Abstract
The importance of salicylic acid (SA) in the signal transduction pathway of plant disease resistance has been well documented in many incompatible plant–pathogen interactions, but less is known about signalling in compatible interactions. In this type of interaction, tomato plants have been found to accumulate high levels of 2,5-dihydroxybenzoic acid (gentisic acid, GA), a metabolic derivative of SA. Exogenous GA treatments induce in tomato plants a set of PR proteins that differ from those induced by salicylic acid. While SA accumulates in tomato plants mainly as 2-O-β-D-glucoside, GA has only been found as 5-O-β-D-xyloside. To characterize this step of the GA signalling pathway further, the present work focuses on the study of the GA-conjugating activity in tomato plants. A gentisate glycosyltransferase (GAGT) cDNA has been isolated and overexpressed in Pichia pastoris, and GA-conjugating activity was confirmed by detecting the xylosylated GA. The purified plant protein is highly specific for GA, showing no activity toward many other phenolic compounds, including SA. In addition, it shows an outstanding selectivity for UDP-xylose as the sugar donor, which differentiates this enzyme from most glycosyltransferases. Both the GA-conjugating activity and the corresponding mRNA show a strong, rapid, and transient induction upon treatment of tomato plants with GA or SA. Furthermore, its expression is rapidly induced by compatible infections. However, neither the gene nor the activity seems to respond to incompatible infections or wounding. The unique properties of this new glycosyltransferase suggest a specific role in regulating the free GA levels in compatible plant–pathogen interactions.
Keywords: Compatible interactions, gentisic acid, glycosylation, plant pathogens, salicylic acid, Solanum lycopersicum, xylosyltransferase
Introduction
Because of their sessile condition, plants have evolved a very efficient defence system against all sorts of potential environmental aggressions, either of a biotic or an abiotic nature. Some of these defence mechanisms are constitutive, and are present in the plant before pathogen entrance, whereas others are pathogen-inducible (van Loon et al., 2006). In the latter case, once the plant recognizes pathogen arrival, a very complex signalling network is established which involves signal molecules such as salicylic acid (SA), jasmonic acid (JA), or ethylene (ET) (Lorenzo and Solano, 2005; Broekaert et al., 2006; Loake and Grant, 2007). Recent studies indicate that other hormones such as abscisic acid, auxins, gibberellic acid, cytokinins, brassinosteroids, and peptide hormones are also implicated in different aspects of plant defence signalling pathways (Bari and Jones, 2009).
Depending on the nature of the plant–pathogen interaction, the resulting infection can be localized or systemic. When the specific gene-for-gene recognition occurs between the plant and the pathogen, an incompatible interaction takes place (Flor, 1971). In this case, the plant activates the so-called hypersensitive response (HR), which mainly consists in rapid cell death around the infection point, causing pathogen confinement, and the infection is referred to as necrotizing. This defence response is very often associated with the activation of systemic acquired resistance (SAR), which is non-specific and long-lasting along the whole plant. This SAR-mediated protection is based on a selective and co-ordinated activation of a number of genes (SAR genes) that are directly implicated in the establishment and maintenance of this resistance (Ryals et al., 1996; Grant and Lamb, 2006). Among these SAR genes, Pathogenesis-Related Proteins (PRs) are low-molecular-weight proteins that not only display antimicrobial properties, but also accumulate locally and systemically in the plant upon infection (Granell et al., 1987; Rodrigo et al., 1993; Sels et al., 2008). On the other hand, when no gene-for-gene recognition occurs, the resulting interaction is considered as compatible. In this case, although the plant may activate an antipathogenic response, the pathogen escapes from local defences and a systemic infection is established (Staskawicz et al., 1995).
To date, HR and SAR have been broadly studied, and SA has been proposed as the signal molecule that mediates these defence responses in incompatible interactions (Gaffney et al., 1993; Delaney et al., 1994; Loake and Grant, 2007). However, very little is known about the signalling of the defence response in compatible interactions. SA accumulates in compatible interactions (O'Donnell et al., 2001; Huang et al., 2003), but a general role has not been established in tomato. This contrasts with findings in Arabidopsis, where SA-deficient plants are generally more susceptible to pathogens (Nawrath and Métraux, 1999). Gentisic acid (GA) has been described to accumulate at higher levels than SA in tomato, Gynura, and cucumber plants subjected to different compatible infections (Bellés et al., 1999, 2006). Moreover, exogenous GA elicits the accumulation of the antifungal PR proteins P23, P32, and P34 in tomato (García Breijo et al., 1990; Rodrigo et al., 1993; Bellés et al., 1999). These proteins are not induced by exogenous SA, which is able to elicit other PR proteins in the same plant. Thus, GA has been proposed to play a role as an intermediary in compatible, non-necrotizing interactions (Bellés et al., 1999, 2006). Interestingly, GA is an effective antifungal plant compound (Lattanzio et al., 1994), and GA behaves as a strong antioxidant molecule in mammalian cells, exerting a protective effect against certain bacteria (Belicova et al., 2001).
Similar to other hydroxybenzoates, GA accumulates in the plant as a glycoconjugate. However, unlike other related phenolics such as SA or benzoic acid, which are conjugated to glucose after their accumulation upon infection (Silverman et al., 1995; Lee and Raskin, 1998, 1999; Chong et al., 2001), GA accumulates exclusively as 5-O-β-D-xylopyranoside (Fayos et al., 2006). This xylose conjugate of GA has recently been found to be the most important induced metabolite in tomato plants upon viroid infection (López-Gresa et al., 2010).
In plants, glycosylation is one of the most common modifications of secondary metabolites, which is implicated in stabilization, the increase of solubility, and in the storage and regulation of levels of certain hormones and signal molecules as well as in the detoxification of xenobiotics (Yalpani et al., 1992; Szerszen et al., 1994; Gachon et al., 2005). Glycosylation is carried out by glycosyltransferases (GTs) which transfer nucleotide-diphosphate-activated sugars (known as the ‘glycosyl donor’) to low-molecular-weight substrates. Increasing evidence suggests that glycosylation is an important mechanism to regulate plant cellular homeostasis with the identification of a large variety of GTs capable of recognizing many different compounds (Bowles et al., 2006). According to the CAZy database (http://www.cazy.org/), glycosyltransferases can be classified into 91 families, depending on substrate specificity and sequence similarity (Osmani et al., 2009). Currently, many GTs have been sequenced, although only a few of them have been characterized biologically.
In this work, the focus is on the purification and characterization of the tomato xylosyltransferase responsible for the conjugation of GA. A cDNA clone was isolated (AJ889012) and expressed in Pichia pastoris. The enzyme displays outstanding selectivity toward the sugar donor, using mainly UDP-xylose. Furthermore, gentisic acid seems to be the only phenolic compound specifically to accept the sugar. The protein and its mRNA show a rapid and transient induction upon systemic infections, and GA and SA treatments. Nevertheless, tomato GAGT apparently does not respond to incompatible interactions or wounding. The unique properties of this novel glycosyltransferase suggest a very specific role for this protein in the regulation of GA levels in compatible plant–pathogen interactions.
Materials and methods
Plant materials, chemicals, and pathogen treatments
Tomato (Solanum lycopersicum L. cv. Rutgers or Rio Grande) plants were grown under standard greenhouse conditions (20–25 °C and 16/8 h light/dark photoperiods).
Treatments and wounding were performed with 3–4-week-old plants. For the SA and GA treatments, fully expanded leaves were excised and immersed by the petiole in 2 mM SA or GA solutions. Ethylene treatments of full plants were carried out in air-tight plexiglass chambers under a continuous flow of gas at 50 ppm. Methyl jasmonate was applied by spraying plants with a 2 mM solution in water containing 0.02% (v/v) TWEEN-20. Wounding was performed by crushing one composite leaf per plant using forceps. The immediate upper leaves were also used to analyse the systemic response. Plant material was harvested at different times, then used immediately or stored frozen at –80 °C.
Inoculation of Rutgers tomato plants with Citrus Exocortis Viroid (CEVd) or with Tomato Mosaic Virus (ToMV) was carried out according to the indications of Granell et al. (1987) and Bellés et al. (1999), respectively. Rio Grande (PtoR) tomato plants were inoculated with Pseudomonas syringae pv. tomato (AvrPto+) at 108 cfu ml−1 to produce a necrotizing infection. The bacterial culture was infiltrated into leaves, as previously described (Anderson et al., 2006). Rio Grande tomato plants and bacteria were kindly supplied by GB Martin (The Boyce Thompson Institute for Plant Research, Ithaca, NY).
Extraction and quantification of SA and GA from tomato leaves
The preparation and analysis of free and conjugated SA and GA were performed according to Bellés et al. (1999, 2006). An HPLC analysis of phenolics was done following the protocol detailed in Yalpani et al. (1992). A 20 μl aliquot from the final methanolic sample was injected into a reverse-phase Symmetry 5 μm C18 (4.6×150 mm; Waters) column equilibrated in 1% acetic acid. Eluents were 1% acetic acid (eluent A) and 100% methanol (eluent B). A lineal gradient starting with 100% eluent A and 0% eluent B and ending with 0% of eluent A and 100% eluent B was applied over 20 min at a flow rate of 1 ml min−1. SA and gentisic acid were detected with a Waters 470 fluorescence detector (λ excitation=313 nm; λ emission=405 nm), and were quantified with the Waters Millennium32 software using authentic standards.
Xylosyltransferase activity assay and detection
The standard assay for GA xylosyltransferases was performed as follows: the reaction mixture contained an appropriate volume of the protein extract and a final concentration of 0.5 mM GA (Sigma) and 1 mM UDP-xylose (acquired from CarboSource Services, Complex Carbohydrate Research Center, University of Georgia, USA). In the radioactive assays, the reaction mixture contained 21 μM GA (Sigma) and 21 μM UDP-[14C]-xylose (American Radiolabeled Chemicals Inc.). To test substrate specificity, 21 μM GA were replaced with the same concentration of the different acceptor substrates: salicylic acid, benzoic acid, 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, 2,3-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, 2,3,4-trihydroxybenzoic acid, 2,4,6-trihydroxybenzoic acid, caffeic acid, ferulic acid, coumaric acid, scopoletin, esculetin, or umbelliferone. All these phenolics were purchased from Sigma. To study the specificity of the glycosyl donor substrate, UDP-[14C]-xylose was replaced with UDP-[14C]-glucose (American Radiolabeled Chemicals Inc.). The different preparations were incubated at 37 °C for 15–60 min. Then, samples were centrifuged for 15 min and analysed either by high performance liquid chromatography (HPLC, Waters), according to the indications of Yalpani et al. (1993) and Bellés et al. (1999) or thin-layer chromatography (TLC) on silica gel (Alugram SIL G/UV plates, Macherey-Nagel). To detect the conjugated products by HPLC, 40 μl of reaction volume were injected into a C18 reverse-phase column (5 μm, 4.6×150 mm; Waters, Milford, MA) with a linear gradient of methanol (0–100%) at a flow rate of 1.5 ml min−1 for 20 min. Conjugated phenolics were detected with either a spectrofluorescence detector as described above (λ excitation=313 nm; λ emission=405 nm) or a radioactivity detector (LB 509 EGG Berthold, Bad Wildbad-Germany). For the TLC analysis, 1–5 μl of each sample was applied to silica gel plates and separated using a solvent consisting of 1-butanol/acetic acid/water (4:1:1 by vol.). Sugars were detected by spraying the dried TLC plates with 15% (v/v) sulphuric acid containing 5 mM ceric sulphate, and were developed at 120 °C for 15 min. Radioactive spots were visualized by autoradiography.
Cloning of tomato GAGT
A sequence alignment of the different GTs of the Solanaceae family was carried out to build a set of degenerate oligonucleotide primers: sense (5′-GTTT(AC)(CT)GAT(AC)(CT)(AG)TT(CT)CTTCC-3′) and antisense (5′-TGGC(AC)(AT)TG(CT)(AC)A(CT)CATTGGTAC-3′). Five μg of total RNA from GA-treated tomato leaves were reverse-transcribed in a final volume of 50 μl using oligo(dT)18 and M-MLV reverse transcriptase (Promega). Then, 5 μl of RT product was amplified using Pfu DNA polymerase (TaKaRa) and the degenerate primers described. The amplified DNA (about 700 bp) was recovered from the agarose gel using the QIAquick Gel Extraction kit (Qiagen) and cloned in pGEM-T Easy (Promega) according to the manufacturer's recommendations. Several random clones were picked up and sequenced. To obtain the full-length cDNA clone, the selected DNA sequence was labelled with [32P]-dCTP using the Ready-To-Go kit (GE Healthcare), and was used as a probe to screen a λ-ZAP (Stratagene) cDNA library constructed in our laboratory from the mRNA of gentisic acid-treated tomato leaves (our unpublished results).
RNA isolation, blotting, and hybridization
Total RNA was prepared by using the TRIzol reagent (Invitrogen) following the manufacturer's instructions. For the Northern blot analysis, 30 μg of RNA were separated in formaldehyde-agarose gels and transferred onto Nytran membranes (Schleicher & Schuell). Hybridization with [32P]-labelled probes and washing conditions were performed as described in Church and Gilbert (1984).
Overexpression and purification of the recombinant GAGT protein in Pichia pastoris
The coding region of the tomato GAGT cDNA was amplified by PCR using the primer pairs: sense (5′-CCGGTACCAGTATGGCCATGACTACTCACAAAGCTC-3′) and antisense (5′-CCGGGCCCGGAAATAGTAACCAACTTGG-3′); and the Expand High Fidelity PCR system (Roche) under the following conditions: one cycle of 95 °C for 3 min, 40 cycles of 1 min at 55 °C, 3 min at 72 °C, and 1 min at 95 °C, with a final extension step at 72 °C for 7 min. The PCR fragment was gel-purified and digested with KpnI and ApaI. The pPICZ plasmid (Invitrogen) was cut under the same conditions and treated with shrimp alkaline phosphatase (Roche). The cDNA sequence was then ligated into pPICZ using T4 DNA ligase (Promega). After transformation into E. coli DH5α cells and plating on LB/half-salt agar containing zeocin (25 μg ml−1, Invitrogen), positive clones were selected and sequenced to confirm the reading frame. Two to 10 μg of the expression construct plasmid DNA were linearized according to the supplier's instructions, and were used to electroporate the P. pastoris competent cells, obtained as described by Gietz and Woods (2002). The expression of the recombinant protein was induced by methanol according to the manufacturer's directions. Yeast cells were centrifuged and the pellet was resuspended in extraction buffer (20 mM sodium phosphate, pH 7.5, 500 mM NaCl, and 25 mM imidazole). Cells were mechanically broken using glass beads (0.5 mm diameter), and the suspension was centrifuged for 20 min at 10 000 g to obtain the crude protein extract for both the uninduced and methanol-induced yeast cells.
Xylosyltransferase activity purification
Preparation of the crude leaf extract:
One hundred grams of frozen tomato leaves were ground to a fine dust in liquid nitrogen, and resuspended in 200 ml extraction buffer consisting of 25 mM MES (pH 6.5), 1 mM PMSF, 0.2% β-mercaptoethanol, and 0.05% PVP. The plant material was then homogenized using a Polytron. The tissue debris was removed by centrifugation at 15 000 g for 20 min and the supernatant was filtered through Miracloth. The filtrate was used for further purification.
Ammonium sulphate fractionation:
The protein fraction precipitating between 35% and 65% saturation of ammonium sulphate was recovered by centrifugation. The pellet was resuspended in 25 mM MES (pH 6.5) containing 0.2% β-mercaptoethanol (MES-M buffer), and was desalted through PD-10 columns (Amersham Pharmacia Biotech) equilibrated with MES-M buffer.
Anion exchange chromatography:
After desalting, protein samples were chromatographed through a Q-Sepharose Fast Flow column (Amersham Pharmacia Biotech), which was equilibrated with 25 mM MES-M buffer containing 0.05 M NaCl. Proteins were eluted with a 0.05–0.6 M lineal NaCl gradient in MES-M buffer at a flow rate of 1 ml min−1. Fractions were collected and assayed for enzyme activity and protein concentration. The fractions containing enzyme activity were pooled, desalted using a PD-10 column, concentrated using an Amicon Ultra-15 Centrifugal Filter Unit (Millipore) and then rechromatographed through Q-Sepharose under the same conditions.
HiTrap Blue affinity chromatography:
The fractions recovered from the previous step were further purified with a HiTrap Blue affinity column (Amersham Pharmacia Biotech) equilibrated with 25 mM MES-M buffer. Proteins were eluted with a 0.2–0.6 M NaCl gradient in MES-M buffer at a flow rate of 2 ml min−1. Active fractions were desalted in a PD-10 column and concentrated using Amicon Ultra-15 filter units.
Protein analysis
Samples from each protein purification step were separated by SDS-PAGE and stained with Coomassie Brilliant Blue R-250 as described by Conejero and Semancik (1977). The Bradford method (1976) was employed for protein quantification using bovine serum albumin as a standard.
Protein modelling
The amino acid sequence of tomato GAGT was submitted to the SWISS-MODEL server (http://swissmodel.expasy.org) using the crystal structure data of Medicago truncatula UGT85H2 glycosyltransferase (Li et al., 2007) as a template (PDB ID 2PQ6). The DeepView (http://spdbv.vital-it.ch/) software was used to visualize, align, and prepare the structures for submission. Final rendering was done with the UCSF Chimera software (http://www.cgl.ucsf.edu/chimera).
Results
Conjugation of gentisic acid in tomato
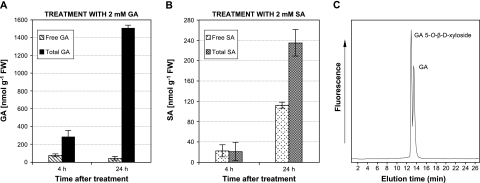
Upon a systemic infection, tomato plants undergo a strong accumulation of GA, whose levels increase considerably more than the corresponding SA levels (Bellés et al., 1999). The production of GA or SA in the plant has been reported to be followed by a rapid conjugation of these phenolics (Lee and Raskin, 1998; Bellés et al., 1999; Schuhegger et al., 2006). Like most hydroxybenzoates, SA accumulates mainly as 2-O-β-D-glucoside (Edwards, 1994; Lee and Raskin, 1999), whereas GA is conjugated as 5-O-β-D-xyloside (Fayos et al., 2006). To study the GA-conjugating activity in tomato, tomato leaves were treated with 2 mM GA, and the levels of the free and conjugated GA were analysed at 4 h and 24 h of treatment, since glycosyltransferases have been shown to be induced very quickly (Lee and Raskin, 1999; Park et al., 2003). As shown in Fig. 1A, more than half the total GA was conjugated 4 h after treatment. By 24 h, most of the GA was conjugated, reaching up to 97%. An analogous experiment was carried out by treating plants with 2 mM SA (Fig. 1B). After 4 h, no conjugated SA was detected, and only half the total SA was conjugated at 24 h. All this indicates in relative and absolute terms that GA conjugates to a much greater extent than SA at 4 h and 24 h after the corresponding treatments.
Fig. 1.
Conjugation of GA and SA in tomato. Tomato leaves were treated with 2 mM gentisic acid or salicylic acid for 4 h and 24 h, and the contents of the free and total GA and SA are shown in (A) and (B), respectively. Results are the means of three independent assays ±SD (standard deviation). (C) HPLC detection of the GA conjugate. Gentisic acid and UDP-xylose were incubated with crude tomato leaf extracts as described in the Material and methods. After 10 min, phenolics were extracted and analysed by HPLC. The peak corresponding to the more polar GA 5-O-β-D-xyloside appears at 13.1 min, while free GA peaks later at 13.8 min.
In order to detect the corresponding GA xylosyltransferase activity, crude extracts of tomato leaves were incubated with UDP-xylose and GA as substrates. After 15 min, the accumulation of phenolics was analysed by HPLC. The chromatograms showed two peaks (Fig. 1C): one corresponds to free gentisic acid (13.8 min), while the more polar one corresponds to GA 5-O-β-D-xyloside (13.1 min). The standard for GA 5-O-β-D-xyloside was obtained in our laboratory (Fayos et al., 2006). Using this activity assay, it was possible to study the induction pattern of the GA xylosyltransferase activity present in tomato leaves upon different treatments or infections.
Cloning the cDNA of GAGT
To obtain a cDNA corresponding to the xylosyltransferase activity detected, a comparative sequence analysis between different glycosyltransferases was performed. Since our activity was induced by either GA or SA (see below), several GTs that have been described to be induced by salicylic acid or by other phenolic compounds were used to perform a DNA sequence alignment. Specifically, the GTs used for the sequence comparison were SAGT, IS5, and Togt1 from Nicotiana tabacum, and Twi1, from Solanum lycopersicum, both species belong to the Solanaceae family (Horvath and Chua, 1996; Fraissinet-Tachet et al., 1998; O'Donnell et al., 1998; Lee and Raskin, 1999). Based on this analysis, a set of degenerate primers was designed and used in a RT-PCR of the RNA of tomato plants which were either healthy or infected by the Citrus Exocortis Viroid. In both cases, a band of the predicted 700 bp was obtained and was more intense in the infected plants (not shown), which is in accordance with an increase in the accumulation of GA 5-O-β-D-xyloside in plants infected with this viroid as compared with control plants (Fayos et al., 2006). This PCR band was cloned in a pGEM-T vector and, since the primers used were highly degenerated, a number of clones were sequenced. The sequences obtained fell into two different categories: the previously described Twi1, a salicylic acid- and wound-induced glycosyltransferase (O'Donnell et al., 1998) and a new clone (GenBank accession number AJ889012). We focused on this clone as the putative GA glycosyltransferase (GAGT). To obtain the complete cDNA of GAGT, the PCR product was used as a probe to screen a cDNA library constructed from the mRNAs of GA-treated tomato leaves which had been previously obtained in our laboratory. The complete cDNA obtained contains an ORF of 1370 bp that codes for a 51.5 kDa protein (see Supplementary Fig. S1A at JXB online). This size is similar to that of most of the GTs implicated in secondary metabolism (Vogt and Jones, 2000). The protein has a deduced isoelectric point of approximately 5.7 and a net charge of –7.8 at pH 7. It contains the consensus sequence PSPG (Plant Secondary Product Glycosyltransferase motif) described by Hughes and Hughes (1994). This sequence has been proposed to be the binding site for the sugar donor UDP (Shao et al., 2005). According to the CAZy database (http://www.cazy.org/), GAGT belongs to family 1 of the glycosyltransferases (GT1). This is the largest family and includes GTs involved in many different processes, such as conjugation and the regulation of signalling molecules (indole acetic acid, zeatin or SA). The GAGT sequence displays an 85% identity with the tobacco SAGT (Lee and Raskin, 1999), which also belongs to the GT1 family. However, its similarity to the rest of the GTs included in this family hardly exceeds 30%, and its identity with tomato Twi1 is also very low (see the phylogenetic tree in Supplementary Fig. S1B at JXB online). In addition to its high similarity to tobacco SAGT, tomato GAGT appears close to another SA glycosyltransferase from rice (accession number BAD34358) and two glycosyltransferases induced by jasmonic acid from maize (Szerszen et al., 1994) and tobacco (accession number AB000623).
Expression and enzyme activity of the recombinant protein
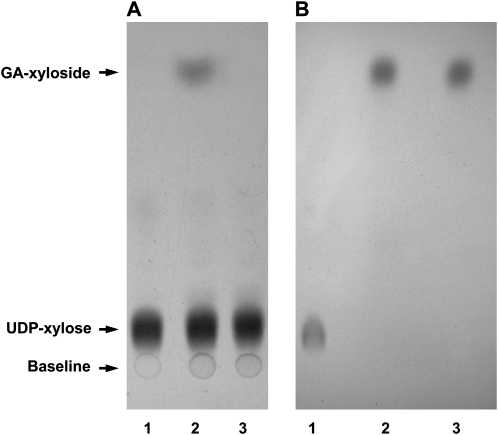
In order to determine whether the isolated putative glycosyltransferase sequence encodes an active GA xylosyltransferase, the coding region of the corresponding cDNA was expressed in Pichia pastoris using the pPICZ overexpression vector. The transformed yeast was grown in a methanol-induced media culture to express the recombinant protein. Detection of the enzyme activity was performed by using TLC and autoradiography as described for the other GTs expressed in yeast (Bencúrová et al., 2003) and for many others expressed in E. coli, such as the tobacco SAGT (Lee and Raskin, 1999; Kohara et al., 2007). Following the addition of UDP-[14C]-xylose and GA, a [14C]-xylosylated metabolite of GA was formed only in the reaction mixture corresponding to the methanol-induced yeast lysates containing the recombinant protein (Fig. 2A). The spot corresponding to the [14C]-xylosylated GA shows the same mobility as the standard 5-O-β-D-xylopyranoside of GA obtained in our laboratory (Fayos et al., 2006) and the xylosylated GA produced using crude plant extracts (Fig. 2B, lanes 2 and 3, respectively). No GA glycoside was produced when UDP-[14C]-glucose was used as a sugar donor. In addition, no activity was detected when SA was tested as a sugar acceptor using UDP-[14C]-xylose or UDP-[14C]-glucose (data not shown). Therefore, these results indicate that the isolated clone encodes a GA-specific xylosyltransferase.
Fig. 2.
Xylosyltransferase activity of recombinant GAGT. Left panel: GAGT cDNA was expressed in Pichia pastoris cells under the control of a methanol-inducible promoter (see the Materials and methods). Extracts from the uninduced (lane 1) and methanol-induced cells (lane 2) were incubated with UDP[14C]-xylose and GA. Lane 3 corresponds to UDP[14C]-xylose. Right panel: samples of UDP-xylose (lane 1), standard GA-5-O-β-D-xyloside, previously obtained in our laboratory (lane 2; Fayos et al., 2006) and the GA xyloside produced using the crude tomato leaf extracts (lane 3) were separated by TLC under the same conditions, and were chemically revealed as described in the text.
Expression of GAGT mRNA and induction of activity
The tomato GAGT cDNA was used as a probe to study the effect of different signal molecules, as well as compatible and incompatible pathogen interactions, on the induction of the mRNA, and gentisate-5-O-β-D-xylosyltransferase enzyme activity was measured in parallel for all treatments.
Effect of GA and SA:
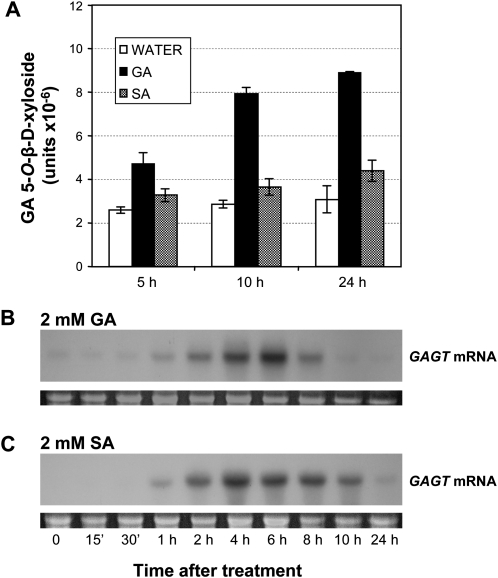
Xylosyltransferase activity was enhanced by both GA and SA treatments as compared to the control water-treated plants (Fig. 3A). This enhanced activity remained 24 h later. Interestingly, the effect of GA on enzyme activity was much stronger than that produced by SA treatment. This early induction pattern matches the behaviour described for many GTs induced by SA (Yalpani et al., 1992; Fraissinet-Tachet et al., 1998; O'Donell et al., 1998; Lee and Raskin, 1999; Park et al., 2003; Griesser et al., 2008). In general, GTs are rapidly inducible enzymes when compared with other proteins that respond to SA, such as PR defence proteins, which typically begin to accumulate later (Granell et al., 1987; Sels et al., 2008). The Northern blot analysis indicates a rapid and transient induction of GAGT mRNA (Fig. 3B, C). Very low levels of GAGT mRNA were constitutively present prior to treatment. Message accumulation began at approximately 1 h after treatment with SA or GA, and reached a peak at between 4 h and 6 h, then returned to the basal levels at 24 h. These results are in agreement with the enzyme activity profiles. However, a high xylosyltransferase activity was still detected 24 h after starting treatments when the mRNA levels had returned to the basal levels.
Fig. 3.
GAGT induction by SA or GA treatment. Tomato leaves were incubated with water, 2 mM salicylic acid (SA) or 2 mM gentisic acid (GA), and plant material was collected at the indicated times. (A) The GAGT activity of the crude protein extracts was measured at 5, 10 or 24 h after treatment. The crude leaf extracts were incubated for 15 min with GA and UDP-xylose, and the amount of 5-O-β-D-xyloside formed was determined by fluorescence HPLC as described in the Materials and methods. Results are the means of three independent assays ±SD (standard deviation). (B, C) Northern blot analysis of GAGT mRNA accumulation in tomato leaves in response to GA (B) and SA (C) treatments. Samples were harvested at 0, 15 min, 30 min, 1, 2, 4, 6, 8, 10, and 24 h after treatment.
Compatible interactions:
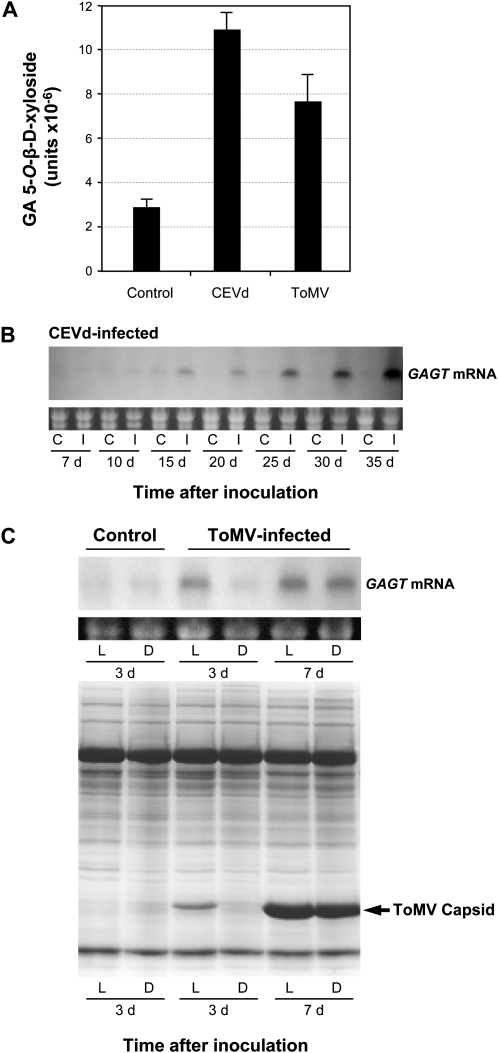
Citrus Exocortis Viroid (CEVd) and Tomato Mosaic Virus (ToMV), which produce a systemic non-necrotizing infection in tomato, have been shown strongly to induce the accumulation of free and conjugated GA (Bellés et al., 1999; Fayos et al., 2006). This has also been observed in other plant–pathogen compatible interactions (Bellés et al., 2006). The GA-xylosyltransferase activity was measured in tomato plants infected with CEVd or ToMV. Tissue samples were collected 4 weeks after inoculation for CEVd-infected plants and 7 d after inoculation for ToMV-infected plants. As shown in Fig. 4A, a dramatic increase in activity was observed in both the virus- and viroid-infected plants, and higher 5-O-β-D-xylosyltransferase levels were found in the CEVd-infected plants as compared to the ToMV-infected tissues. These results are in agreement with those obtained by Bellés et al. (1999), where levels of total GA upon viroid infection were higher than the GA levels present in the virus-infected plants. Thus, there seems to be a correlation between GA accumulation and the enhanced GA xylosyltransferase activity in these plants. The Northern blot analysis shows that the GAGT mRNA apparently followed the severity of symptoms caused by viroid infection (Fig. 4B). Viroid disease symptoms appeared in the ‘Rutgers’ tomato plants 2 weeks after inoculation with CEVd. Accordingly, no accumulation of GAGT mRNA was detected 7–10 d after CEVd inoculation, when the mRNA levels progressively accumulated along the viroid disease. ToMV inoculation was performed in 5-week-old tomato plants. Samples of local and distal leaves were collected at 3 d and 7 d post-inoculation, coinciding with the absence or presence of disease symptoms, respectively. The presence of the viral capsid was confirmed by SDS/PAGE, and disease progress was concomitant with the induction of the GAGT mRNA (Fig. 4C). These results indicate that, in both the CEVd and ToMV infections, the induction of the GAGT runs parallel to the progress of the disease, resulting in enhanced GA 5-O-β-D-xylosyltransferase activity.
Fig. 4.
GAGT induction by compatible infections. Tomato plants were inoculated with Citrus Exocortis Viroid (CEVd) or Tomato Mosaic Virus (ToMV), and infected leaves were collected at different days post-inoculation. (A) The GAGT activity of the tomato protein extracts was measured 35 d after CEVd inoculation or on day 7 after ToMV inoculation. The crude leaf extracts were incubated for 15 min with GA and UDP-xylose, and the amount of 5-O-β-D-xyloside formed was determined by fluorescence HPLC as described in the Materials and methods. Results are the means of three independent assays ±SD (standard deviation). (B) The time-course analysis by Northern blot of the GAGT mRNA accumulation in tomato leaves, in response to CEVd infection. C: control plants; I: CEVd-infected plants. Samples were collected on days 7, 10, 15, 20, 25, 30, and 35 post-inoculation. (C) Upper panel: time-course analysis by Northern blot of the GAGT mRNA accumulation. The local (L) or immediate upper (Distal, D) leaves were collected on days 3 or 7 post-inoculation. The first lanes correspond to the control, non inoculated plants. Lower panel: SDS-PAGE analysis of the total leaf proteins from the ToMV-infected tomato plants for the same samples shown in the upper panel. The protein band corresponding to the ToMV capsid is indicated by n arrow.
Incompatible interactions:
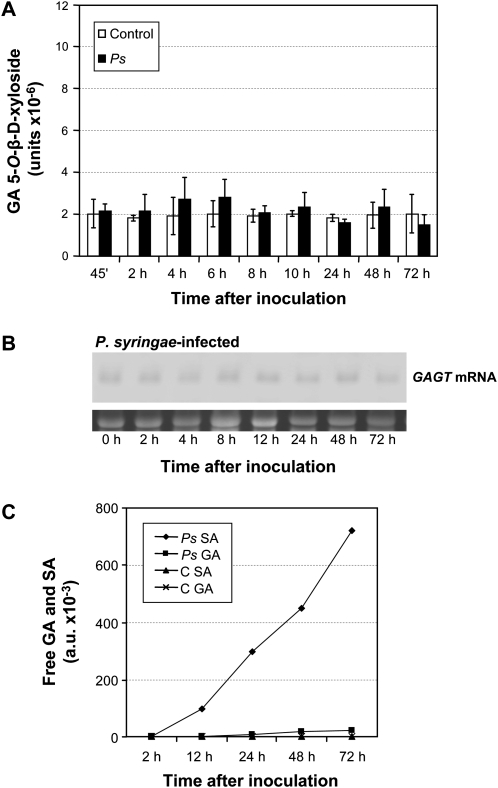
In order to establish a necrotizing infection, tomato plants were inoculated with Pseudomonas syringae. Samples were taken at several times post-inoculation and tested for GA xylosyltransferase activity. As Fig. 5A shows, Pseudomonas infection induced no increase in GAGT activity. Consequently, only basal levels of GAGT mRNA were detected by Northern blot analysis (Fig. 5B). Since the appearance of local, necrotic lesions is accompanied by an increase in SA (Malamy et al., 1990; Métraux et al., 1990; Uknes et al., 1993), the content of free SA and GA was determined throughout the bacterial infection. Figure 5C shows that, although the infection led to an expected increase in the SA levels, the GA levels remained practically unchanged. However, SA treatment did induce GAGT (Fig. 3C). The results obtained with P. syringae question the effect of SA on GAGT, and suggest that induction by SA may occur through GA, which is a metabolic derivative of SA. This is in contrast with many glycosyltransferases which are induced in an SA-dependent way after infection with Pseudomonas syringae pv. tomato (Langlois-Meurinne et al., 2005).
Fig. 5.
GAGT induction by incompatible infections. (A) The GAGT activity of tomato protein extracts was measured at different times (45 min, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h) post-inoculation with Pseudomonas syringae. Enzyme reactions were incubated for 15 min and 5-O-β-D-xyloside was analysed by fluorescence HPLC. Results are the means of three independent assays ±SD (standard deviation). (B) The time-course analysis by Northern blot of the GAGT mRNA accumulation in tomato leaves, in response to Pseudomonas syringae infection. Samples were harvested at 0, 2, 4, 6, 8, 10, 12, 24, 48, and 72 h post-inoculation. (C) The free gentisic acid and salicylic acid levels in the control or the Pseudomonas syringae-infected tomato leaves at 2, 12, 24, 48, and 72 h post-inoculation.
Wound and jasmonic acid treatment:
Contrary to the results reported for other GTs such as tomato Twi1 (O'Donnell et al., 1998), whose mRNA is accumulated by wounding, no increase in GA xylosyltransferase activity was detected upon wounding or treatment with jasmonic acid (Fig. 6A); furthermore, the Northern blot analyses (Fig. 6B, C, D) confirm this observation.
Fig. 6.
GAGT induction by wounding or MeJ treatment. Tomato plants were wounded or treated with 2 mM methyl-jasmonate (MeJ, and leaves were collected at different times. (A) The GAGT activity present in the tomato protein extracts was measured at 5, 10, or 24 h after wounding or MeJ treatment. Activity was measured in the control leaves, MeJ-treated leaves, wounded leaves (local), and immediate upper leaves (distal). Enzyme reactions were incubated for 15 min and 5-O-β-D-xyloside was determined by fluorescence HPLC. Results are the means of three independent assays ±SD (standard deviation). (B) Northern blot analysis of the GAGT mRNA accumulation in tomato leaves in response to MeJ treatment. The mRNAs from tomato leaves were harvested at 15 min, 30 min, 1, 2, 4, 6, 8, 10, and 24 h after spraying plants with a 2 mM MeJ solution. The TCI21 probe was used as a positive control (Lisón et al., 2006). (C, D). Northern blot analysis of the GAGT mRNA accumulation in tomato leaves in response to wounding. The total mRNAs from tomato wounded leaves (C) or immediate upper leaves (D) were extracted from the plant material harvested at the indicated times. TCI21 was used as a positive control.
Substrate specificity
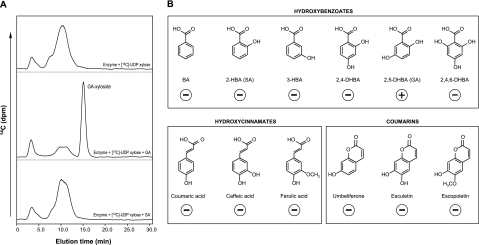
The in vitro substrate specificity of recombinant GTs often differs from the native activity. The ability of the recombinant protein to conjugate diverse substrates makes it difficult to study the physiological role of GT in the plant (Jones and Vogt, 2001; Achnine et al., 2005; Bowles et al., 2006). Consequently, the GA 5-O-β-D-xylosyltransferase activity from Rutgers tomato plants was purified to study its substrate specificity properly. The enzyme in the crude extracts was stable for months when stored at –80 °C. However, the enzyme was sensitive to oxidation; therefore, a reducing agent (β-mercaptoethanol) was always included in the extraction buffer. Activity was purified as described in the Materials and methods, and resulted in a 68-fold purification factor (Table 1). The purified enzyme preparation was assayed for substrate specificity towards a variety of phenolic sugar acceptors, using UDP-[14C]-xylose as a sugar donor (Fig. 7A, B). When UDP-[14C]-glucose was used as a sugar donor, the purified enzyme showed no conjugating activity. This is in agreement with most GTs described to date, which are fairly specific to the sugar donor substrate (Warnecke and Heinz, 1994; Lee and Raskin, 1999; Vogt and Jones; 2000). By contrast, GTs are usually less selective toward sugar acceptors (Warnecke and Heinz, 1994; Fraissinet-Tachet et al., 1998; Lee and Raskin, 1999; Jackson et al., 2001; Griesser et al., 2008). It is worth noting that, in our case, tomato GAGT shows a surprisingly narrow specificity toward GA as the sugar acceptor. A similar specificity has been reported for another glycosyltransferase purified from Catharanthus roseus, which efficiently conjugates GA among other phenolics, but this enzyme uses UDP-glucose as the sugar donor instead of UDP-xylose (Yamane et al., 2002).
Table 1.
Purification of GAGT from tomato leaves
| Step | Total protein | Total activity | Specific activity | Purification | Yield |
| (mg) | (milliunits) | (milliunits mg−1) | (Fold) | (%) | |
| Crude extract | 527.4 | 7700.1 | 14.60 | 1.0 | – |
| (NH4)2SO4 (35–65%) precipitation | 301.3 | 6544.2 | 21.72 | 1.5 | 84.9 |
| Q-Sepharose, first step | 3.7 | 1852.1 | 500.56 | 34.3 | 24.1 |
| Q-Sepharose, second step | 1.1 | 1082.4 | 983.96 | 67.4 | 14.1 |
| HiTrap Blue | 0.2 | 199.5 | 997.46 | 68.3 | 2.6 |
GAGT was extracted from 100 g of tomato leaves and activity was measured by integrating the HPLC fluorescence peak area corresponding to the GA-xyloside as detailed in the Materials and methods.
Fig. 7.
Substrate specificity assay of sugar acceptors for GAGT. The purified plant enzyme was tested for substrate specificity using UDP-[14C]-xylose and the following phenolics were used as sugar acceptors: benzoic acid (BA), 2-hydroxybenxoic acid (salicylic acid, SA), 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, 2,3-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid (gentisic acid, GA), 2,6-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, 2,3,4-trihydroxybenzoic acid, 2,4,6-trihydroxybenzoic acid, caffeic acid, ferulic acid, coumaric acid, escopoletin, esculetin, and umbelliferone. Samples were HPLC-analysed and the eluted radioactivity was monitored. (A) Typical elution profiles obtained with UDP-[14C]-xylose alone (top panel) or in the presence of GA (middle panel) or SA (bottom panel). A distinctive radioactive peak was detected using GA as a sugar acceptor, but not when the acceptor was SA. The same negative result was obtained with the rest of the phenolics assayed. The qualitative results for some representative phenolics are shown in (B).
Protein modelling
To date, the crystal structure of four plant glycosyltransferases is available: two from Medicago truncatula (MtUGT71G1 and MtUGT85H2), one from Vitis vinifera (VvGT1), and another one from Arabidopsis (AtUGT71B2) (reviewed in Osmani et al., 2009). Our sequence data were submitted to the SWISS-MODEL server by considering these four GTs as a reference. No valid model was retrieved when AtUGT71B2 or VvGT1 were used as a template. The modelling using M. truncatula UGT71G1 provided only a partial folding at the C-terminal domain (not shown). However, a fairly good structure was obtained with M. truncatula UGT85H2 glycosyltransferase (see Supplementary Fig. S2 at JXB online). Although this procedure does not necessarily describe the real three-dimensional structure of the protein, the proposed model for tomato GAGT shares the structural features described for the Family-1 GTs by adopting the so-called GT B-fold formed by the C-terminal and N-terminal domains separated by an interdomain linker (Osmani et al., 2009). The sugar donor is deeply buried in a narrow groove in the C-terminal domain and interacts with the highly conserved PSPG motif. Remarkably, tomato GAGT and M. truncatula UGT85H2 only share a 32% amino acid identity, but the model proposed for tomato GAGT fits the described structure of MtUGT85H2 well.
Discussion
Gentisic acid has been described as a very efficient antifungal compound in plants (Lattanzio et al., 1994). Besides, GA has been proposed as a signal molecule in the activation of the plant defence response in systemic infections (Bellés et al., 1999, 2006). Although in lower levels than GA, in these compatible interactions the plant accumulates SA along with the appearance of symptoms, and both compounds conjugate quickly and efficiently (Bellés et al., 1999, 2006; Fayos et al., 2006; Schuhegger et al., 2006). The same occurs with many other hydroxybenzoic acids derived from secondary metabolism. The sugar moiety with which these metabolites conjugate varies depending on plant species. Thus, in tobacco plants, SA has been found as either a glucosyl-ester or an O-β-D-glucoside (Lee and Raskin, 1998, 1999). In rice plants however, SA accumulates only as the 2-O-β-D-glucoside (Silverman et al., 1995), which is the predominant and most stable conjugated form of SA in many plants (Edwards, 1994; Lee and Raskin, 1999). As regards GA, it has been seen to accumulate as 5-O-β-D-glucoside in both Catharanthus roseus (Yamane et al., 2002) and Fagopyrum esculentum, where SA is detoxified by turning into GA which, in turn, is quickly glucosylated (Schulz et al., 1993). Gentisic acid has also been found as 2-O-β-D-glucoside in Cotoneaster orbicularis (El-Mousallamy et al., 2000). Similar to what has been described above, the acceptor substrate is bound to a glucose molecule in most cases. Very interestingly, GA accumulates as 5-O-β-D-xylopyranoside in systemic infections of tomato plants (Fayos et al., 2006), and this compound is the principal differential metabolite in viroid-infected plants (López-Gresa et al., 2010).
The conjugation of these phenolic compounds is carried out by glycosyltransferases (GTs). The main role of these enzymes is to regulate the free and active levels of different metabolites (Yalpani et al., 1992; O'Donnell et al., 1998; Lee and Raskin, 1998). To date, a large number SA-conjugating GTs have been characterized in plants. Some are able to use different hydroxybenzoic acids as a substrate, including GA (Yalpani et al., 1992; Fraissinet-Tachet et al., 1998; Lee and Raskin, 1999; Lim et al., 2002). A GT that conjugates GA as a preferred phenolic substrate has been described in Catharanthus roseus (Yamane et al., 2002). In addition, O'Donnell et al. (1998) have characterized a putative tomato GT gene (twi1), which is rapidly induced by SA and wounding. Nonetheless, no biochemical characterization of a tomato GT that conjugates SA and/or GA has been performed to date.
The main objective of this work is to characterize the glycosyltransferase responsible for the conjugation of GA to xylose in tomato in order to gain a better understanding of the role of this phenolic compound in the signalling of the plant defence response. To study the GA conjugation in tomato, the glycosylation of GA and/or SA in tomato leaves treated with these two phenolics were compared first. Our results reveal how GA accumulates mainly as a glycoconjugate, whereas only a small fraction of the total GA is present as a free form (Fig. 1). By contrast, no conjugated SA is detected at 4 h of treatment, and only half the total SA takes a conjugated form after 24 h. Such a glycosylation might be performed by the same enzyme or by different enzyme activities. According to our data, if a single enzyme activity is responsible for conjugating both phenolics, GA will be the preferential substrate. Alternatively, we could speculate about the existence of a specific gentisate-5-O-β-D-xylosyltransferase. In any case, these results encouraged us to characterize this potent glycosyltransferase which is responsible for the conjugation of GA to xylose in tomato.
The GA conjugating activity was monitored by the HPLC detection of GA 5-O-β-D-xyloside. In many cases, glycosyltransferases have been seen to be rapidly induced by their own substrate (Fraissinet-Tachet et al., 1998; Lee and Raskin, 1999; Lim et al., 2002). An increase in tomato GA xylosyltransferase was detected at 5 h of GA or SA exogenous treatments. This increase was much higher in the GA-treated plants (Fig. 3A) while the mRNA levels are similar (Fig. 3B, C). This could indicate that there is another gene which is only activated by GA. However, the possibility that the enzyme activity or stability is positively regulated by GA cannot be disregarded. Enzyme activity also increased in those tomato plants systemically infected with CEVd or ToMV, and the conjugation of the GA was much higher in the CEVd-infected plants (Fig. 5A). These results are in accordance with those previously obtained in our laboratory (Bellés et al., 1999). In that former work, the free and conjugated GA levels in the tomato leaves infected with CEVd were higher than the levels detected in the ToMV-infected plants. GA-conjugating activity is not apparently induced in incompatible infections, such as the Rio Grande tomato plants infected with Pseudomonas syringae pv. tomato AvrPto (Fig. 5A). In this kind of infection, the analysis of phenolic compounds did not reveal an increase in free or conjugated GA, although an outstanding increase in free and conjugated SA has been widely described in the HR response (Malamy et al., 1990; Métraux et al., 1990; Uknes et al., 1993). These results indicate that the induction of the GA conjugating activity observed in SA-treated tomato plants (Fig. 3A) may not be due to the SA itself, but to the conversion of SA into GA, which could be the effective inducer of the xylosyltransferase. In fact, similar to the results previously described by Schulz et al. (1993), it was observed that the SA-treated tomato leaves accumulate conjugated GA (Fig. 3A).
The complete cDNA sequence of the tomato GA-glycosyltransferase was isolated from a λ-ZAP library built from GA-treated tomato plants, and codes for a 51.5 kDa protein whose amino acid sequence is very similar to the tobacco SA-glycosyltransferase described previously by Lee and Raskin (1998, 1999). GAGT cDNA has been expressed in Pichia pastoris and the recombinant protein was active when GA and UDPX were used. However, it showed no activity toward SA when either UDPX or UDPG were used as sugar donors.
The substrate specificity of recombinant proteins may differ from the specificity of the native protein, which makes it difficult to ascertain the physiological role of the glycosyltransferase in planta (Jones and Vogt, 2001; Achnine et al., 2005; Bowles et al., 2006). Consequently, it was decided to purify the corresponding activity from tomato plants in order to perform specificity studies. A large number of phenolic compounds, such as sugar acceptors, were tested using either UDPG or UDPX as sugar donors, and it was observed that the purified tomato glycosyltransferase was only active when GA and UDPX were used as substrates. The results obtained with both the purified and the recombinant protein show that, unlike the rest of the GTs previously characterized (Warnecke and Heinz, 1994; Fraissinet-Tachet et al., 1998; Lee and Raskin, 1999; Jackson et al., 2001; Griesser et al., 2008), tomato GAGT displays very high substrate specificity. In addition, these results indicate that the glycosylation of GA and SA would be carried out by different enzymes. Such an outstanding specificity would allow the protein selectively to regulate the free and conjugated GA levels. Why the plant is so selective in the conjugation of GA remains an interesting open question.
Tomato GAGT seems to be regulated by pathogen signalling. In parallel with GA xylosyltransferase activity, the GAGT mRNA is induced quickly by an exogenous treatment of either SA or GA, and declines a few hours later. It is also induced in tomato plants infected with ToMV or CEVd while symptoms appear. This mRNA induction explains not only the accumulation of 5-O-β-D-xyloside previously described in these infections (Bellés et al., 1999; Fayos et al. 2006), but also the increased GAGT activity detected. However, this tomato GA xylosyltransferase is not apparently involved in the response to wounding, even though the phylogenetic study indicates that GAGT is in close vicinity to the other GT genes induced by jasmonic acid.
Taken together, our results indicate that the GA-conjugating xylosyltransferase that we have characterized is involved in the plant defence response, specifically in non-necrotizing compatible interactions where GA has been described as a major signal molecule. Unlike this tomato GAGT, most of the previously described GTs have been implicated in incompatible or necrotizing interactions (Fraissinet-Tachet et al., 1998; O'Donnell et al., 1998; Lee and Raskin, 1999; Park et al., 2003), where SA has been involved in the induction of PR proteins and the stablishment of SAR (Delaney et al., 1994; Sticher et al., 1997). SA appears to be the immediate precursor of GA biosynthesis (Bellés et al., 1999). The absence of a GA signal in incompatible interactions, despite the SA levels being high, could indicate that the activity which converts SA into GA (a salicylate-5-hydroxylase) would not be induced or would be inhibited. Should this be the case, the expression of both SA-5-hydroxylase and GAGT could be co-ordinated and implicated in systemic infections. Therefore, it is hypothesized that a rapid induction of the salicylate-5-hydroxylase during compatible interactions would occur, thus provoking the accumulation of GA. After having induced PR proteins or other defence genes, GA would be quickly inactivated by GAGT, thus preventing its possible toxicity.
To gain a better understanding of the role of GA in plant defence, the generation of transgenic tomato plants that either overexpress or silence GAGT would be a powerful tool. In this respect, some results have been reported in the literature. Transgenic tobacco plants that overexpress or down-regulate the biosynthesis of a tobacco glucosyltransferase (TOGT1), which acts on the hydroxycoumarin scopoletin, have been obtained. The down-regulation of TOGT1 led to a reduced accumulation of scopoletin glucoside, enhanced oxidative stress, and weakened virus resistance (Chong et al., 2002). Conversely, the overexpression of TOGT1 led to precocious lesion formation during the hypersensitive response to tobacco mosaic virus (Gachon et al., 2004), and also to increased resistance against Potato virus Y (Matros and Mock, 2004). In potato, the ectopic expression of an anthocyanin 5-O-glucosyltransferase (5-UGT) improved plant defence against Erwinia carotovora subsp. carotovora (Lorenc-Kukuła et al., 2005). In Arabidopsis thaliana, the overexpression of a deoxynivalenol-glucosyltransferase (DOGT1) led to enhanced tolerance against deoxynivalenol, which is a mycotoxin from Fusarium, and the T-DNA tagged mutants (ugt73b3 and ugt73b5) exhibited less resistance to P. syringae pv. tomato-AvrRpm1, indicating that the expression of the corresponding UGT genes is necessary during the hypersensitive response (Langlois-Meurinne et al., 2005). Recently, the down-regulation of a Capsicum annuum UGT by VIGS suggests the implication of this gene in the resistance response against TMV infection by controlling SA accumulation (Lee et al., 2009). All these results emphasize the importance of plant secondary metabolite glycosyl transferases in plant–pathogen interactions.
The expression pattern of tomato GAGT and its narrow substrate specificity suggest that this protein plays a specific role in defence signalling. As speculated for other glycosyl transferases (O'Donnell et al., 1998; Roberts et al., 1999), the substrate of such a rapidly induced, defence-related enzyme may be an important signal molecule. Since gentisic acid has been found to be involved in different systemic plant–pathogen interactions, the early and transient induction of this novel xylosyltransferase may indicate its important role in selectively regulating the free levels of this phenolic in plants.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. (A) Sequence alignment of tomato GAGT and different plant glycosyltransferases; (B) phyllogenetic tree for the sequence alignment of Fig. S1A.
Supplementary Fig. S2. Proposed folding for tomato GAGT.
Supplementary Material
Acknowledgments
The authors would like to thank Gregory B Martin (The Boyce Thompson Institute for Plant Research, Ithaca, NY) for kindly providing the Rio Grande tomato seeds and the Pseudomonas bacterial strains. This work has been supported by the Spanish Ministry of Science and Innovation (grant numbers BMC2003-07837 and BFU2006-11546).
References
- Achnine L, Huhman DV, Farag MA, Sumner LW, Blount JW, Dixon RA. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. The Plant Journal. 2005;41:875–887. doi: 10.1111/j.1365-313X.2005.02344.x. [DOI] [PubMed] [Google Scholar]
- Anderson JC, Pascuzzi PE, Xiao F, Sessa G, Martin GB. Host-mediated phosphorylation of Type III effector AvrPto promotes Pseudomonas virulence and avirulence in tomato. The Plant Cell. 2006;18:502–514. doi: 10.1105/tpc.105.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Jones JD. Role of plant hormones in plant defence responses. Plant Molecular Biology. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- Belicova A, Krizkova L, Nagy M, Krajcovic J, Ebringer L. Phenolic acids reduce the genotoxicity of acridine orange and ofloxacin in Salmonella typhimurium. Folia Microbiologica (Praha) 2001;46:511–514. doi: 10.1007/BF02817994. [DOI] [PubMed] [Google Scholar]
- Bellés JM, Garro R, Fayos J, Navarro P, Primo J, Conejero V. Gentisic acid as a pathogen-inducible signal, additional to salicylic acid for activation of plant defences in tomato. Molecular Plant–Microbe Interactions. 1999;12:227–235. [Google Scholar]
- Bellés JM, Garro R, Pallás V, Fayos J, Rodrigo I, Primo J, Conejero V. Accumulation of gentisic acid is associated with systemic infections but not with the hypersensitive response in plant–pathogen interactions. Planta. 2006;223:500–511. doi: 10.1007/s00425-005-0109-8. [DOI] [PubMed] [Google Scholar]
- Bencúrová M, Rendić D, Fabini G, Kopecky EM, Altmann F, Wilson IBH. Expression of eukaryotic glycosyltransferases in the yeast Pichia pastoris. Biochimie. 2003;85:413–422. doi: 10.1016/s0300-9084(03)00072-5. [DOI] [PubMed] [Google Scholar]
- Bowles D, Lim EK, Poppenberger B, Vaistij FE. Glycosyltransferases of lipophylic small molecules. Annual Review of Plant Biology. 2006;57:567–597. doi: 10.1146/annurev.arplant.57.032905.105429. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broekaert WF, Delaure SL, De Bolle MF, Cammue BP. The role of ethylene in host–pathogen interactions. Annual Review of Phytopathology. 2006;44:393–416. doi: 10.1146/annurev.phyto.44.070505.143440. [DOI] [PubMed] [Google Scholar]
- Chong J, Baltz R, Schmitt C, Beffa R, Fritig B, Saindrenan P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. The Plant Cell. 2002;14:1093–1107. doi: 10.1105/tpc.010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Pierrel MA, Atanassova R, Werck-Reichhart D, Fritig B, Saindrenan P. Free and conjugated benzoic acid in tobacco plants and cell cultures. Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiology. 2001;125:318–328. doi: 10.1104/pp.125.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church GM, Gilbert W. Genomic sequencing. Proceedings of the National Academy of Sciences, USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conejero V, Semancik JS. Analysis of the proteins in crude plant extracts by polyacrylamide gel electrophoresis. Phytopathology. 1977;67:1424–1426. [Google Scholar]
- Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negretto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E. A central role of salicylic acid in plant disease resistance. Science. 1994;266:1247–1249. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- Edwards R. Conjugation and metabolism of salicylic acid in tobacco. Journal of Plant Physiology. 1994;143:609–614. [Google Scholar]
- El-Mousallamy AMD, Hussein SAM, Merfort I, Nawwar MAM. Unusual phenolic glycosides from Cotoneaster orbicularis. Phytochemistry. 2000;53:699–704. doi: 10.1016/s0031-9422(99)00598-1. [DOI] [PubMed] [Google Scholar]
- Fayos J, Bellés JM, López-Gresa MP, Primo J, Conejero V. Induction of gentisic acid 5-O-β-d-xylopyranoside in tomato and cucumber plants infected by different pathogens. Phytochemistry. 2006;67:142–148. doi: 10.1016/j.phytochem.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Flor H. Current status of the gene-for-gene concept. Annual Review of Phytopathology. 1971;9:257–296. [Google Scholar]
- Fraissinet-Tachet L, Baltz R, Chong J, Kauffmann S, Fritig B, Saindrenan P. Two tobacco genes induced by infection, elicitor and salicylic acid encode glucosyltransferases acting on phenylpropanoids and benzoic acid derivatives, including salicylic acid. FEBS Letters. 1998;437:319–323. doi: 10.1016/s0014-5793(98)01257-5. [DOI] [PubMed] [Google Scholar]
- Gachon C, Baltz R, Saindrenan P. Over-expression of a scopoletin glucosyltransferase in Nicotiana tabacum leads to precocious lesion formation during the hypersensitive response to tobacco mosaic virus but does not affect virus resistance. Plant Molecular Biology. 2004;54:137–146. doi: 10.1023/B:PLAN.0000028775.58537.fe. [DOI] [PubMed] [Google Scholar]
- Gachon CM, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends in Plant Science. 2005;10:542–549. doi: 10.1016/j.tplants.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Ukness S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- García-Breijo FJ, Garro R, Conejero V. C7 (P32) and C6 (P34) PR proteins induced in tomato leaves by citrus exocortis viroid infection are chitinases. Physiological and Molecular Plant Pathology. 1990;36:249–260. [Google Scholar]
- Gietz RD, Woods RA. Transformation of yeast by the LiAc/ss carrier DNA/PEG method. Methods in Enzymology. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- Granell A, Bellés JM, Conejero V. Induction of pathogenesis-related proteins in tomato by citrus exocortis viroid, silver ion and ethephon. Physiological and Molecular Plant Pathology. 1987;31:83–90. [Google Scholar]
- Grant M, Lamb C. Systemic immunity. Current Opinion in Plant Biology. 2006;9:414–420. doi: 10.1016/j.pbi.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Griesser M, Vitzthum F, Fink B, Bellido ML, Raasch C, Munoz-Blanco J, Schwab W. Multi-substrate flavonol O-glucosyltransferases from strawberry (Fragaria×ananassa) achene and receptacle. Journal of Experimental Botany. 2008;59:2611–2625. doi: 10.1093/jxb/ern117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath DM, Chua NH. Identification of an immediate-early salicylic acid-inducible tobacco gene and characterization of induction by other compounds. Plant Molecular Biology. 1996;31:1061–1072. doi: 10.1007/BF00040724. [DOI] [PubMed] [Google Scholar]
- Huang J, Cardoza YJ, Schmelz EA, Raina R, Engelberth J, Tumlinson JH. Differential volatile emissions and salicylic acid levels from tobacco plants in response to different strains of Pseudomonas syringae. Planta. 2003;217:767–775. doi: 10.1007/s00425-003-1039-y. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hughes MA. Multiple secondary plant product UDP-glucose glucosyltransferase genes expressed in cassava (Manihot esculenta Crantz) cotyledons. DNA Sequence. 1994;5:41–49. doi: 10.3109/10425179409039703. [DOI] [PubMed] [Google Scholar]
- Jackson RG, Lim E-K, Li Y, Kowalczyk M, Sandberg G, Hoggett J, Ashford DA, Bowles DJ. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. Journal of Biological Chemistry. 2001;276:4350–4356. doi: 10.1074/jbc.M006185200. [DOI] [PubMed] [Google Scholar]
- Jones P, Vogt T. Glycosyltransferases in secondary plant metabolism: tranquilizers and stimulant controllers. Planta. 2001;213:164–174. doi: 10.1007/s004250000492. [DOI] [PubMed] [Google Scholar]
- Kohara A, Nakajima C, Yoshida S, Muranaka T. Characterization and engineering of glycosyltransferases responsible for steroid saponin biosynthesis in Solanaceous plants. Phytochemistry. 2007;68:478–486. doi: 10.1016/j.phytochem.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Langlois-Meurinne M, Gachon CM, Saindrenan P. Pathogen-responsive expression of glycosyltransferase genes UGT73B3 and UGT73B5 is necessary for resistance to Pseudomonas syringae pv. tomato in Arabidopsis. Plant Physiology. 2005;139:1890–1901. doi: 10.1104/pp.105.067223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio V, De Cicco D, Venere D, Lima G, Salerno M. Antifungal activity of phenolics against fungi commonly encountered during storage. Italian Journal of Food Science. 1994;6:23–30. [Google Scholar]
- Lee BJ, Kim SK, Choi SB, Bae J, Kim KJ, Kim YJ, Paek KH. Pathogen-inducible CaUGT1 is involved in resistance response against TMV infection by controlling salicylic acid accumulation. FEBS Letters. 2009;583:2315–2320. doi: 10.1016/j.febslet.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Lee HI, Raskin I. Glucosylation of salicylic acid in Nicotiana tabacum cv. Xanthi-nc. Phytopathology. 1998;88:692–697. doi: 10.1094/PHYTO.1998.88.7.692. [DOI] [PubMed] [Google Scholar]
- Lee HI, Raskin I. Purification, cloning, and expression of a pathogen inducible UDPglucose:salicylic acid glucosyltransferase from tobacco. Journal of Biological Chemistry. 1999;274:36637–36642. doi: 10.1074/jbc.274.51.36637. [DOI] [PubMed] [Google Scholar]
- Li L, Modolo LV, Escamilla-Trevino LL, Achnine L, Dixon RA, Wang X. Crystal structure of Medicago truncatula UGT85H2: insights into the structural basis of a multifunctional (iso)flavonoid glycosyltransferase. Journal of Molecular Biology. 2007;370:951–963. doi: 10.1016/j.jmb.2007.05.036. [DOI] [PubMed] [Google Scholar]
- Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ. The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. Journal of Biological Chemistry. 2002;277:586–592. doi: 10.1074/jbc.M109287200. [DOI] [PubMed] [Google Scholar]
- Lisón P, Rodrigo I, Conejero V. A novel function for the cathepsin D inhibitor in tomato. Plant Physiology. 2006;142:1329–1339. doi: 10.1104/pp.106.086587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G, Grant M. Salicylic acid in plant defence-the players and protagonists. Current Opinion in Plant Biology. 2007;10:466–472. doi: 10.1016/j.pbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- López-Gresa MP, Maltese F, Bellés JM, Conejero V, Kim HK, Choi YH, Verpoorte R. Metabolic response of tomato leaves upon different plant–pathogen interactions. Phytochemical Analysis. 2010;21:89–94. doi: 10.1002/pca.1179. [DOI] [PubMed] [Google Scholar]
- Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Current Opinion in Plant Biology. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Lorenc-Kukuła K, Jafra S, Oszmiański J, Szopa J. Ectopic expression of anthocyanin 5-O-glucosyltransferase in potato tuber causes increased resistance to bacteria. Journal of Agricultural and Food Chemistry. 2005;53:272–281. doi: 10.1021/jf048449p. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DF, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to tobacco mosaic virus. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Matros A, Mock HP. Ectopic expression of a UDP-glucose phenylpropanoid glucosyltransferase leads to increased resistance of transgenic tobacco plants against infection with potato virus Y. Plant and Cell Physiology. 2004;45:1185–1193. doi: 10.1093/pcp/pch140. [DOI] [PubMed] [Google Scholar]
- Métraux J-P, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverdi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Nawrath C, Métraux JP. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. The Plant Cell. 1999;11:1393–1404. doi: 10.1105/tpc.11.8.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Jones JB, Antoine FR, Ciardi J, Klee HJ. Ethylene-dependent salicylic acid regulates an expanded cell death response to a plant pathogen. The Plant Journal. 2001;25:315–323. doi: 10.1046/j.1365-313x.2001.00968.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Truesdale MR, Calvert CM, Dorans A, Roberts MR, Bowles DJ. A novel tomato gene that rapidly responds to wound- and pathogen-related signals. The Plant Journal. 1998;14:137–142. doi: 10.1046/j.1365-313X.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Osmani SA, Bak S, Møller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009;70:325–347. doi: 10.1016/j.phytochem.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Park YS, Min HJ, Ryang SH, Oh KJ, Cha JS, Kim HY, Cho TJ. Characterization of salicylic acid-induced genes in Chinese cabbage. Plant Cell Reports. 2003;21:1027–1034. doi: 10.1007/s00299-003-0606-9. [DOI] [PubMed] [Google Scholar]
- Roberts MR, Warner SAJ, Darby R, Lim EK, Draper J, Bowles DJ. Differential regulation of a glucosyl transferase gene homologue during defence responses in tobacco. Journal of Experimental Botany. 1999;50:407–410. [Google Scholar]
- Rodrigo I, Vera P, Tornero P, Hernández-Yago J, Conejero V. cDNA cloning of viroid-induced tomato pathogenesis-related protein P23. Characterization as a vacuolar antifungal factor. Plant Physiology. 1993;102:939–945. doi: 10.1104/pp.102.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryals JA, Neuenschwander UH, Willits MG, Molina A, Steiner HY, Hunt MD. Systemic acquired resistance. The Plant Cell. 1996;8:1809–1819. doi: 10.1105/tpc.8.10.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuhegger R, Ihring A, Gantner S, et al. Induction of systemic resistance in tomato by N-acyl-l-homoserine lactone-producing rhizosphere bacteria. Plant, Cell and Environment. 2006;29:909–918. doi: 10.1111/j.1365-3040.2005.01471.x. [DOI] [PubMed] [Google Scholar]
- Schulz M, Schnabl H, Manthe B, Schweihofen B, Casser I. Uptake and detoxification of salicylic acid by Vicia faba and. Fagopyrum esculentum. Phytochemistry. 1993;33:291–294. [Google Scholar]
- Sels J, Mathys J, De Coninck BM, Cammue BP, De Bolle MF. Plant pathogenesis-related (PR) proteins: a focus on PR peptides. Plant Physiology and Biochemistry. 2008;46:941–950. doi: 10.1016/j.plaphy.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Shao H, He X, Achnine L, Blount JW, Dixon RA, Wang X. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. The Plant Cell. 2005;17:3141–3154. doi: 10.1105/tpc.105.035055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman P, Seskar M, Kanter D, Schweizer P, Metraux JP, Raskin I. Salicylic acid in rice (biosynthesis, conjugation, and possible role) Plant Physiology. 1995;108:633–639. doi: 10.1104/pp.108.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskawicz BJ, Ausubel FM, Baker BJ, Ellis JG, Jones JD. Molecular genetics of plant disease resistance. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- Sticher L, Mauch-Mani B, Métraux JP. Systemic acquired resistance. Annual Review of Phytopathology. 1997;35:235–270. doi: 10.1146/annurev.phyto.35.1.235. [DOI] [PubMed] [Google Scholar]
- Szerszen JB, Szczyglowski K, Bandurski RS. Iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science. 1994;265:1699–1701. doi: 10.1126/science.8085154. [DOI] [PubMed] [Google Scholar]
- Uknes S, Winter AN, Delaney T, Vernooij B, Morse A, Friedrich L, Nye G, Potter S, Ward E, Ryals J. Biological induction of systemic acquired resistance in Arabidopsis. Molecular Plant–Microbe Interactions. 1993;6:692–698. [Google Scholar]
- van Loon LC, Rep M, Pieterse CM. Significance of inducible defence-related proteins in infected plants. Annual Review of Phytopathology. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Vogt T, Jones P. Glycosyltransferases in plant natural product synthesis: characterization of a supergene family. Trends in Plant Science. 2000;5:380–386. doi: 10.1016/s1360-1385(00)01720-9. [DOI] [PubMed] [Google Scholar]
- Warnecke DC, Heinz E. Purification of a membrane-bound UDP-glucose:sterol β-D-glucosyltransferase based on its solubility in diethyl ether. Plant Physiology. 1994;105:1067–1073. doi: 10.1104/pp.105.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Leon J, Lawton MA, Raskin I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiology. 1993;103:315–321. doi: 10.1104/pp.103.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Schulz M, Davies MP, Balke NE. Partial purification and properties of an inducible uridine 5′-diphosphate-glucose:salicylic acid glucosyl transferase from oat roots. Plant Physiology. 1992;100:457–463. doi: 10.1104/pp.100.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S, Shimoda K, Watanabe K, Hirata T. Purification and characterization of gentisic acid glucosyltransferase from the cultured cells of Catharanthus roseus. Journal of Molecular Catalysis B: Enzymatic. 2002;17:59–63. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.