Abstract
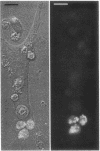
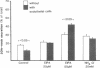
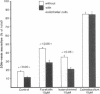
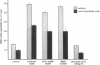
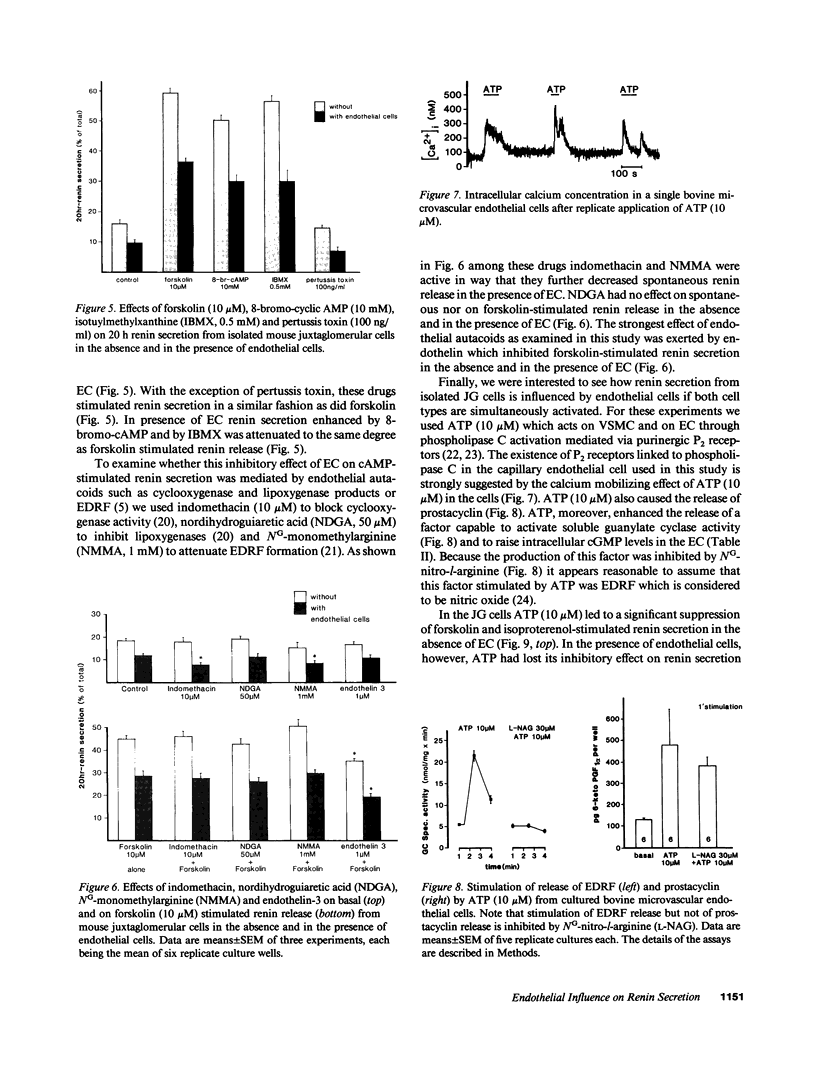
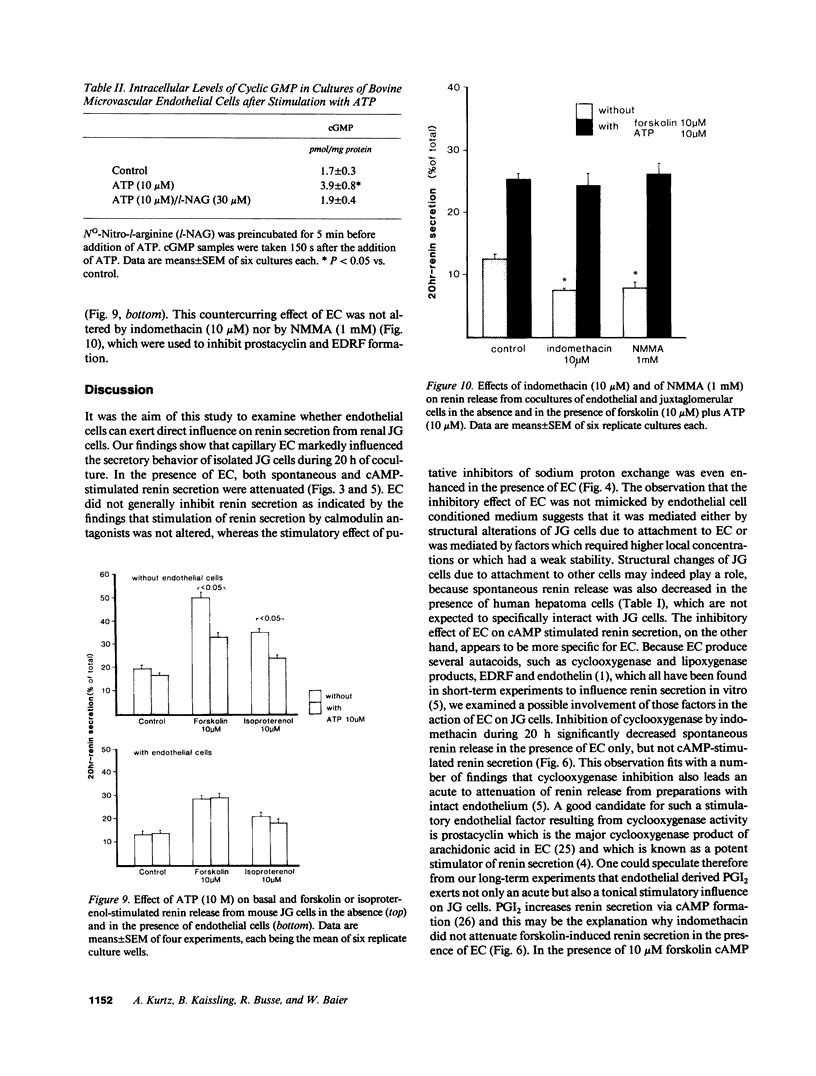
Utilizing cocultures of mouse renal juxtaglomerular cells with bovine microvascular endothelial cells, we have examined whether endothelial cells exert direct influence on renin secretion from renal juxtaglomerular cells. In the presence of endothelial cells both spontaneous and forskolin (10 microM) or isoproterenol (10 microM) stimulated renin release were markedly attenuated. The stimulatory effect of the calmodulin antagonist calmidazolium (10 microM) on renin secretion was not altered by endothelial cells, whereas the stimulatory effect of ethylisopropylamiloride (50 microM) an inhibitor of sodium-proton exchange was enhanced in the presence of endothelial cells. Indomethacin (10 microM) and NG-monomethyl-l-arginine (NMMA) (1 mM) used to inhibit cyclooxygenase activity and production of endothelium-derived relaxing factor (EDRF) decreased spontaneous renin release in the presence of endothelial cells only, but had no effect on forskolin stimulated renin secretion. Endothelin (1 microM) inhibited cAMP stimulated renin release both in the absence and in the presence of endothelial cells. ATP (10 microM) which acts on both endothelial and juxtaglomerular cells via purinergic P2 receptors inhibited cAMP stimulated renin release only in the absence but not in the presence of endothelial cells. This modulatory effect of endothelial cells was no altered by indomethacin nor by NMMA. Taken together, our findings provide first evidence for a local control function of the endothelium on cAMP stimulated renin secretion from renal juxtaglomerular cells, which could in part be mediated by endothelin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol. 1979 Nov;237(5):F333–F343. doi: 10.1152/ajprenal.1979.237.5.F333. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bührle C. P., Scholz H., Hackenthal E., Nobiling R., Taugner R. Epithelioid cells: membrane potential changes induced by substances influencing renin secretion. Mol Cell Endocrinol. 1986 Apr;45(1):37–47. doi: 10.1016/0303-7207(86)90080-8. [DOI] [PubMed] [Google Scholar]
- Campbell W. B., Henrich W. L. Endothelial factors in the regulation of renin release. Kidney Int. 1990 Oct;38(4):612–617. doi: 10.1038/ki.1990.250. [DOI] [PubMed] [Google Scholar]
- Ferrier C. P., Kurtz A., Lehner P., Shaw S. G., Pusterla C., Saxenhofer H., Weidmann P. Stimulation of renin secretion by potassium-channel activation with cromakalim. Eur J Clin Pharmacol. 1989;36(5):443–447. doi: 10.1007/BF00558067. [DOI] [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Furie M. B., Cramer E. B., Naprstek B. L., Silverstein S. C. Cultured endothelial cell monolayers that restrict the transendothelial passage of macromolecules and electrical current. J Cell Biol. 1984 Mar;98(3):1033–1041. doi: 10.1083/jcb.98.3.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hackenthal E., Paul M., Ganten D., Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990 Oct;70(4):1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- Kurtz A. Cellular control of renin secretion. Rev Physiol Biochem Pharmacol. 1989;113:1–40. doi: 10.1007/BFb0032674. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984 Oct 30;124(2):359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Hutter A., Bührle C., Nobiling R., Taugner R., Hackenthal E., Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986 Apr;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Calcium influx into endothelial cells and formation of endothelium-derived relaxing factor is controlled by the membrane potential. Pflugers Arch. 1990 May;416(3):305–311. doi: 10.1007/BF00392067. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Nakase K., Ikegawa R., Hayashi K., Ohyama T., Morimoto S. The endothelium-derived vasoconstrictor peptide endothelin inhibits renin release in vitro. Life Sci. 1989;44(2):149–157. doi: 10.1016/0024-3205(89)90533-x. [DOI] [PubMed] [Google Scholar]
- Moe O., Tejedor A., Campbell W. B., Alpern R. J., Henrich W. L. Effects of endothelin on in vitro renin secretion. Am J Physiol. 1991 Apr;260(4 Pt 1):E521–E525. doi: 10.1152/ajpendo.1991.260.4.E521. [DOI] [PubMed] [Google Scholar]
- Murayama T., Ui M. Loss of the inhibitory function of the guanine nucleotide regulatory component of adenylate cyclase due to its ADP ribosylation by islet-activating protein, pertussis toxin, in adipocyte membranes. J Biol Chem. 1983 Mar 10;258(5):3319–3326. [PubMed] [Google Scholar]
- Mülsch A., Böhme E., Busse R. Stimulation of soluble guanylate cyclase by endothelium-derived relaxing factor from cultured endothelial cells. Eur J Pharmacol. 1987 Mar 17;135(2):247–250. doi: 10.1016/0014-2999(87)90620-0. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Phaneuf S., Berta P., Casanova J., Cavadore J. C. ATP stimulates inositol phosphates accumulation and calcium mobilization in a primary culture of rat aortic myocytes. Biochem Biophys Res Commun. 1987 Mar 13;143(2):454–460. doi: 10.1016/0006-291x(87)91375-1. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Nakamaru M., Saito H., Higaki J., Ogihara T. Endothelin inhibits renin release from isolated rat glomeruli. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1244–1247. doi: 10.1016/s0006-291x(88)81273-7. [DOI] [PubMed] [Google Scholar]
- Satoh H., Satoh S. Prostaglandin E2 and I2 production in isolated dog renal arteries in the absence or presence of vascular endothelial cells. Biochem Biophys Res Commun. 1984 Feb 14;118(3):873–876. doi: 10.1016/0006-291x(84)91476-1. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Takagi M., Matsuoka H., Atarashi K., Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1164–1168. doi: 10.1016/s0006-291x(88)80996-3. [DOI] [PubMed] [Google Scholar]
- Taugner R., Bührle C. P., Hackenthal E., Mannek E., Nobiling R. Morphology of the juxtaglomerular apparatus and secretory mechanisms. Contrib Nephrol. 1984;43:76–101. doi: 10.1159/000409945. [DOI] [PubMed] [Google Scholar]
- Vidal M. J., Romero J. C., Vanhoutte P. M. Endothelium-derived relaxing factor inhibits renin release. Eur J Pharmacol. 1988 May 10;149(3):401–402. doi: 10.1016/0014-2999(88)90679-6. [DOI] [PubMed] [Google Scholar]