Abstract
Ischemic cardiomyopathy refers to a significant impairment of left ventricular function, a condition resulting from atherosclerotic coronary artery disease. The left ventricular ejection fraction is usually 35% or less, and electron microscopy shows an increased deposition of collagen in the space between the capillaries and the myocytes. The present study shows the alteration in collagen concentration and phenotypes in ischemic cardiomyopathy, and the effect captopril treatment has on these parameters. In patients with ischemic cardiomyopathy, collagen concentration estimated from hydroxyproline increased from 7.96 +/- 1.24 mg/g to 13.9 +/- 1.30 mg/g, P less than 0.05. Ischemic cardiomyopathic patients given captopril therapy had a significantly lower collagen concentration of 10.03 +/- 1.46 mg/g, P less than 0.05. The collagen type I:III ratio decreased from 1.93 +/- 0.52 to 1.23 +/- 0.27 in patients with ischemic cardiomyopathy. Of these patients, those receiving captopril had a collagen type I:III ratio of 1.49 +/- 0.38, which did not differ significantly from the ratio of individuals with normal myocardium. There was no significant difference in type I collagen concentration in the myocardium of normal individuals, patients with ischemic cardiomyopathy, and patients with ischemic cardiomyopathy receiving captopril therapy. The type III collagen concentration increased significantly from 2.56 +/- 0.21 mg/g in normal myocardium to 6.10 +/- 0.58 mg/g in ischemic cardiomyopathic myocardium. Patients receiving captopril had a myocardial collagen type III concentration of 4.87 +/- 0.64 mg/g, which was significantly lower than that found in patients with ischemic cardiomyopathy. An increased deposition of type III collagen may be partly responsible for altering the compliance of the myocardium, resulting in dilatation of the heart and possibly leading to eventual heart failure.
Full text
PDF

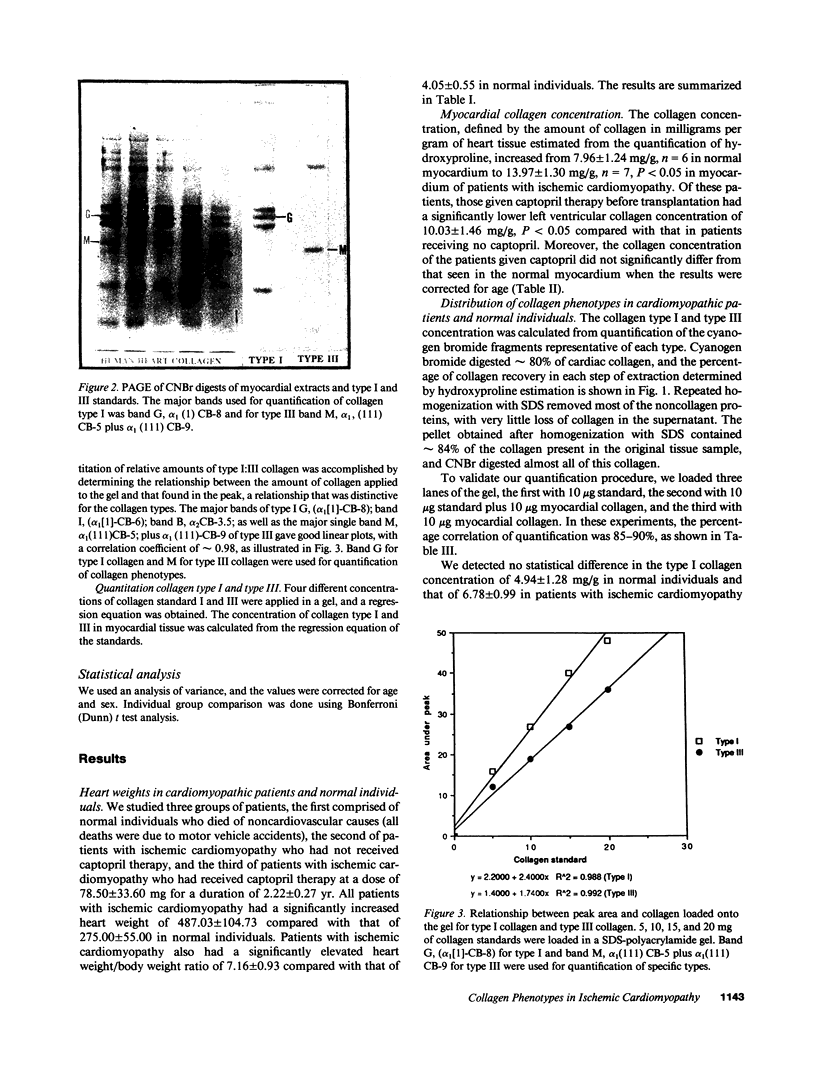
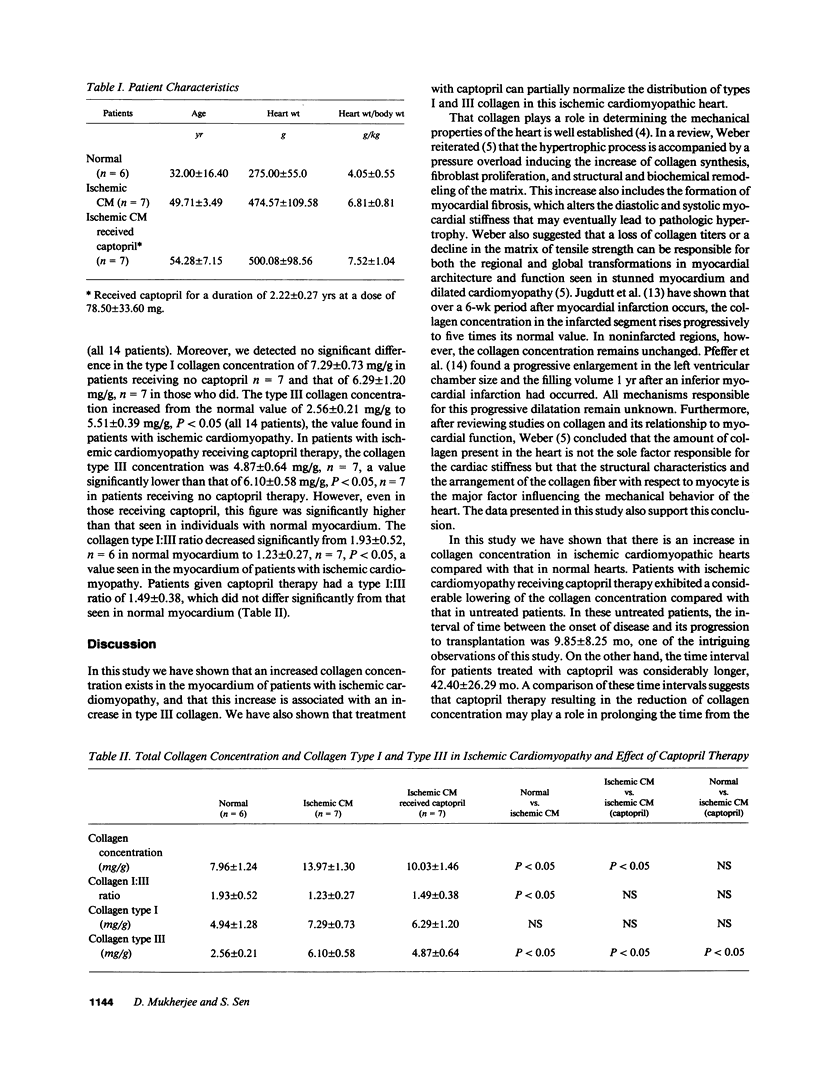


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartosová D., Chvapil M., Korecký B., Poupa O., Rakusan K., Turek Z., Vízek M. The growth of the muscular and collagenous parts of the rat heart in various forms of cardiomegaly. J Physiol. 1969 Feb;200(2):285–295. doi: 10.1113/jphysiol.1969.sp008693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch G. E., Giles T. D., Colcolough H. L. Ischemic cardiomyopathy. Am Heart J. 1970 Mar;79(3):291–292. doi: 10.1016/0002-8703(70)90416-3. [DOI] [PubMed] [Google Scholar]
- Burch G. E., McDonald C. D. Prolonged bed rest in the treatment of ischemic cardiomyopathy. Chest. 1971 Nov;60(5):424–430. doi: 10.1378/chest.60.5.424. [DOI] [PubMed] [Google Scholar]
- Burch G. E., Tsui C. Y., Harb J. M. Ischemic cardiomyopathy. Am Heart J. 1972 Mar;83(3):340–350. doi: 10.1016/0002-8703(72)90435-8. [DOI] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Munderloh N. H. Isolation and characterization of CNBr peptides of human (alpha 1 (III) )3 collagen and tissue distribution of (alpha 1 (I) )2 alpha 2 and (alpha 1 (III) )3 collagens. J Biol Chem. 1975 Dec 25;250(24):9304–9312. [PubMed] [Google Scholar]
- Faxon D. P. ACE inhibition for the failing heart: experience with captopril. Am Heart J. 1988 May;115(5):1085–1093. doi: 10.1016/0002-8703(88)90081-6. [DOI] [PubMed] [Google Scholar]
- Jugdutt B. I., Amy R. W. Healing after myocardial infarction in the dog: changes in infarct hydroxyproline and topography. J Am Coll Cardiol. 1986 Jan;7(1):91–102. doi: 10.1016/s0735-1097(86)80265-0. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Cockerill P., McAnulty R. J., Hastings J. R. A simplified method for quantitation of the relative amounts of type I and type III collagen in small tissue samples. Anal Biochem. 1981 May 15;113(2):301–312. doi: 10.1016/0003-2697(81)90081-6. [DOI] [PubMed] [Google Scholar]
- Medugorac I., Jacob R. Characterisation of left ventricular collagen in the rat. Cardiovasc Res. 1983 Jan;17(1):15–21. doi: 10.1093/cvr/17.1.15. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Sen S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res. 1990 Dec;67(6):1474–1480. doi: 10.1161/01.res.67.6.1474. [DOI] [PubMed] [Google Scholar]
- Pfeffer M. A., Lamas G. A., Vaughan D. E., Parisi A. F., Braunwald E. Effect of captopril on progressive ventricular dilatation after anterior myocardial infarction. N Engl J Med. 1988 Jul 14;319(2):80–86. doi: 10.1056/NEJM198807143190204. [DOI] [PubMed] [Google Scholar]
- Sen S., Bumpus F. M. Collagen synthesis in development and reversal of cardiac hypertrophy in spontaneously hypertensive rats. Am J Cardiol. 1979 Oct 22;44(5):954–958. doi: 10.1016/0002-9149(79)90228-5. [DOI] [PubMed] [Google Scholar]
- Shekhonin B. V., Domogatsky S. P., Idelson G. L., Koteliansky V. E. Participance of fibronectin and various collagen types in the formation of fibrous extracellular matrix in cardiosclerosis. J Mol Cell Cardiol. 1988 Jun;20(6):501–508. doi: 10.1016/s0022-2828(88)80077-4. [DOI] [PubMed] [Google Scholar]
- Weber K. T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989 Jun;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]




