., Bernerd F., Blumenberg M. Epidermal growth factor and transforming growth factor alpha specifically induce the activation- and hyperproliferation-associated keratins 6 and 16. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6786–6790. doi: 10.1073/pnas.90.14.6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz I., Murphy G. F., Yan H. C., Herlyn M., Albelda S. M. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993 Nov;143(5):1458–1469. [PMC free article] [PubMed] [Google Scholar]
- Kokai Y., Cohen J. A., Drebin J. A., Greene M. I. Stage- and tissue-specific expression of the neu oncogene in rat development. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8498–8501. doi: 10.1073/pnas.84.23.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Issing W., Miki T., Popescu N. C., Aaronson S. A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus M. A., Quaranta V., Jones J. C. Surface relocation of alpha 6 beta 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991 Dec;115(6):1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H., Salo T., Haapasalmi K., Kramer R. H., Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993 Sep;92(3):1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. C., Lotz M. M., Steele G. D., Jr, Mercurio A. M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992 May;117(3):671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire H. C., Jr, Jaworsky C., Cohen J. A., Hellman M., Weiner D. B., Greene M. I. Distribution of neu (c-erbB-2) protein in human skin. J Invest Dermatol. 1989 Jun;92(6):786–790. doi: 10.1111/1523-1747.ep12696796. [DOI] [PubMed] [Google Scholar]
- Marchese C., Rubin J., Ron D., Faggioni A., Torrisi M. R., Messina A., Frati L., Aaronson S. A. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: differentiation response distinguishes KGF from EGF family. J Cell Physiol. 1990 Aug;144(2):326–332. doi: 10.1002/jcp.1041440219. [DOI] [PubMed] [Google Scholar]
- Marchionni M. A., Goodearl A. D., Chen M. S., Bermingham-McDonogh O., Kirk C., Hendricks M., Danehy F., Misumi D., Sudhalter J., Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993 Mar 25;362(6418):312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Matias J. R., Orentreich N. Stimulation of hamster sebaceous glands by epidermal growth factor. J Invest Dermatol. 1983 Jun;80(6):516–519. doi: 10.1111/1523-1747.ep12535112. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983 Jan;205(1):47–55. doi: 10.1002/ar.1092050107. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Morishima C., Deuel T. F. Growth factor-induced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991 Feb;87(2):694–703. doi: 10.1172/JCI115048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Chiu M. L., Payne R. E., Jr Stimulation of growth of keratinocytes by basic fibroblast growth factor. J Invest Dermatol. 1988 May;90(5):767–769. doi: 10.1111/1523-1747.ep12560956. [DOI] [PubMed] [Google Scholar]
- Orr-Urtreger A., Trakhtenbrot L., Ben-Levy R., Wen D., Rechavi G., Lonai P., Yarden Y. Neural expression and chromosomal mapping of Neu differentiation factor to 8p12-p21. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1867–1871. doi: 10.1073/pnas.90.5.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Pierce G. F., Yanagihara D., Klopchin K., Danilenko D. M., Hsu E., Kenney W. C., Morris C. F. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994 Mar 1;179(3):831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Culouscou J. M., Whitney G. S., Green J. M., Carlton G. W., Foy L., Neubauer M. G., Shoyab M. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1746–1750. doi: 10.1073/pnas.90.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman G. D., Green J. M., Culouscou J. M., Carlton G. W., Rothwell V. M., Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993 Dec 2;366(6454):473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- Plowman G. D., Whitney G. S., Neubauer M. G., Green J. M., McDonald V. L., Todaro G. J., Shoyab M. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4905–4909. doi: 10.1073/pnas.87.13.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M. F., Cordon-Cardo C., Slamon D. J. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990 Jul;5(7):953–962. [PubMed] [Google Scholar]
- Prigent S. A., Lemoine N. R., Hughes C. M., Plowman G. D., Selden C., Gullick W. J. Expression of the c-erbB-3 protein in normal human adult and fetal tissues. Oncogene. 1992 Jul;7(7):1273–1278. [PubMed] [Google Scholar]
- Prigent S. A., Lemoine N. R. The type 1 (EGFR-related) family of growth factor receptors and their ligands. Prog Growth Factor Res. 1992;4(1):1–24. doi: 10.1016/0955-2235(92)90002-y. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Shing Y., Christofori G., Hanahan D., Ono Y., Sasada R., Igarashi K., Folkman J. Betacellulin: a mitogen from pancreatic beta cell tumors. Science. 1993 Mar 12;259(5101):1604–1607. doi: 10.1126/science.8456283. [DOI] [PubMed] [Google Scholar]
- Shoyab M., Plowman G. D., McDonald V. L., Bradley J. G., Todaro G. J. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science. 1989 Feb 24;243(4894 Pt 1):1074–1076. doi: 10.1126/science.2466334. [DOI] [PubMed] [Google Scholar]
- Sliwkowski M. X., Schaefer G., Akita R. W., Lofgren J. A., Fitzpatrick V. D., Nuijens A., Fendly B. M., Cerione R. A., Vandlen R. L., Carraway K. L., 3rd Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994 May 20;269(20):14661–14665. [PubMed] [Google Scholar]
- Spivak-Kroizman T., Rotin D., Pinchasi D., Ullrich A., Schlessinger J., Lax I. Heterodimerization of c-erbB2 with different epidermal growth factor receptor mutants elicits stimulatory or inhibitory responses. J Biol Chem. 1992 Apr 25;267(12):8056–8063. [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Physiological effects of transforming growth factor in the newborn mouse. Science. 1985 Aug 16;229(4714):673–675. doi: 10.1126/science.3860952. [DOI] [PubMed] [Google Scholar]
- Wen D., Peles E., Cupples R., Suggs S. V., Bacus S. S., Luo Y., Trail G., Hu S., Silbiger S. M., Levy R. B. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992 May 1;69(3):559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Wen D., Suggs S. V., Karunagaran D., Liu N., Cupples R. L., Luo Y., Janssen A. M., Ben-Baruch N., Trollinger D. B., Jacobsen V. L. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994 Mar;14(3):1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates R. A., Nanney L. B., Gates R. E., King L. E., Jr Epidermal growth factor and related growth factors. Int J Dermatol. 1991 Oct;30(10):687–694. doi: 10.1111/j.1365-4362.1991.tb02609.x. [DOI] [PubMed] [Google Scholar]
- Zeder-Lutz G., Altschuh D., Geysen H. M., Trifilieff E., Sommermeyer G., Van Regenmortel M. H. Monoclonal antipeptide antibodies: affinity and kinetic rate constants measured for the peptide and the cognate protein using a biosensor technology. Mol Immunol. 1993 Feb;30(2):145–155. doi: 10.1016/0161-5890(93)90086-q. [DOI] [PubMed] [Google Scholar]