Abstract
In a previous work, the immunolocation of the chickpea XTH1 (xyloglucan endotransglucosylase/hydrolase 1) protein in the cell walls of epicotyls, radicles, and stems was studied, and a role for this protein in the elongation of vascular cells was suggested. In the present work, the presence and the location of the XTH1 protein in embryonic axes during the first 48 h of seed imbibition, including radicle emergence, were studied. The presence of the XTH1 protein in the cell wall of embryonic axes as early as 3 h after imbibition, before radicle emergence, supports its involvement in germination, and the fact that the protein level increased until 24 h, when the radicle had already emerged, also suggests its participation in the elongation of embryonic axes. The localization of XTH1 clearly indicates that the protein is related to the development of vascular tissue in embryonic axes during the period studied, suggesting that the role of this protein in embryonic axes is the same as that proposed for seedlings and plants.
Keywords: Cell wall, Cicer arietinum, embryonic axes, germination, vascular tissues, xyloglucan endotransglucosylase/hydrolase
Introduction
XTH (xyloglucan endotransglucosylase/hydrolase) belongs to a subgroup of the glycoside hydrolase family 16 (GH16). XTHs exhibit one or both of two enzymatic activities—xyloglucan:xyloglucosyl transferase (EC 2.4.1.207) and xyloglucan-specific endo β-1,4- glucanase (EC 3.2.1.151) activity—which are currently referred to as xyloglucan endotransglucosylase (XET) and xyloglucan endohydrolase (XEH) activity, respectively (Nishitani, 1997; Rose et al., 2002; Fry, 2004).
The XTH gene products from GH16 have been classified into three major phylogenetic subgroups (groups 1–3) on the basis of sequence similarity, although these do not appear to predict XTH enzymatic activity or tissue specificity (Rose et al., 2002). New bioinformatic analyses have indicated that group 3 can be subdivided into two predominant clades, designated group 3-A and group 3-B (Baumann et al., 2007).
The genome sequencing of several model plants has revealed that higher plants typically contain a large group of XTH proteins. For example, in the genome of Arabidopsis thaliana (L.) ecotype Columbia, 33 open reading frames encoding XTH proteins have been detected (Yokoyama and Nishitani, 2001). The genomic database completed for rice (Oryza sativa L. ‘Nipponbare’) contains 29 members of the XTH gene family (Yokoyama et al., 2004), while the genome of Populus trichocarpa contains 41 XTH genes (Geisler-Lee et al., 2006). The important role of XTH in secondary wall construction processes (Bourquin et al., 2002; Matsui et al., 2005), which are more sophisticated in woody plants, could explain the higher number of XTH family proteins in woody plants than in Arabidopsis and rice.
The relationship between enzyme activity and cell elongation has been extensively investigated. XTHs participate in the loosening and rejoining of primary cell wall xyloglucans, leading to cell wall expansion (Fry, 1995; Xu et al., 1996; Nishitani, 1997; Thompson et al., 1998), and in the integration of newly secreted xylogluan chains in the cell wall (Fry et al., 1992; Nishitani and Tominaga, 1992; Thompson et al., 1998; Thompson and Fry, 2001; Rose et al., 2002).
XET activity has often been correlated with the growth rate (Fry et al., 1992; Potter and Fry, 1994; Palmer and Davies, 1996; Catala et al., 1997), although it has also been detected in vegetative tissues that have ceased to elongate (Smith et al., 1996; Barrachina and Lorences, 1998); in ripening fruit (Redgwell and Fry, 1993; Maclachlan and Brady, 1994; Ishimaru and Kobayashi, 2002; Miedes and Lorences, 2009) and, as mentioned above, during secondary cell wall formation (Bourquin et al., 2002; Matsui et al., 2005). Currently, the role of XTHs as wall-modifying enzymes seems to be clear, but the existence of many different isoenzymes in the XTH family of proteins hinders the determination of the role of any given individual XTH protein in xyloglucan dynamics, which is involved not only in the loosening but also in the construction, stiffening, and disassembly of the cell wall. To elucidate the roles of individual XTHs, it is necessary to focus on the function of each protein and gene in specific cell types or tissues.
Like other cell wall-loosening enzymes related to cell elongation, such as α-expansins, XTHs have also been studied in relation to the elongation of embryonic axes during seed germination. However, the role of these enzymes in germination is still unclear, and most studies only refer to gene expression at different times after seed imbibition. Thus, in Arabidopsis several cell wall hydrolases, including a xyloglucan endotransglycosylase, were up-regulated by gibberellic acid (GA) in seeds following imbibition (Ogawa et al.. 2003). In tomato, the XTH gene LeXET4 (renamed SlXTH4) has been related to the control of germination, since it is specifically expressed in the endosperm cap of germinating tomato seeds prior to radicle emergence (Chen et al., 2002). In germinating chickpea seeds, transcripts of CaXTH1, encoding a putative XTH (XTH1), were detected from the first hour after imbibition (Hernández-Nistal et al., 2006), suggesting that its reported role in epicotyl elongation (Romo et al., 2005; Jimenez et al., 2006) could be extended to embryonic axes.
Study of the XTH mRNA accumulation patterns and of the tissue specificity of these enzymes is suggestive of the physiological process in which any given XTH enzyme is involved. Moreover the location of the protein could facilitate current insight into the precise function of these proteins.
Regarding the above-mentioned XTH1 from Cicer arietinum, the location of the chickpea XTH1 protein in the cell walls of epicotyls, radicles, and stems has previously been reported, indicating a function for this protein in the extension of the parenchyma cells of epicotyls and also in developing vascular tissue, in turn suggesting a role in the elongation of vascular cells (Jiménez et al., 2006). The aim of the present work was to elucidate the location of the XTH1 protein in embryonic axes during the first 48 h of seed imbibition, including radicle emergence, in order to determine whether the role of this protein in embryonic axes is the same as that proposed for seedlings and plants.
Materials and methods
Plant material and germination conditions
Chickpea seeds (C. arietinum ‘Pedrosillano’) sterilized in 0.1% (v/v) sodium hypochlorite were germinated in water on glass plates covered with filter paper in the dark at 25 °C and 80% relative humidity. Embryonic axes of chickpea seeds imbibed for up to 48 h were used. The embryonic axes were collected at 1, 3, 12, 36, and 48 h after the start of imbibition, frozen in liquid N2, and stored at –70 °C until used.
Cell wall protein extraction and western blotting
Cell walls were isolated from embryonic axes at different times after the start of imbibition, as described in Hernández-Nistal et al. (2006). Proteins were extracted from freshly isolated cell walls with 1 M NaCl in Na-citrate/phosphate (10 mM, pH 5.5) at 4 °C for 48 h. The wall suspension was filtered through Miracloth (Calbiochem, USA), and the protein extract was dialysed against Na-acetate (20 mM, pH 5.0). The dialysed protein was centrifuged for 25 min at 6500 g and concentrated using an Amicon PM3 ultrafiltration cell (Amicon Corporation, USA). The protein was evaluated by Protein Assay (Bio-Rad, Baltimore, MD, USA).
For western blotting, proteins were separated by SDS–PAGE (Laemmli, 1970) and electrotransferred onto polyvinyldifluoridene (PVDF) membranes (Amersham Biosciences, USA). Immunoblots were prepared essentially according to the procedure of Harlow and Lane (1988), using anti-XTH1 IgGs at 1:10 000 dilution and a horseradish peroxidase-conjugated secondary antibody (goat anti-rabbit, BioRad, Germany) at 1:100 000. The blots were developed by chemiluminescence, using the ECL Advanced Western Blotting Detection Kit (Amersham Biosciences, USA).
The anti-XTH1 antibodies were previously generated (Jiménez et al., 2006) against purified recombinant XTH1 protein. The specificity of anti-XTH1 IgGs for XTH proteins has been verified previously by western blot and MALDI-TOF (matrix-assisted laser desorption ionization-time of flight) analyses (Jiménez et al., 2006).
Immunocytochemical labelling
Whole embryonic axes from 1 h to 36 h after imbibition, and hooks, epicotyls, and the apical and basal halves of roots from 48-h-old embryonic axes were fixed and dehydrated prior to being embedded in paraffin (Paraplast Plus, Sigma). The embedded tissues were sliced into 12 μm sections using a microtome (Leica Instruments GmbH, Germany) and affixed to slides pre-coated with high molecular weight poly-L-lysine. Samples were deparaffinized with xylene and rehydrated through a graded ethanol series. The sections were then incubated for 5 min in 10 mM citrate buffer, pH 6.0, at 100 °C to inactivate endogenous alkaline phosphatase activity, since an alkaline phosphatase-conjugated secondary antibody was used to develop the reaction. The samples were then washed twice in TRIS-buffered saline (TBS: 0.1 M TRIS, 0.1 M NaCl, pH 7.4). Free binding sites were blocked for 45 min with 5% bovine serum albumin (BSA) and 3% normal swine serum in TBS. Anti-XTH1 IgGs (1:100 dilution in TBS with 3% BSA) were applied to sections for 2 h at room temperature. Excess antibody was removed with extensive washing in 0.5% Tween-20, 1% BSA in TBS. After a second blocking, secondary antibody (goat anti-rabbit IgG conjugated with alkaline phosphatase, at 1:300 dilution in TBS with 3% BSA) was applied and the preparations were then extensively washed as above. The colour reaction used to visualize the antigen–antibody complexes was performed in TBS supplemented with 50 mM MgCl2, pH 9.5, containing 5-bromo-4-chloro-3-indolyl-phosphate and 4-nitroblue tetrazolium chloride. Sections were dehydrated in a graded ethanol series, dipped in xylene, and mounted in Entellan (Merck, Darmstadt, Germany). Photographs were taken using a microscope (DMLS2, Leica, Germany) equipped with a Canon Power Shot S50 camera.
Accession number
The nucleotide sequence CaXTH1, reported in this paper has been submitted to the EMBL/GenBank database under accession number AJ004917.
Results
Immunodetection of the XTH1 protein in cell wall protein extracts from embryonic axes
The distribution of the XTH1 protein was studied in embryonic axes from 1 h to 48 h after seed imbibition. In C. arietinum seeds, the completion of germination (i.e. radicle emergence) begins at 12 h and is almost completed 24 h after the start of water uptake, when the percentage of seed germination is close to 100% (Hernández Nistal et al., 2006). Between 18 h and 24 h radicles grow exponentially and epicotyl development begins at 36 h. The length of the embryonic axes during the time studied is shown in Table 1.
Table 1.
Length of embryonic axes during the germination of Cicer arietinum seeds from 1 h to 48 h after the start of water uptake
| 1 h | 3 h | 6 h | 12 h | 18 h | 24 h | 36 h | 48 h | |
| Length (mm) | 3.67±0.59 | 4.33±0.49 | 4.67±0.49 | 4.97±0.3 | 6.17±0.45 | 9.27±1.28 | 18.11±1.56 | 32.00±4.83 |
Seeds were germinated in water in darkness at 25 °C and 80% relative humidity. Each data point represents the average of three replicates ±SD.
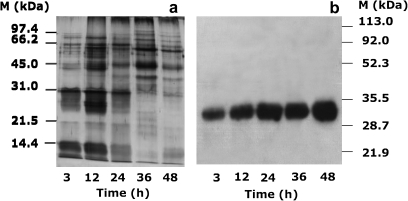
To check whether XTH1 was also located in the cell walls of embryonic axis cells, as happens in epicotyls and stems (Jiménez et al., 2006), western blot analyses were carried out with the specific anti-XTH1 antibodies and cell wall protein extracts obtained from embryonic axes imbibed for 3, 12, 24, 36, and 48 h. When the total cell wall protein extract from embryonic axes was separated by SDS–PAGE (Fig. 1A), the anti-XTH1 IgGs recognized a 32 kDa polypeptide, consistent with the molecular weight of mature XTH1, and no other protein band was detected (Fig. 1B). The chickpea XTH1 protein was detected in embryonic axes excised from seeds from 3 h to 48 h after seed imbibition. The intensity of the immunoreaction increased with the time of imbibition, the highest value being reached at 24 h after the start of water uptake (Fig. 1B), in agreement with the trend of CaXTH1 transcripts (Hernández-Nistal et al., 2006). The protein level was similar at 36 h and increased again at 48 h, coinciding with epicotyl emergence. The purified IgGs from the pre-immune serum did not recognize any cell wall protein (data not shown).
Fig. 1.
Immunodetection by western blot of XTH1 in cell wall protein extracts from embryonic axes excised from seeds imbibed in darkness for the indicated times. Cell wall proteins from 3- to 48-h-old embryonic axes were separated by SDS–PAGE and (a) silver stained or (b) incubated with anti-XTH1 IgGs.
Immunolocation of the XTH1-cross-reacting protein
In order to determine the tissue and cellular location of the XTH1 protein in embryonic axes excised from seeds imbibed for 3–48 h, immunocytochemical studies were conducted using the anti-XTH1 antibodies, as described in the Materials and methods. The immunocytochemical assays were conducted on longitudinal sections of 3-, 12-, 24-, and 36-h-old embryonic axes (Figs 2–4), and in different parts of 48-h-old axes (Fig. 5).
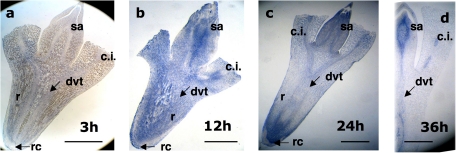
Fig. 2.
Immunolocation of the XTH1 protein in embryonic axes excised from seeds imbibed in darkness for the indicated times. Longitudinal sections were taken from (a) 3-h-, (b) 12-h-, (c) 24-h-, and (d) 36-h-old embryonic axes treated with anti-XTH1 IgGs. c.i., cotyledon insertion region; dvt, differentiating vascular tissue; r, radicle; rc, root cap; sa, shoot apex. Bars=100 μm.
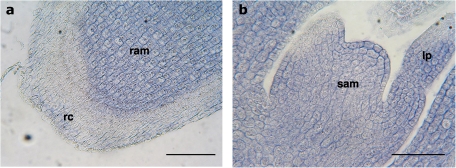
Fig. 3.
Immunolocation of the XTH1 protein in meristematic areas of 24-h-old chickpea embryonic axes. (a) Apical zone of the radicle showing the division zone of the radicle. (b) Shoot apical meristem and developing primary leaf. lp, leaf primordium; ram, radicle apical meristem; rc, root cap; sam, shoot apical meristem. Bars (a) 200 μm; (b) 100 μm.
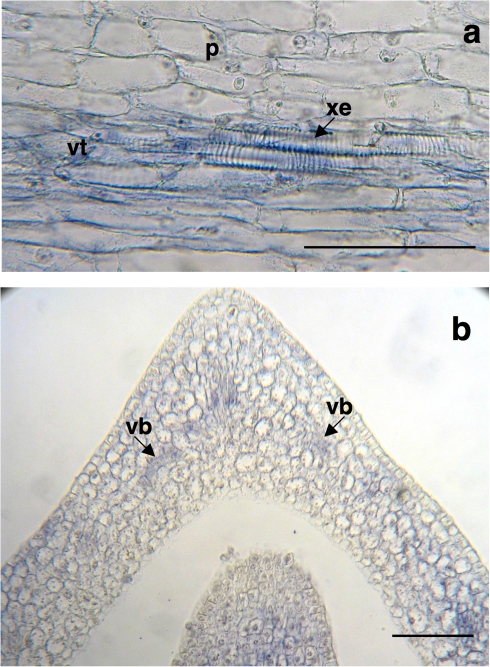
Fig. 4.
Immunolocation of the XTH1 protein in vascular tissue of 36-h-old chickpea embryonic axes. (a) Longitudinal section of embryonic axes showing differentiating vascular tissue. (b) Initial vascular bundle when the leaf primordium begins its development. p, parenchyma; vb, vascular bundle; vt, vascular tissue; xe, xylem elements. Bars=100 μm.
Fig. 5.
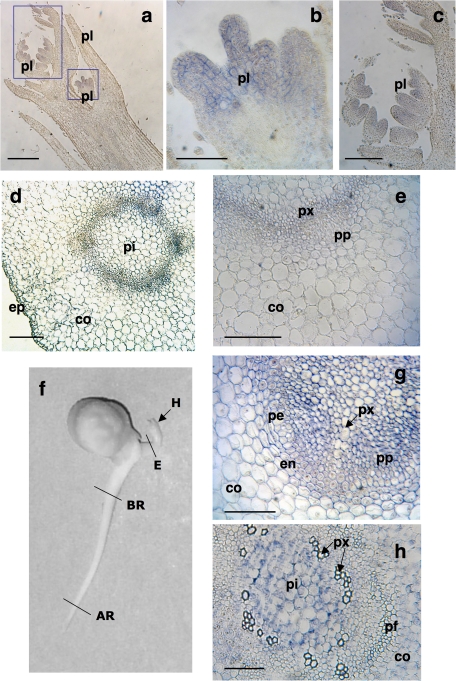
Immunolocation of XTH1 protein in embryonic axes after 48 h of imbibition. Immunocytochemical studies were performed separately in hooks (a, b, c), epicotyls (d, e), and radicles (g, h) treated with anti-XTH-1 IgGs. Longitudinal section of the meristematic shoot (a) and magnification of leaf primordia (b, c). Cross-section of the epicotyl (d) and its magnification (e). Cross-section from the apical region (g) and from the basal region (h) of the root. The black and white photograph (f) shows a 48-h-old chickpea seedling where the zones used for immunocytochemical studies are indicated: H, hook; E, epicotyl, BR, basal region of the root; AR, apical region of the root; co, cortex; en, endodermis; ep, epidermis; pe, pericycle; pi, pith; pf, phloem fibres pl, primary leaves; pp, primary phloem; px, primary xylem. Bars (a, d) 500 μm; (b) 100 μm; (c, g, h) 250 μm; (e) 300 μm.
Early on in germination, after 3 h of imbibition, the immunodetection of XTH1 protein was very low and only a slight bluish colour was observed in the initial vascular tissue (Fig. 2a). The labelling of XTH1 protein had increased by 12 h and, although the labelling appears rather homogeneous in all tissue, it was more intense in differentiating vascular tissue (Fig. 2b). By 24 h (Fig. 2c), the protein was mainly located in the vascular tissue throughout the embryonic axes, whereas the labelling in parenchyma cells decreased as time progressed, except in the meristematic zone of the radicle, where a very intense blue colour was observed. By 36 h, the location of the protein was restricted to the vascular system and meristematic regions (Fig. 2d).
The specific labelling of the XTH1 protein in different meristematic areas of 24-h-old chickpea axes appeared not only in the division zone of the radicle above the calyptra (Fig. 3a), where labelling in cells undergoing division can be clearly seen, but also in the meristematic zones of developing primary leaves and in the shoot apical meristem (Fig. 3b), indicating a consistent role for the protein in all meristematic tissues.
The marked presence of the XTH1 protein in vascular cells can be seen in detail in Fig. 4. By 36 h after imbibition, the protein was mainly located in differentiating vascular tissue (Fig. 4a) and in the initial vascular bundle of the leaf primordium when it started to develop (Fig. 4b).
Owing to the large size of the 48-h-old axes, immunocytochemical studies were performed separately in hooks, epicotyls, and roots. In the meristematic hook, whose longitudinal apical section was used, the XTH1 protein was located in the cell walls of meristematic cells, clearly in leaf primordia (Fig. 5a–c). The labelling of developing epicotyls from 48-h-old seedlings revealed the presence of the XTH1 protein in the cell wall of cortex and pith cells, although the most intense labelling appeared in the bundles of vascular tissues (Fig. 5d, e).
Since the aim of the present study was to establish a putative relationship between the location of the protein and growth, in the immunolocalization studies root sections with different growth activity were used. After removing the first 3 mm of the root tip, where almost no elongation was occurring, 2 mm sections were separated (as indicated in Fig. 5f) from the apical region, with a rapid and extensive cell elongation, and from the basal region (the root region closest to insertion of the cotyledon), which does not show any elongation activity. The XTH1 protein was clearly bound to the cell walls of the cortex and pith cells in the apical and basal sections, detection being stronger in the apical sections. In these, the XTH1 protein was mainly located in the cell walls of the endodermis layer, pericycle, and differentiating vascular elements (i.e. the primary xylem and primary phloem) (Fig. 5g), whereas in the basal section the signal was restricted to the pith and cortex cells closest to the vascular system (Fig. 5h).
Discussion
Germination begins with water uptake by seeds and ends with the initial elongation of embryonic axes (Bewley, 1997). The completion of germination is visible as from the emergence of the radicle. While a weakening of embryo-enclosing tissues is a prerequisite for germination in many seeds, embryo growth is essential for the germination of all of them. Both processes—the weakening of the embryo covers and embryo growth—may be associated with cell wall-loosening activities.
XTH is one of the cell wall enzymes with cell wall-loosening activities, although the precise role of XTHs in cell elongation is still subject to debate, as is their putative involvement in the elongation of embryonic axes during germination. Since XTHs represent a large gene family, it is to be expected that the different gene products would be active in different aspects of cell wall metabolism, from seed germination through flowering, and in all plant organs, as suggested by Becnel et al. (2006). Regarding germination and the growth of embryonic axes, in situ analyses revealed that one of the Arabidopsis XTH genes (AtXTH5) was only expressed in the embryonic axis, suggesting that the enzyme is potentially involved in the cell wall loosening associated with embryo growth (Ogawa et al., 2003). In tomato, where 25 XTHs have been identified, only LeXET4 (renamed SlXTH4) has been related to the control of seed germination prior to radicle emergence (Chen et al., 2002) although, according to the searches for tomato XTH made in databases, several of them are represented in libraries from germinating seeds (Saladié et al., 2006).
In germinating chickpea seeds, the transcript accumulation of CaXTH1 in embryonic axes has previously been reported as early as 1 h after seed imbibition, i.e. even earlier than when α-expansin transcripts are detected and before radicle elongation takes place (Hernández-Nistal et al., 2006). The XTH encoded by CaXTH1 has been implicated in the chickpea plant elongating process based on several observations regarding its pattern of expression, such as the high level of CaXTH1 transcripts in elongating organs, its up-regulation by growth-promoting hormones, or its down-regulation by growth inhibition treatment (Romo et al., 2005).
Although gene expression studies are useful for checking the involvement of a protein in a specific process, the actual location of this protein, encoded by a given gene, could provide more accurate information about its role in the process. Thus, to gain insight into the role of XTHs in the chickpea germination process, the localization of the XTH1 protein in embryonic axes of C. arietinum during germination and post-germination was studied by using polyclonal antibodies raised against the Escherichia coli-expressed recombinant protein encoded by CaXTH1. The specificity of antibodies to XTH proteins has been checked previously with MALDI-TOF analysis (Jiménez et al., 2006).
The presence of the XTH1 protein in the cell wall of embryonic axes as early as 3 h after imbibition, before radicle emergence, supports its involvement in germination sensu stricto. The fact that the protein level increased until 24 h, when the radicle had already emerged, also suggests its participation in the elongation of embryonic axes (Fig. 1).
The localization of XTH1 clearly indicates that the protein is related to the development of vascular tissue throughout the period studied and, although at 12 h after imbibition its location was general in the embryonic axes, as time progressed it became more specifically associated with proliferating cells and differentiating vascular tissue (Figs 2–4).
The consistent presence of the protein in meristematic tissue, cells undergoing division in the division zone of the radicle (Fig. 3a), in the shoot apical meristem (Fig. 3b), and in meristematic zones of developing primary leaves (Fig. 5b, c) indicates that XTH tended to be located in rapidly dividing and expanding tissues, suggesting that it functions in the development and morphogenesis of tissues close to the meristem. In other species, specific XTHs have also been related to meristematic tissue differentiation and vascular development in roots, pointing to its involvement in seedling growth. Thus in Arabidopsis, AtXTH19, which is up-regulated by auxin, is expressed in the apical dividing and elongating primary root regions as well as in the differentiation zone (Yokoyama and Nishitani, 2001) and XTH9 is expressed in the shoot apices, where cell division is most active (Hyodo et al., 2003). More recently, Wang et al. (2010) reported an XTH gene highly expressed in the bamboo meristem.
The location of the XTH1 protein in vascular tissue was clear in embryonic axes at 24 h and 36 h after the start of imbibition. In particular, vascular tissues are labelled at 36 h after imbibition, whereas the label in parenchyma tissues decreases (Figs 2d, 4a). This could be linked to the ongoing elongation growth of the individual cells in the vascular tissues, compared with the parenchyma tissues at the same stage of development. Thus, at 36 h the parenchyma cells are 58 μm long whereas xylem cells reach 81 μm, as can be seen in Fig. 4a. XTH1 protein was also found in xylem and phloem cells in 48-h-old roots, but only in the younger areas during the early stages of differentiation (Fig. 5g, h). In support of the involvement of this protein in vascular differentiation, it is worth noting that in the basal region of the 48-h-old roots characterized by its loss of primary growth, no XTH1 was detected either in xylem or in phloem mature elements (Fig. 5h). Thus, the protein did not appear in the xylem or phloem cells in the basal region, where vascular development had already ended, but it is still present in those cells that maintain differentiation capacity, such as the pith cells (Fig. 5h). A similar involvement of XTHs in early phloem differentiation in poplar stems was reported by Bourquin et al. (2002) upon evaluating XET activity. In Arabidopsis, an essential role for AtXTH27 in the elongation of tracheary elements during vascular development has also been indicated (Matsui et al., 2005; Liu et al., 2007).
Xyloglucans in the primary cell walls are thought to be degraded during the elongation process of the immature tracheary elements. The chickpea XTH1 protein could mediate the degradation of xyloglucans in the primary cell wall of such tracheary elements. Alternatively, it is possible that the XTH1 protein could mediate the fragmentation of xyloglucans by converting the large split fragment of the donor xyloglucan into small xyloglucan fragments, as suggested by Matsui et al. (2005) for AtXTH27.
XTH1 was also present in the vascular bundles of primary leaves, as can be seen in Fig. 4b, showing a transverse section of a developing leaf after 36 h of seed imbibition. This indicates that chickpea XTH1 could have a similar function in different organs and could be responsible for the vascular differentiation observed not only in radicles but also in leaves. Consistent with this, the same function for this protein in seedling epicotyls and radicles as well as in stem internodes has been proposed previously. XTH1 was located in immature, growing vascular elements of young epicotyls, roots, and young stem internodes (Jimenez et al., 2006), suggesting that XTH1 could play an essential role in the process of xyloglucan degradation in growing xylem and phloem cells. Thus, the role of XTH1 in the differentiation of vascular tissue is similar in embryonic axes, seedlings, and plants in C. arietinum.
Acknowledgments
This work was supported by grants from the Ministerio de Ciencia e Innovación, Spain (BFU2009-08769) and from the Junta de Castilla y León (SA023A10-2).
Glossary
Abbreviation
- XTH
xyloglucan endotransglucosylase/hydrolase
References
- Barrachina C, Lorences EP. Xyloglucan endotransglycosylase activity in pine hypocotyls. Intracellular localization and relationship with endogenous growth. Physiologia Plantarum. 1998;102:55–60. doi: 10.1034/j.1399-3054.1998.1020108.x. [DOI] [PubMed] [Google Scholar]
- Baumann MJ, Eklöf JM, Michel G, Kallas AM, Teeri TT, Czjzek M, Brume H. Structural evidence for the evolution of xyloglucanase activity from xyloglucan endo-transglycosylases: biological implications for cell wall metabolism. The Plant Cell. 2007;19:1947–1963. doi: 10.1105/tpc.107.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becnel J, Natarajan M, Kipp A, Braam J. Developmental expression patterns of Arabidopsis XTH genes reported by transgenes and Genevestigator. Plant Molecular Biology. 2006;61:451–467. doi: 10.1007/s11103-006-0021-z. [DOI] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. The Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin V, Nishikubo N, Abe H, Brumer H, Denman S, Eklund M, Christiernin M, Teeri TT, Sundberg B, Mellerowicz EJ. Xyloglucan endotransglycosylases have a function during the formation of secondary cell walls of vascular tissues. The Plant Cell. 2002;14:3073–3088. doi: 10.1105/tpc.007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá C, Rose JKC, Bennett AB. Auxin regulation and spatial localization of an endo-1,4-β-d-glucanase and a xyloglucan endotransglycosylase in expanding tomato hypocotyls. The Plant Journal. 1997;12:417–426. doi: 10.1046/j.1365-313x.1997.12020417.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. Journal of Experimental Botany. 2002;53:215–223. doi: 10.1093/jexbot/53.367.215. [DOI] [PubMed] [Google Scholar]
- Fry SC. Polysaccharide-modifying enzymes in the plant cell wall. Annual Review of Plant Physiology and Plant Molecular Biology. 1995;46:498–520. [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytology. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler-Lee J, Geisler M, Coutinho PM, et al. Poplar carbohydrate-active enzymes. gene identification and expression analyses. Plant Physiology. 2006;140:946–962. doi: 10.1104/pp.105.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane DC. Antibodies: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- Hernández-Nistal J, Labrador E, Martín I, Jiménez T, Dopico B. Transcriptional profiling of cell wall protein genes in chickpea embryonic axes during germination and growth. Plant Physiology and Biochemistry. 2006;44:684–692. doi: 10.1016/j.plaphy.2006.10.017. [DOI] [PubMed] [Google Scholar]
- Hyodo H, Yamakawa S, Takeda Y, Tsuduki M, Yolota A, Nishitani K, Kohchi T. Active gene expression of a xyloglucan endotransglucosylase/hydrolase gene, XTH9, in inflorescence apices is related to cell elongation in Arabidopsis thaliana. Plant Molecular Biology. 2003;52:473–482. doi: 10.1023/a:1023904217641. [DOI] [PubMed] [Google Scholar]
- Ishimaru M, Kobayashi S. Expression of a xyloglucan endo-transglycosylase gene is closely related to grape berry softening. Plant Science. 2002;162:621–628. [Google Scholar]
- Jiménez T, Martín I, Labrador E, Dopico B. The immunolocalization of a xyloglucan endotransglucosylase/hydrolase specific to elongating tissues in Cicer arietinum suggests a role in the elongation of vascular cells. Journal of Experimental Botany. 2006;57:3979–3988. doi: 10.1093/jxb/erl169. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu Y-B, Lu S-M, Zhang J-F, Liu S, Lu Y-T. A xyloglucan transglucosylase/hydrolase is involved in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta. 2007;226:1547–1560. doi: 10.1007/s00425-007-0591-2. [DOI] [PubMed] [Google Scholar]
- Maclachlan G, Brady C. Endo-1,4-β-glucanase, xyloglucanase, and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiology. 1994;105:965–974. doi: 10.1104/pp.105.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Yokoyama R, Seki M, Ito T, Shinozaki K, Takahashi T, Komeda Y, Nishitani K. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. The Plant Journal. 2005;42:525–534. doi: 10.1111/j.1365-313X.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- Miedes E, Lorences E. Xyloglucan endotransglucosylase/hydrolase (XTH) during tomato fruit growth and ripening. Journal of Plant Physiology. 2009;166:489–498. doi: 10.1016/j.jplph.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Nishitani K. The role of endoxyloglucan transferase in the organization of plant cell walls. International Review of Cytology. 1997;173:157–206. doi: 10.1016/s0074-7696(08)62477-8. [DOI] [PubMed] [Google Scholar]
- Nishitani K, Tominaga R. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Ogawa M, Hanada A, Yamauchi Y, Kuwahara A, Kamiya Y, Yamaguchi S. Gibberellin biosynthesis and response during Arabidopsis seed germination. The Plant Cell. 2003;15:1591–1604. doi: 10.1105/tpc.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SJ, Davies WJ. An analysis of relative elemental growth rate, epidermal cell size and xyloglucan endotransglycosylase activity through the growing zone of ageing maize leaves. Journal of Experimental Botany. 1996;47:339–347. [Google Scholar]
- Potter I, Fry SC. Changes in xyloglucan endotransglycosylase (XET) activity during hormone-induced growth in lettuce and cucumber hypocotyls and spinach cell suspension cultures. Journal of Experimental Botany. 1994;45:1703–1710. [Google Scholar]
- Redgwell RJ, Fry SC. Xyloglucan endotransglycosylase activity increases during kiwifruit (Actinidia deliciosa) ripening. Plant Physiology. 1993;103:1399–1406. doi: 10.1104/pp.103.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo S, Jiménez T, Labrador E, Dopico B. The gene for a xyloglucan endotransglucosylase/hydrolase from Cicer arietinum is strongly expressed in elongating tissues. Plant Physiology and Biochemistry. 2005;43:169–176. doi: 10.1016/j.plaphy.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant and Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Saladié M, Rose JKC, Cosgrove DJ, Catalá C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. The Plant Journal. 2006;47:282–295. doi: 10.1111/j.1365-313X.2006.02784.x. [DOI] [PubMed] [Google Scholar]
- Smith RC, Matthews PR, Schünmann PHD, Chandler PM. The regulation of leaf elongation and xyloglucan endotransglycosylase by gibberellin in ‘Himalaya’ barley (Hordeum vulgare L.) Journal of Experimental Botany. 1996;47:1395–1404. [Google Scholar]
- Thompson JE, Fry SC. Density-labelling of cell wall polysaccharides in cultured rose cells: comparison of incorporation of 3H and 13C from exogenous glucose. Carbohydrate Research. 2001;332:175–182. doi: 10.1016/s0008-6215(01)00064-7. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Smith RC, Fry SC. Xyloglucan undergoes inter-polymeric transglycosylation during binding to the plant cell wall in vivo: evidence from 13C/3H dual labelling and isopycnic centrifugation in caesium trifluoroacetate. Biochemical Journal. 1998;327:699–708. doi: 10.1042/bj3270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Peng H, Lin E, et al. Identification of genes related to the development of bamboo rhizome bud. Journal of Experimental Botany. 2010;61:551–561. doi: 10.1093/jxb/erp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Campbell P, Vargheese AK, Braam J. The Arabidopsis XET-related gene family: environmental and hormonal regulation of expression. The Plant Journal. 1996;9:879–889. doi: 10.1046/j.1365-313x.1996.9060879.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Nishitani K. A comprehensive expression analysis of all members of a gene family encoding cell-wall enzymes allowed us to predict cis-regulatory regions involved in cell wall construction in specific organs of Arabidopsis. Plant and Cell Physiology. 2001;42:1025–1033. doi: 10.1093/pcp/pce154. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Rose JK, Nishitani K. A surprising diversity and abundance of xyloglucan endotransglycosylase/hydrolases in rice. Classification and expression analysis. Plant Physiology. 2004;134:1088–1099. doi: 10.1104/pp.103.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]