Abstract
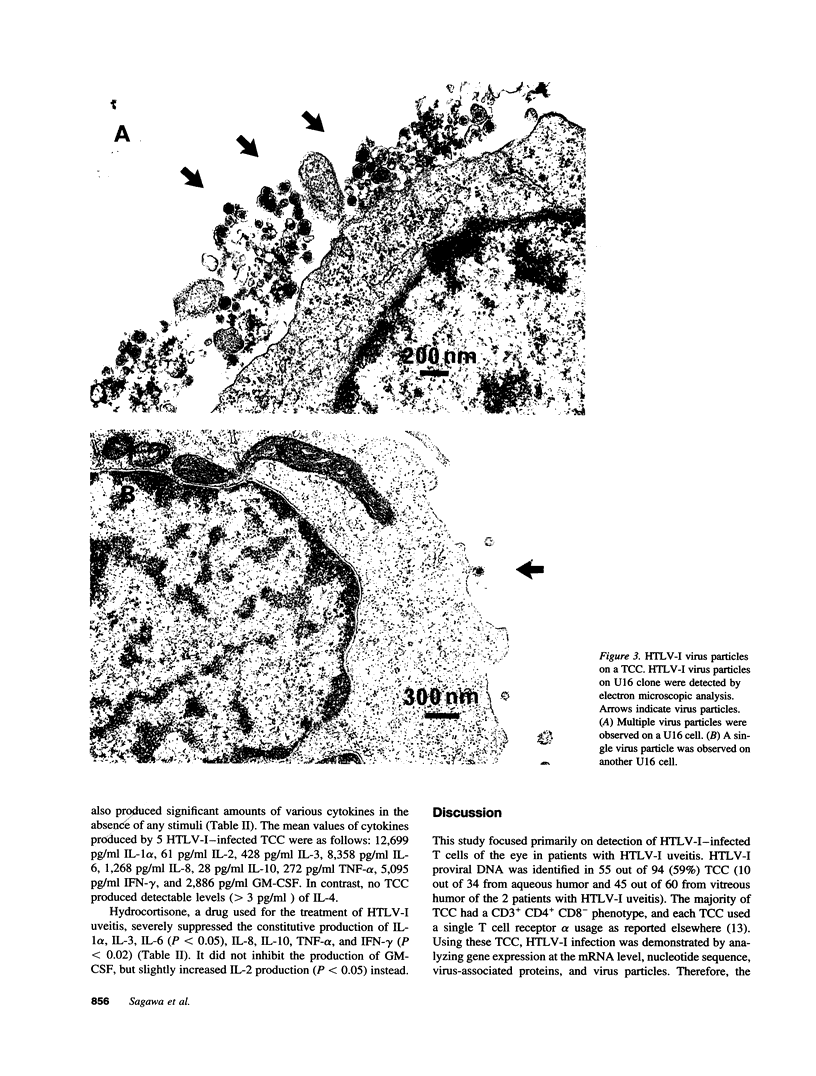
The immunopathology of human T cell lymphotropic virus type 1 (HTLV-I) uveitis was addressed by using T cell clones (TCC) established from the intraocular fluid of patients with HTLV-I uveitis. Proviral DNA of HTLV-I was identified in 55 out of 94 (59%) or 13 out of 36 (36%) TCC from the ocular fluid or the peripheral blood of these patients, respectively. Most of HTLV-I-infected TCC had a CD3+ CD4+ CD8- phenotype. HTLV-I infection on TCC was confirmed by analysis of the viral mRNA, nucleotide sequence, virus-associated proteins, and virus particles. HTLV-I-infected TCC, but not HTLV-I negative TCC, constitutively produced high amounts of IL-6 (1,336 +/- 1,050 pg/ml) and TNF-alpha (289 +/- 237 pg/ml) in the absence of any stimuli. HTLV-I-infected TCC from the ocular lesion also constitutively produced high amounts of IL-1 alpha (12,699 pg/ml), IL-2 (61 pg/ml), IL-3 (428 pg/ml), IL-8 (1,268 pg/ml), IL-10 (28 pg/ml), IFN-gamma (5,095 pg/ml), and GM-CSF (2,886 pg/ml). Hydrocortisone, a drug effective in vivo for the treatment of HTLV-I uveitis, severely depressed cytokine production in vitro in most cases. In summary, the results demonstrated direct evidence of HTLV-I infection of the eye and suggest that cytokines produced by HTLV-I-infected T cells are responsible for the intraocular inflammation in patients with HTLV-I uveitis.
Full text
PDF

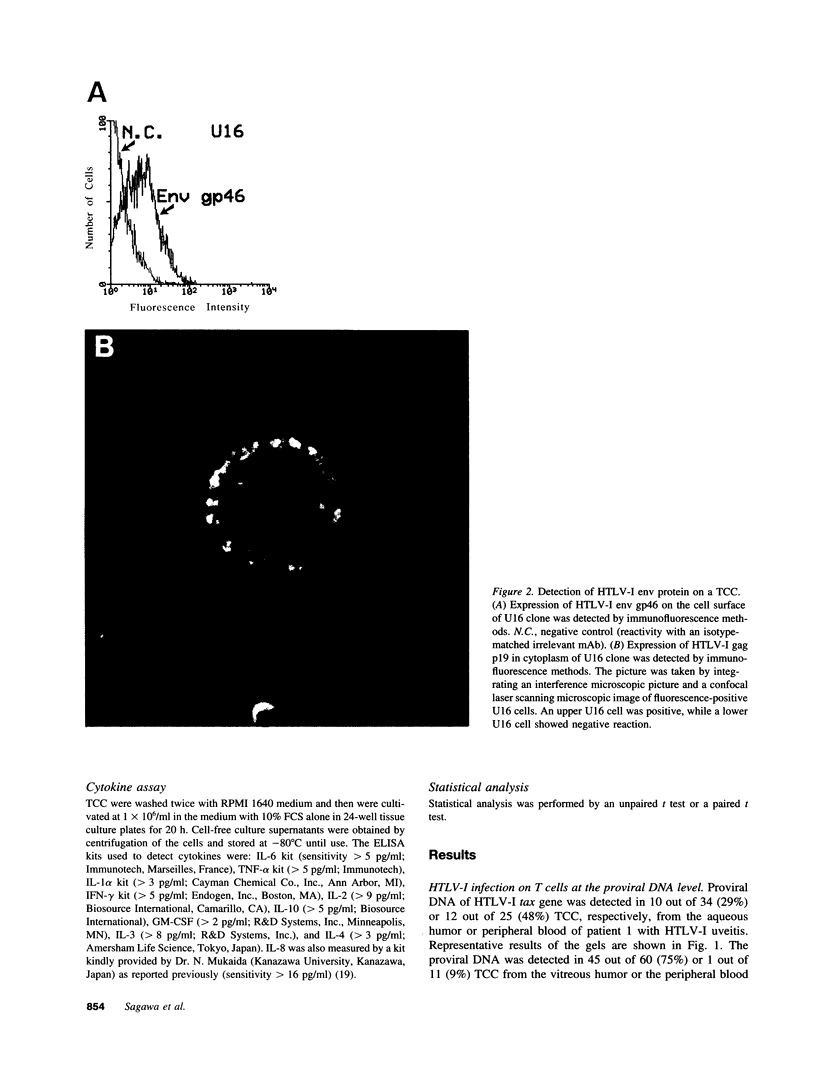


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujii M., Sugamura K., Hinuma Y. A monoclonal antibody that defines p24, a core protein of adult T-cell leukemia virus, and its precursor. Gan. 1984 Jul;75(7):595–602. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessain A., Barin F., Vernant J. C., Gout O., Maurs L., Calender A., de Thé G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985 Aug 24;2(8452):407–410. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K., Platsoucas C. D., Balch C. M. Autologous tumor-specific cytotoxic T lymphocytes in the infiltrate of human metastatic melanomas. Activation by interleukin 2 and autologous tumor cells, and involvement of the T cell receptor. J Exp Med. 1988 Oct 1;168(4):1419–1441. doi: 10.1084/jem.168.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannagi M., Harada S., Maruyama I., Inoko H., Igarashi H., Kuwashima G., Sato S., Morita M., Kidokoro M., Sugimoto M. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int Immunol. 1991 Aug;3(8):761–767. doi: 10.1093/intimm/3.8.761. [DOI] [PubMed] [Google Scholar]
- Katsikis P. D., Chu C. Q., Brennan F. M., Maini R. N., Feldmann M. Immunoregulatory role of interleukin 10 in rheumatoid arthritis. J Exp Med. 1994 May 1;179(5):1517–1527. doi: 10.1084/jem.179.5.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y. C., Mukaida N., Ishiyama S., Tokue A., Kawai T., Matsushima K., Kasahara T. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993 Apr;61(4):1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Tanaka Y., Tozawa H. Monoclonal antibody defining tax protein of human T-cell leukemia virus type-I. Tohoku J Exp Med. 1989 Jan;157(1):1–11. doi: 10.1620/tjem.157.1. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Furukawa S., Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990 Jul;56(1):29–36. doi: 10.1016/0090-1229(90)90166-n. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Tajima K., Watanabe T., Yamaguchi K. Human T lymphotropic virus type 1 uveitis. Br J Ophthalmol. 1994 Feb;78(2):149–154. doi: 10.1136/bjo.78.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Takatsuki K., Yoshimura K., Shirao M., Nakashima S., Mori S., Araki S., Miyata N. HTLV-I uveitis: a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res. 1992 Mar;83(3):236–239. doi: 10.1111/j.1349-7006.1992.tb00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki M., Watanabe T., Yamaguchi K., Yoshimura K., Nakashima S., Shirao M., Araki S., Takatsuki K., Mori S., Miyata N. Uveitis associated with human T-cell lymphotropic virus type I. Am J Ophthalmol. 1992 Aug 15;114(2):123–129. doi: 10.1016/s0002-9394(14)73974-1. [DOI] [PubMed] [Google Scholar]
- Mochizuki M., Yamaguchi K., Takatsuki K., Watanabe T., Mori S., Tajima K. HTLV-I and uveitis. Lancet. 1992 May 2;339(8801):1110–1110. doi: 10.1016/0140-6736(92)90699-4. [DOI] [PubMed] [Google Scholar]
- Osame M., Usuku K., Izumo S., Ijichi N., Amitani H., Igata A., Matsumoto M., Tara M. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986 May 3;1(8488):1031–1032. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Pober J. S., Bevilacqua M. P., Mendrick D. L., Lapierre L. A., Fiers W., Gimbrone M. A., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J Immunol. 1986 Mar 1;136(5):1680–1687. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Lapierre L. A., Mendrick D. L., Fiers W., Rothlein R., Springer T. A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986 Sep 15;137(6):1893–1896. [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshwar P., Ganea D., Gascón P. Induction of IL-3 and granulocyte-macrophage colony-stimulating factor by substance P in bone marrow cells is partially mediated through the release of IL-1 and IL-6. J Immunol. 1994 Apr 15;152(8):4044–4054. [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Lindner S. G., Gootenberg J., Popovic M., Hemmi H., Sarin P. S., Gallo R. C. Lymphokine production by cultured human T cells transformed by human T-cell leukemia-lymphoma virus-I. Science. 1984 Feb 17;223(4637):703–707. doi: 10.1126/science.6320367. [DOI] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hikikoshi A., Taniguchi T., Yoshida M. Expression of the pX gene of HTLV-I: general splicing mechanism in the HTLV family. Science. 1985 Jun 28;228(4707):1532–1534. doi: 10.1126/science.2990031. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Hirano T., Ishibashi K., Nakano N., Taga T., Sugamura K., Yamamura Y., Kishimoto T. Immortalization of BGDF (BCGF II)- and BCDF-producing T cells by human T cell leukemia virus (HTLV) and characterization of human BGDF (BCGF II). J Immunol. 1985 Mar;134(3):1728–1733. [PubMed] [Google Scholar]
- Sugamura K., Fujii M., Ueda S., Hinuma Y. Identification of a glycoprotein, gp21, of adult T cell leukemia virus by monoclonal antibody. J Immunol. 1984 Jun;132(6):3180–3184. [PubMed] [Google Scholar]
- Sugamura K., Matsuyama M., Fujii M., Kannagi M., Hinuma Y. Establishment of human cell lines constitutively producing immune interferon: transformation of normal T cells by a human retrovirus. J Immunol. 1983 Oct;131(4):1611–1612. [PubMed] [Google Scholar]
- Tanaka Y., Koyanagi Y., Chosa T., Yamamoto N., Hinuma Y. Monoclonal antibody reactive with both p28 and p19 of adult T-cell leukemia virus-specific polypeptides. Gan. 1983 Jun;74(3):327–330. [PubMed] [Google Scholar]
- Tanaka Y., Yasumoto M., Nyunoya H., Ogura T., Kikuchi M., Shimotohno K., Shiraki H., Kuroda N., Shida H., Tozawa H. Generation and characterization of monoclonal antibodies against multiple epitopes on the C-terminal half of envelope gp46 of human T-cell leukemia virus type-I (HTLV-I). Int J Cancer. 1990 Oct 15;46(4):675–681. doi: 10.1002/ijc.2910460421. [DOI] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Wano Y., Hattori T., Matsuoka M., Takatsuki K., Chua A. O., Gubler U., Greene W. C. Interleukin 1 gene expression in adult T cell leukemia. J Clin Invest. 1987 Sep;80(3):911–916. doi: 10.1172/JCI113152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Mochizuki M., Araki S., Miyata N., Yamaguchi K., Tajima K., Watanabe T. Clinical and immunologic features of human T-cell lymphotropic virus type I uveitis. Am J Ophthalmol. 1993 Aug 15;116(2):156–163. doi: 10.1016/s0002-9394(14)71279-6. [DOI] [PubMed] [Google Scholar]