Abstract
In Arabidopsis thaliana, chloroplasts move towards the periclinal cell walls upon exposure to low blue light intensities and to anticlinal walls under high light. The regulation of these chloroplast movements involves members of both the phototropin and phytochrome families of photoreceptors. Examination of fluence-rate response dependencies in phot1 and phot2 mutants revealed that although both photoreceptors are capable of inducing chloroplast accumulation under low-light conditions, the signals from these photoreceptors appear to be antagonistic. Chloroplast movements in wild-type plants were intermediate between those of the single phot mutants, consistent with each operating through separate signalling cascades. Mutants in phot2 showed transient chloroplast avoidance responses upon exposure to intense blue light, and slow but sustained chloroplast avoidance under intense white light, indicating that in the absence of phot2, phot1 is capable of generating both a low and a high-light response signal. Mutations in phytochrome B (phyB) caused an enhanced avoidance response at intermediate and high light intensities. Examination of phyB, phot1phyB, and phot2phyB mutants indicated that this enhancement is caused by PhyB inhibition of the high-light avoidance response in wild-type plants. In addition, our results suggest that the inhibition by PhyB is not exclusive to either of the phot1 or phot2 signalling pathways.
Keywords: Arabidopsis thaliana, chloroplast movement, phototropin, phytochrome
Introduction
As their sole source of energy, sunlight is one of the most important resources for plants, and they have evolved many responses to facilitate its capture (Franklin et al., 2005). Low-light conditions can limit plant growth due to an inadequate energy supply, while too much light can damage plants through the production of reactive oxygen species, and lead to the breakdown of the photosynthetic apparatus (Melis, 1999). Plants have evolved many developmental and physiological strategies to regulate light absorption under these less than ideal conditions, such as phototropism, shade avoidance, variations in size of light-harvesting complexes, changes in cell density, and leaf morphology, but most occur slowly and are not easily reversed (Boardman, 1977; Galen et al., 2004; Vandenbussche et al., 2005).
Plants have also evolved mechanisms to achieve more rapid and reversible responses to changing light intensities. Light-induced chloroplast rearrangements are one example of a rapid response that contributes to a plant's abilty to regulate the amount of light captured (Zurzycki, 1961; Wagner et al., 1972; Inoue and Shibata, 1974; Lechowski, 1974; Walczak and Gabrýs, 1980; Augustynowicz and Gabrýs, 1999). Specifically, under low-intensity light, chloroplasts accumulate along periclinal cell walls, where they cover more of the surface area that is being illuminated. This is referred to as the accumulation, or low-light response. When exposed to high-intensity light, the chloroplasts relocate along anticlinal cell walls, reducing their exposure and allowing more light to pass through the cells. This is referred to as the avoidance, or high-light response. At intermediate light intensities between those that cause robust accumulation and avoidance responses, the chloroplasts display biphasic movement, which involves a brief period of avoidance followed by an extended accumulation phase (Kagawa and Wada, 1999; DeBlasio et al., 2003). These light-induced chloroplast movements are thought to represent an adaptive advantage for plants exposed to varying light conditions (Zurzycki, 1955; Augustynowicz and Gabrýs, 1999). Consistent with this hypothesis, mutants that are unable to move their chloroplasts showed severe bleaching and developed necrotic lesions more rapidly than the wild type after transfer to high-intensity light (Jeong et al., 2002; Kasahara et al., 2002; Koniger et al., 2008).
In some non-flowering plant species, chloroplast movement can be initiated by both blue and red light, with the signals acting additively during simultaneous exposure (Kraml and Herrmann, 1991; Kagawa and Wada, 1996; Augustynowicz and Gabrýs, 1999; Kadota et al., 2000). The response to red light is far-red reversible, indicating that a phytochrome serves as the photoreceptor (Yatsuhashi et al., 1987; Kagawa and Wada, 1996; Nozue et al., 1998) Available mutants in fern and moss indicate that the phototropin family of blue-light photoreceptors is required for the response to blue light in these species (Kagawa et al., 2004). In addition, mutations in three of the four phototropin genes in the moss Physcomitrella patens also caused reduced chloroplast movement in response to red light, indicating that the phototropins may play a role in the downstream signalling from phytochrome in addition to acting as blue-light photoreceptors (Kagawa et al., 2004).
In angiosperms such as Arabidopsis thaliana, chloroplast movement is strictly a blue-light response, mediated by the phot1 and phot2 blue-light photoreceptors (Kagawa et al., 2001; Sakai et al., 2001). The phototropins are also involved in phototropism, light-regulated hypocotyl elongation, stomatal opening, leaf expansion and localization of the nucleus (Liscum and Briggs, 1995; Kinoshita et al., 2001; Folta et al., 2003; Kosei et al., 2007). Knockout of both phototropins is sufficient to eliminate all chloroplast movement in Arabidopsis (Sakai et al., 2001), although each has a unique impact on the modulation of the response. Partially irradiated phot1 single mutants exposed to fluence rates of blue light from 16 μmol m−2 s−1 to 100 μmol m−2 s−1 were able to induce an avoidance response while phot2 mutants were not, suggesting that phot2 is responsible for the high-light response (Sakai et al., 2001). In addition, increases in cellular levels of PHOT2 have been correlated with an increased rate of chloroplast movement during the avoidance response (Kimura and Kagawa, 2009). By contrast, both photoreceptors were able to induce the low-light response, with phot1 and phot2 mutants showing accumulation between 2 μmol m−2 s−1and 16 μmol m−2 s−1 and 0.4–100 μmol m−2 s−1 of blue light, respectively (Kagawa et al., 2001; Sakai et al., 2001). Measurements of the change in light transmittance through leaves of phot2 mutants indicated that phot1 was able to initiate a low-light response under as little as 0.08 μmol m−2 s−1 blue light and continued to produce chloroplast accumulation at light intensities up to 120 μmol m−2 s−1 (Jarillo et al., 2001).
The mechanism of signal transduction from the phototropins to downstream effectors involved in chloroplast movements is unknown, but it has been hypothesized that the signals for accumulation and avoidance are distinct and act through separate pathways. The low- and high-light movement responses have been shown to display different kinetic characteristics, with the low-light response signal being generated slower and lasting longer than the high-light response signal (Malec, 1994; Trojan and Gabrýs, 1996; Kagawa and Wada, 1999). However, results from phot1 mutants indicate that phot2 can induce both chloroplast accumulation and avoidance, and therefore may feed into both of these pathways (Sakai et al., 2001). Phot1 has only been shown to cause a low-light response, but it is unclear if this is through the same pathway as phot2-induced accumulation.
Although red light has no effect on chloroplast movement in the angiosperms studied (Walczak and Gabrýs, 1980), the red/far-red photoreceptors PhyA and PhyB appear to be involved in the response. Mutants in phyA and phyB showed an enhanced avoidance response after exposure to blue-light intensities from 10–60 μmol m−2 s−1, and overexpression of these photoreceptors caused reduced chloroplast accumulation under low light (DeBlasio et al., 2003). It is unlikely that phytochromes are used as a red-light photoreceptors in this situation, since the presence or absence of red or far-red light had no significant impact on the magnitude or direction of chloroplast movement (DeBlasio et al., 2003). However, the observation that the phytochrome mutants display altered chloroplast avoidance (DeBlasio et al., 2003) and phototropism (Whippo and Hangarter, 2004) suggests that the phytochromes may influence blue-light responses in the absence of red light.
To understand better how the phototropins and phytochromes affect chloroplast movements, we conducted detailed fluence-rate response experiments in phot1, phot2, phyB, and photphy double mutants. Our results indicated that, in phot1 mutants, phot2 signals for an accumulation response at blue-light intensities between 0.1 μmol m−2 s−1 and 5 μmol m−2 s−1 and produces a high-light response at 20 μmol m−2 s−1 and above. In phot2 mutants, light intensities between 0.1 μmol m−2 s−1and 100 μmol m−2 s−1 produced an enhanced low-light response compared with the wild type, but the higher fluence rates caused transient chloroplast avoidance before the onset of accumulation. Exposure of phot2 mutants to intense light during time-lapse microscopy revealed the presence of a slow, attenuated high-light response. In addition, analysis of phyB, phot1phyB, and phot2phyB leaves suggest that the enhancement of the high-light response in phyB mutants is caused by loss of PhyB-dependent inhibition of chloroplast avoidance that may serve a regulatory role in wild-type plants.
Materials and methods
Mutant lines and growth conditions
Arabidopsis ecotype Columbia gl1 was the wild type in this study and all mutants were in the Columbia background. Arabidopsis phyB-9 seeds were obtained from Jason Reed (University of North Carolina, Chapel Hill). Phototropin mutants were obtained from Emmanuel Liscum (University of Missouri, Columbia; phot1-1, phot1-5, and phot2-5) and Takatoshi Kagawa (University of Tsukuba, Tsukuba City, Japan; phot2-1) Unless otherwise noted, phot1 indicates allele phot1-1. True-breeding homozygous double mutants were generated by crossing phyB and either phot1-1 or phot2-1. Double mutants were confirmed by using the following physiological markers: elongated hypocotyl in phyB seedlings grown in red light, chloroplast accumulation under high blue light in phot2-1, and through the low-blue-light phototropism defect in phot1.
Seeds were sown on moist Scott's plug mix (Scott's Sierra, Marysville, OH) and incubated at 4 °C for 48 h. Plants were germinated and grown in a growth room under 60–70 μmol m−2 s−1 of light provided by a combination of warm-white and cool-white fluorescent bulbs (General Electric, Louisville KY) with 12 h photoperiods at 23 °C. Plants were fertilized with K-Grow all-purpose plant food (Kmart, Troy, MI) every 2 weeks after germination.
Photometric measurements of chloroplast movement
Chloroplast movement was measured photometrically as described previously (DeBlasio et al., 2003, 2005). Leaves were excised from 5–6-week-old adult plants at the end of their 12 h night period and placed in a dark humid container with their petioles placed in microfuge tubes filled with water. In order to make transmittance readings, individual leaves were sandwiched between two glass microscope slides (VWR International, West Chester, PA) with the petiole sticking out into a moist paper towel to maintain hydration. The assembly was then arranged on a stage so the leaf covered a 5 mm diameter red Plexiglas window (Rohm and Haas No. 2423; Dayton Plastics, Columbus, OH) above the sensor from a Li-Cor 1800 spectroradiometer (Li-Cor Inc., Lincoln, NE). A red light emitting diode (660 nm) (LED; Radio Shack, Fort Worth, TX) was mounted directly above the leaf to provide 20–30 μmol m−2 s−1 red light for measurements of leaf transmittance. Blue light to induce movement was provided by shining light from a halogen fibre optic light microscope illuminator (Cole Palmer, Chicago, IL) through a blue interference filter 450±25 nm (Melles Griot, 03FIB304) at an angle of 60º relative to the surface of the leaf. Changes in blue-light intensity were produced by placing neutral density filters between the light source and the leaf. In order to measure red-light transmittance through the leaf, a Li-Cor 1800 quantum sensor recorded and integrated the quantum flux between 650 nm and 670 nm for each time point. For each leaf, the change in % red-light transmittance was calculated as ((It/Io)×100/IA) where It and Io are the incident and transmitted red light fluence rate, respectively, and IA is the average red-light transmittance value measured prior to the blue light treatments. Results are presented as the average change in % red-light transmittance ±standard error.
Time-lapse microscopy
Dark-acclimated leaves were excised from 5–6-week-old Arabidopsis plants. Pieces of the leaf blades of approximately 2 mm2 were cut with a razor, excluding the midvein, and mounted abaxial side down, on a slide in water under a cover slip. The mounted leaf pieces were exposed from below to constant white light (about 3000 μmol m−2 s−1) generated from the brightfield lamp on a Nikon E800 microscope (Melville, NY). Images of the adaxial surface of the palisade cell layer were captured every 30 s with MetaMorph software (Universal Imaging, Downingtown, PA) and a Hamamatsu ORCA-ER charge-coupled device camera (Hamamatsu City, Japan).
Results
Chloroplast movements in phototropin mutants
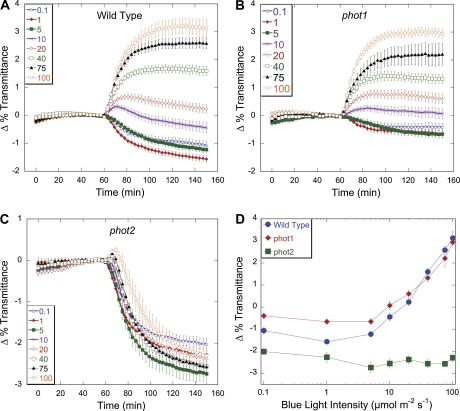
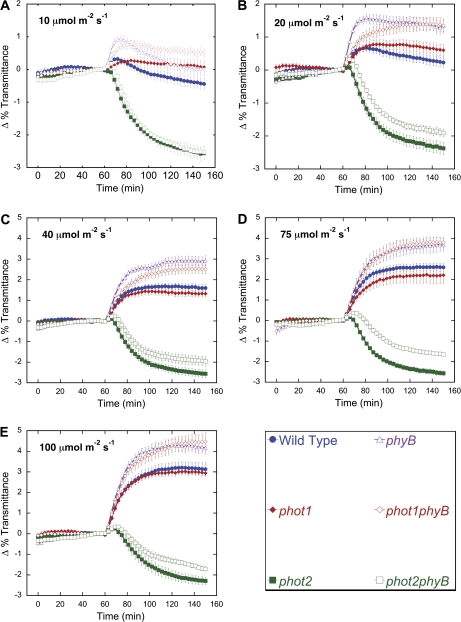
Because red light does not affect chloroplast movement in Arabidopsis, it can be used to monitor the relative position of chloroplasts in cells by measuring the amount of red light transmitted through a leaf (Walczak and Gabrýs, 1980; Jarillo et al., 2001; DeBlasio et al., 2003, 2005; Luesse et al., 2006). In order to understand better the roles of phot1 and phot2 signalling in both the accumulation and avoidance responses, detailed fluence-rate response curves for chloroplast movement were created for the wild type and the phot1-1 and phot2-1 mutants by recording changes in red-light transmittance through leaves during treatment with blue light intensities between 0.1 μmol m−2 s−1 and 100 μmol m−2 s−1. Exposure of wild-type leaves to 0.1 μmol m−2 s−1 or 1 μmol m−2 s−1 of blue-light induced a chloroplast accumulation response which resulted in an average change in red-light transmittance of –1 to –1.6% (Fig. 1A). At 5 μmol m−2 s−1 and 10 μmol m−2 s−1, the magnitude of the change in red-light transmittance was reduced with exposure to 10 μmol m−2 s−1 causing a biphasic response (Figs 1A, 2B). Fluence rates above 20 μmol m−2 s−1 induced avoidance responses that increased in magnitude with higher fluence rates. These findings with wild type are consistent with previously published results (DeBlasio et al., 2005; Luesse et al., 2006).
Fig. 1.
Time-course of fluence responses of light-induced chloroplast movements in leaves of wild type and phot1 and phot2 mutants. (A–C) Red-light transmittance was measured for 60 min in dark-acclimated leaves to establish a baseline before a 90 min blue-light treatment was initiated. Red-light transmittance was recorded every 3 min. The data are the average ±SE of 5–15 leaves per light treatment. (D) Fluence response curves from the final transmittance changes from (A), (B), and (C) of wild type, phot1, and phot2 after 90 min blue light treatments. (This figure is available in colour at JXB online.)
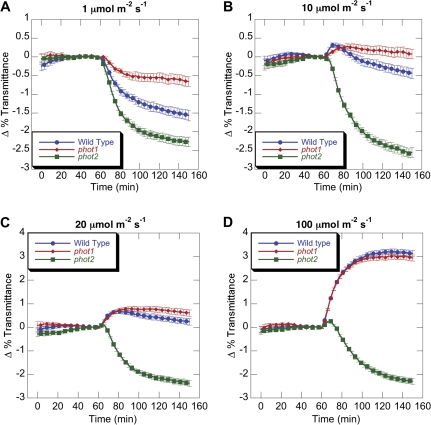
Fig. 2.
Time-course of chloroplast movements in phot1 and phot2 under low and transitional intensities of blue light. Data from Fig. 1 are presented to allow comparisons of chloroplast movement at individual light intensities. (This figure is available in colour at JXB online.)
Compared with the wild type, the phot1 mutant showed a reduced chloroplast accumulation response at fluence rates from 0.1–5 μmol m−2 s−1 (Fig. 1B, D). At 0.1 μmol m−2 s−1 of blue light, the phot1 mutant showed a –0.4% change in transmittance, indicating that although the response is weak, when acting alone phot2 can induce chloroplast accumulation under very low fluence rates of light. Chloroplast avoidance in phot1 mutants was similar to the wild type under high blue light intensities of 20–100 μmol m−2 s−1. The phot2 mutant displayed an accumulation response under all light conditions as previously observed (Fig. 1C) (Jarillo et al., 1998; Kagawa et al., 2001; Sakai et al., 2001), but the magnitude of the light transmittance change in phot2 mutants (about –2.4%) was about twice as large as the maximum seen in the wild-type accumulation response (Fig. 1D). Moreover, the change in red light transmittance induced by low blue light in the wild type was intermediate between the responses observed in the phot1 and phot2 mutants under the same conditions (Figs 1D, 2A). These data suggest that the signals generated by phot1 and phot2 for chloroplast accumulation may be acting antagonistically to one another.
Closer analysis of the kinetics of the chloroplast movement at intermediate fluence rates also supports the existence of distinct phot1 and phot2-mediated accumulation signalling pathways. Upon exposure to 10 μmol m−2 s−1 of continuous blue light, wild-type leaves produced a biphasic change in light transmittance, characterized by an initial phase of chloroplast avoidance followed by a prolonged period of accumulation (Fig. 2B). However, phot1 leaves failed to show a significant biphasic response under any tested light intensity, instead producing a weak chloroplast avoidance response of 0.2% at 10 μmol m−2 s−1. Since phot1 also showed attenuated chloroplast accumulation under low light, it seems likely that signalling from phot1 is responsible for the accumulation phase of the biphasic response seen in the wild type at 10 μmol m−2 s−1. In addition, analysis of phot2 mutants at 20 μmol m−2 s−1 revealed a 6 min lag before the onset of the accumulation response, while at lower fluence rates chloroplast accumulation could be detected within 3 min of the initiation of the light treatment (Fig. 2C). High fluence rates (40–100 μmol m−2 s−1) produced a small biphasic response in phot2, with the length of the initial chloroplast avoidance phase increasing in response to higher light intensities (Figs 1C, 2D; see Supplementary Fig. S1 at JXB online). Similar trends were also seen with the phot2-5 allele (data not shown).
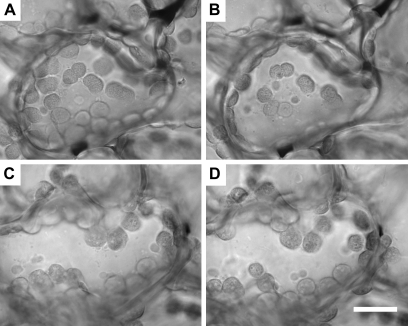
Visual examination of chloroplast movements in phot2 and phot1phot2 double mutants during exposure to 60 min of 3000 μmol m−2 s−1 white light, generated from a brightfield lamp on a microscope, consistently showed slow but steady movement of chloroplasts toward the anticlinal cell walls in phot2 mutants, indicative of an attenuated high-light avoidance response (Fig. 3; see Supplementary video S1 at JXB online). An identical response was also produced by the phot2-5 (data not shown). As expected, phot1phot2 double mutants displayed no chloroplast movement, indicating that phot1 is required to stimulate the weak avoidance response observed in the phot2 mutant.
Fig. 3.
High-light-induced chloroplast movements in phot2 and phot1phot2 mutants. The phot2 mutant (A, B) and phot1phot2 double mutant (C, D) were exposed to intense white light generated by the brightfield lamp on a microscope for 60 min. Images captured at 0 min (A, C) and 60 min (B, D) are shown. Some chloroplasts in the phot2 mutant have moved to the anticlinal cell walls (see Supplementary video S1). The bar is equal to 5 μm.
Chloroplast movements in phyB mutants
Previous work showed that mutations in phyB enhanced the chloroplast avoidance response under both intermediate and high fluence rates of blue light (DeBlasio et al., 2003). To understand better how PhyB regulates light-induced chloroplast movements, detailed fluence rate dependencies were analysed for phyB, phot1phyB, and phot2phyB mutants to allow observation of the loss of PhyB signalling in backgrounds with altered phototropin-dependent chloroplast movements.
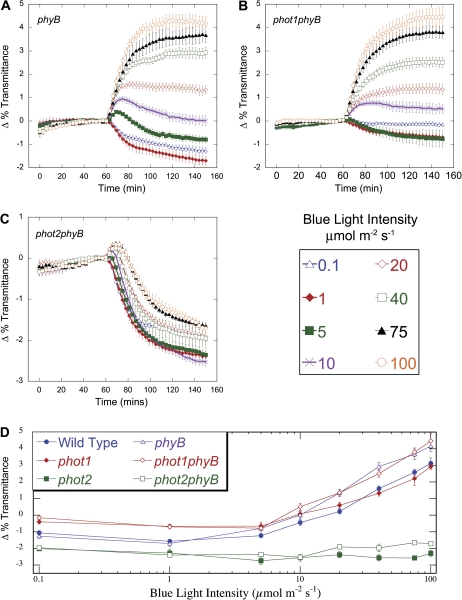
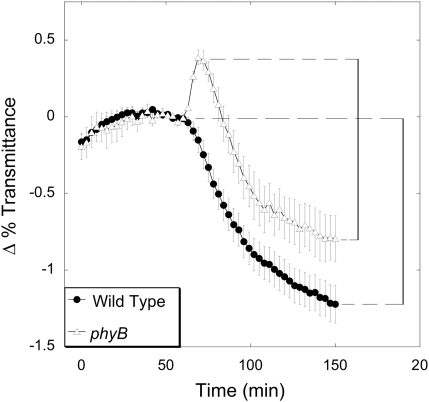
Consistent with previous results, phyB mutants exhibited wild-type levels of chloroplast movement upon exposure to low light (0.1–1 μmol m−2 s−1), and an enhanced avoidance response under high light intensities (20–100 μmol m−2 s−1) (Fig. 4A, D). Like the wild type, phyB mutants also displayed a biphasic response when exposed to 10 μmol m−2 s−1 of blue light, but the phyB avoidance phase was significantly enhanced (Fig. 5A). A robust biphasic response was also observed when phyB leaves were illuminated with 5 μmol m−2 s−1 of blue light (Fig. 6). At this fluence rate, wild-type leaves exhibited a strict accumulation response, suggesting that, in wild-type plants, PhyB acts to repress the avoidance phase of the biphasic response. Furthermore, at 5 μmol m−2 s−1 the overall change in light transmittance (from the highest to the lowest) associated with the accumulation phase of the biphasic response in phyB mutants was almost identical in magnitude to the size of the accumulation response produced by the wild type (Fig. 6). This indicates that, once initiated, the chloroplast accumulation response was unperturbed in the absence of PhyB.
Fig. 4.
Time-course of fluence responses of light-induced chloroplast movements in leaves of wild type and phyB, phot1phyB, and phot2phyB mutants. (A–C) Red-light transmittance was measured for 60 min in dark-acclimated leaves to establish a baseline before a 90 min blue-light treatment was initiated. Red-light transmittance was recorded every 3 min. The data are the average ±SE of 5–15 leaves per light treatment. (D) Final red-light transmittance changes in, phot1, phot2, phyB, phot1phyB, and phot2phyB after 90 min exposures to single fluence-rates of blue light (from Fig. 1A–C and Fig. 4A–C). (This figure is available in colour at JXB online.)
Fig. 5.
Time-courses of chloroplast movements in phyB, phot1phyB, and phot2phyB under transitional and high blue-light intensities. Data were replotted from Fig. 4 to facilitate comparisons between genotypes at individual light intensities.
Fig. 6.
Biphasic chloroplast movement response in phyB at 5 μmol m−2 s−1. Red-light transmittance through wild-type and phyB leaves was measured every 3 min. for 60 min before and during exposure to 5 μmol m−2 s−1 blue light. At this blue light intensity, phyB displays a biphasic response while the wild type shows only a low-light response. The lines drawn on the graph demark the equal magnitude (1.2%) of the accumulation response in both genotypes.
To determine the epistatic relationship of PhyB to phot1 and phot2, the chloroplast movement responses of phot1phyB and phot2phyB double mutants were examined under various light conditions. When exposed to blue light intensities of less than 10 μmol m−2 s−1, phot1phyB and phot2phyB double mutants behaved like the phot1 and phot2 single mutants, respectively (Fig. 4D). Interestingly, the phot1phyB double mutant failed to develop the avoidance phase of the biphasic response seen in the phyB single mutant at 5 μmol m−2 s−1, further supporting the hypothesis that phot1 is required for the accumulation phase of the biphasic response under intermediate light conditions (Fig. 4A, B). At 10 μmol m−2 s−1, phot1phyB displayed elements of both the phyB and phot1 single mutants but with a final change in light transmittance distinct from either of these (Fig. 5A). Like phot1, the phot1phyB mutants failed to initiate the accumulation phase of the biphasic response seen in the wild type and phyB. At 20 μmol m−2 s−1, phyB took only 12 min to reach its maximum transmittance change of 1.5%, while phot1phyB took approximately twice as long, but eventually attained an overall response similar to the phyB mutant (Fig. 5B). Under higher light intensities (>20 μmol m−2 s−1), phyB and phot1phyB mutants displayed similarly enhanced chloroplast avoidance responses when compared with the wild type (Fig. 5B–E).
In phot2 and phot2phyB leaves, low blue light induced similarly enhanced accumulation responses, but under high light the magnitude of the overall change in transmittance was decreased in the double mutant (Figs 4, 5) At blue light intensities greater than 10 μmol m−2 s−1, the initial avoidance portion of the biphasic response normally seen in phot2 showed increased magnitude and duration in the phot2phyB double mutant, indicating an enhancement of chloroplast avoidance and could be the reason for the difference in magnitude of the response between phot2 and phot2phyB mutants (Fig. 5). Since the phyB mutation resulted in the enhancement of chloroplast movement in response to high light in both the phot1 and phot2 mutant backgrounds, it may indicate that PhyB does not work exclusively with either photropin signalling pathways but affects chloroplast avoidance in general.
Discussion
Phototropin signalling for chloroplast movement
In Arabidopsis, chloroplast movements were shown to be mediated somewhat redundantly by the activity of both phot1 and phot2 (Kagawa and Wada, 2000; Sakai et al., 2001). To examine the relationship of the signals produced by these photoreceptors for chloroplast movement, detailed fluence rate response curves were created for phot1 and phot2 mutants. These results indicated that when exposed to low intensity blue light (0.1–5 μmol m−2 s−1), phot1 mutants exhibited attenuated chloroplast accumulation while phot2 mutants displayed an enhanced accumulation response (Fig. 1D). Under the same light conditions, wild-type leaves produced an accumulation response that was intermediate between the responses observed for phot1 and phot2. If the phototropins use the same signalling pathway for chloroplast accumulation, it would be expected that their signals would act additively, and mutations in either phototropin would lead to a reduction in movement under low-light conditions. However, since phot2 mutants showed an enhanced accumulation response compared with the wild type, it suggests that phot2 actually inhibits phot1-dependent chloroplast accumulation under low light. In this case, even though phot1 and phot2 can each induce an accumulation response under low light, their signalling pathways for chloroplast movement appear to be distinct, and the final chloroplast position is a compromise between those two signals.
Analysis of the phototropin mutants also provided information about chloroplast movement at transitional light intensities between those that cause the accumulation response and those that cause avoidance (typically around 10 μmol m−2 s−1 in the wild type). These intermediate light intensities result in a biphasic change in light transmittance, characterized by a rapid onset of chloroplast avoidance followed by a prolonged period of chloroplast accumulation. The biphasic behaviour is probably caused by the simultaneous initiation of both the high and low-light responses, possibly by distinct phot1- and phot2-mediated pathways. Because the signal and movement for chloroplast accumulation are slower than for avoidance (Kagawa and Wada, 1999), the biphasic movement may reflect a rapid initiation of chloroplast avoidance which is eventually reversed by the slower process of accumulation. This is also supported by microbeam irradiation studies of prothallial cells in fern. When a region of a single cell was treated with a blue light microbeam of constant intensity of 5 W m−2 (about 20 μmol m−2 s−1) the chloroplasts initially moved out the light, but after about 10 min returned to the irradiated area, indicating that the response involves antagonism between the signal for the high and low-light responses (Kagawa and Wada, 1999). In our studies, intermediate light intensities failed to induce a biphasic response in phot1 mutants, instead showing only slight chloroplast avoidance (Fig. 2B). This could indicate that, in wild-type plants exposed to blue light intensities of 10 μmol m−2 s−1, phot2 signals for a high-light response while phot1 signals for a low-light response. This suggests that not only is the biphasic response caused by separate signals for accumulation and avoidance but that at 10 μmol m−2 s−1 phot1 and phot2 pathways produce competing signals. Because phot1 mutants do not show robust biphasic movements, the response is probably not caused by desensitization of the photoreceptors.
Signalling by phot1 can induce chloroplast avoidance
It has been thought that the high-light-induced chloroplast avoidance response is mediated completely by phot2 since previous experiments showed that phot2 mutants display a chloroplast accumulation response at blue light intensities between 0.8 μmol m−2 s−1 and 100 μmol m−2 s−1 (Jarillo et al., 2001; Sakai et al., 2001). However, our results provide three pieces of evidence suggesting that phot1 is also capable of signalling for an avoidance response. (i) When exposed to high-intensity light, phot2 mutants displayed a modest but reproducible biphasic response (Figs 1, 2). Although the response was transient, it indicates that, without the aid of phot2, phot1 can induce a weak high-light response. (ii) Double mutants in phot1phyB showed a reduced rate of movement under 10–40 μmol m−2 s−1 when compared to phyB single mutants (Fig. 5), suggesting that phot1 can enhance the rate of avoidance movement in this background. (iii) When exposed to intense white light, the chloroplasts in phot2 mutants moved slowly towards the anticlinal cell walls, while phot1phot2 double mutants failed to show any movement when observed through microscopy (Fig. 3). This response was seen in both phot2-1 and phot2-5 suggesting that it is not allele specific. Taken together, these observations indicate that, under certain light conditions, phot1 can initiate a chloroplast avoidance response in the absence of phot2. However, because phot1 mutants and the wild type show similar chloroplast movements under high light intensities (Fig. 1), phot2 clearly plays the dominant role in driving the avoidance response. It is also possible that the avoidance movement seen in the phot2 mutant, but not in the phot1phot2 double mutant is driven by temperature as opposed to light intensity. It has been shown that phot2 mediates chloroplast relocation to the anticlinal walls of Adiantum capillus-veneris under low-temperature conditions (Kodama et al., 2008). It is possible that phot1 is mediating a similar response under high-temperature conditions in Arabidopsis.
Phyb inhibition of the high-light avoidance response
Although previous work showed that mutants in phyB displayed enhanced chloroplast avoidance in response to intermediate and high blue light (DeBlasio et al., 2003), it was not clear if PhyB acted to inhibit the high-light response or if it enhanced the antagonistic low-light response. The analysis presented here of the response of phyB mutants to transitional blue-light intensities (5–20 μmol m−2 s−1) suggests that PhyB acts to inhibit chloroplast avoidance in wild-type plants rather than to enhance accumulation. Specifically, under 5 μmol m−2 s−1 of blue light, phyB displayed a biphasic response while the wild-type showed only accumulation (Fig. 6). The total change between the maximum and minimum levels of light transmittance was similar in both lines, indicating that the avoidance response was enhanced while the low-light accumulation response was unchanged. The phyB mutant showed a similarly enhanced avoidance response at 10 μmol m−2 s−1. If PhyB were inhibiting chloroplast avoidance by enhancing accumulation, it would be expected that the magnitude of the chloroplast accumulation phase of the biphasic response would be decreased in the phyB mutant. These observations suggest that the PhyB-mediated repression of the high-light response is limited to inhibition of the chloroplast avoidance signalling pathway.
Because phot1 and phot2 appear to act through independent and distinct signalling pathways to regulate chloroplast movement and other blue-light responses (Fig. 1) (Sakai et al., 2000; Inada et al., 2004) it is possible that PhyB activity could interact with one or both of these pathways to inhibit chloroplast avoidance under high light. If PhyB worked with phot1 signalling exclusively, it would be expected that the phot1 mutants would respond exactly like phot1phyB double mutants. The same is true for phot2 and phot2phyB double mutants. However, if PhyB signalling interacts with both phot1 and phot2, or functions independently of them, then the phot1phyB and phot2phyB double mutants would be expected to continue to display the phyB phenotype. Examination of phot1phyB and phot2phyB showed that the enhanced movement caused by the loss of PhyB is present in both double mutants, indicating that the PhyB inhibition of the chloroplast avoidance response is not exclusive to phot1 or phot2 signalling (Figs 4, 5).
The exact role of PhyB in light-dependent chloroplast movements in Arabidopsis remains unclear. Since neither red nor far-red light exposure appears to affect chloroplast movement in angiosperms, it is unlikely that PhyB acts as a red light photoreceptor in its modulation of chloroplast movement (Walczak and Gabrýs, 1980; DeBlasio et al., 2003). However, because blue light can mediate PhyA-dependent very low fluence responses, PhyB may also be activated by blue light to induce suppression of chloroplast avoidance (Hamazato et al., 1997). Although red light does not cause hypocotyl phototropism, phytochrome appears to modulate the response in a similar manner to what has been observed for chloroplast movement. In the absence of red light, PhyB enhanced the response at blue light intensities above 1 μmol m−2 s−1 and PhyA caused inhibition under higher fluence rates (Whippo and Hangarter, 2004). In the fern Adiantum capillus-veneris, mutations in the blue-light-absorbing phototropins inhibited red-light-induced chloroplast movement, suggesting a role for Adiantum phototropins in phytochrome signal transduction. In Arabidopsis, PhyB can function in signal transduction downstream of the phototropins. One potential source of Phot and Phy signalling overlap is found in the PHYTOCHROME KINASE SUBSTRATE (PKS) family of proteins, which have been shown to interact directly with both phot1 and PhyA and play a role in both phot1 and phot2 signalling (de Carbonnel et al., 2010). However, triple knockout mutants in pks1, pks2, and pks4 show normal levels of chloroplast movement, indicating that these three genes are not required for a normal response. It is possible that PKS3, for which no null mutants were available, may be involved.
In conclusion, detailed analysis of fluence response relationships for light-induced chloroplast movments in phot1 and phot2 mutants indicate that, although both phot1 and phot2 can induce chloroplast accumulation, they operate through integrated but antagonistic pathways to control this movement. Evidence is also presented that, under very high-light conditions, the phot1 photoreceptor can signal for chloroplast avoidance, although phot2 is the primary regulator of this response. In addition, results obtained with phot1phyB and phot2phyB double mutants indicates that PhyB acts to attenuate the high light avoidance response in concert with both phot1 and phot2.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary video S1. Time-lapse analysis of high light-induced chloroplast movement in palisade phot2 mutant.
Supplementary Fig. S1. Comparisons between genotypes immediately after exposure to individual fluence rates of blue light for analysis of the biphasic response: data redrawn from Figs 1C and 4C.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Science Foundation (IOB-0416741).
Glossary
Abbreviations
- Col
Arabidopsis thaliana Columbia ecotype
- Ler
Arabidopsis thaliana Landsberg erecta ecotype
- Phot
phototropin
- Phy
phytochrome
References
- Augustynowicz J, Gabrý s H. Chloroplast movements in fern leaves: correlation of movement dynamics and environmental flexibility of the species. Plant, Cell and Environment. 1999;22:1239–1248. [Google Scholar]
- Boardman NK. Comparative photosynthesis of sun and shade plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1977;28:355–377. [Google Scholar]
- DeBlasio SL, Mullen JL, Luesse DR, Hangarter RP. Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Physiology. 2003;133:1471–1479. doi: 10.1104/pp.103.029116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBlasio SL, Luesse DR, Hangarter RP. A plant-specific protein essential for blue-light-induced chloroplast movements. Plant Physiology. 2005;139:101–114. doi: 10.1104/pp.105.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carbonnel M, Davis P, Roelfsema MRG, Inoue S-i, Schepens I, Lariguet P, Geisler M, Shimazaki K-i, Hangarter R, Fankhauser C. The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signalling element that regulates leaf flattening and leaf positioning. Plant Physiology. 2010;152:1391–1405. doi: 10.1104/pp.109.150441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Lieg EJ, Durham T, Spalding EP. Primary inhibition of hypocotyl growth and phototropism depend differently on phototropin-mediated increases in cytoplasmic calcium induced by blue light. Plant Physiology. 2003;133:1464–1470. doi: 10.1104/pp.103.024372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA, Larner VS, Whitelam GC. The signal transducing photoreceptors of plants. International Journal of Developmental Biology. 2005;49:653–664. doi: 10.1387/ijdb.051989kf. [DOI] [PubMed] [Google Scholar]
- Galen C, Huddle J, Liscum E. An experimental test of the adaptive evolution of phototropins: blue-light photoreceptors controlling phototropism in Arabidopsis thaliana. Evolution. 2004;58:515–523. [PubMed] [Google Scholar]
- Hamazato F, Shinomura T, Hanzawa H, Chory J, Furuya M. Fluence and wavelength requirements for arabidopsis CAB gene induction by different phytochromes. Plant Physiology. 1997;115:1533–1540. doi: 10.1104/pp.115.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada S, Ohgishi M, Mayama T, Okada K, Sakai T. RPT2 is a signal transducer involved in phototropic response and stomatal opening by association with phototropin 1 in Arabidopsis thaliana. The Plant Cell. 2004;16:887–896. doi: 10.1105/tpc.019901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Shibata K. Comparative examination of terrestrial plant leaves in terms of light-induced absorption changes due to chloroplast rearrangements. Plant and Cell Physiology. 1974;15:717–721. [Google Scholar]
- Jarillo J, Ahmad M, Cashmore A. NPL1: a second member of the NPH serine/threonine kinase family of Arabidopsis. Plant Physiology. 1998;117:719. [Google Scholar]
- Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- Jeong WJ, Park YI, Suh K, Raven JA, Yoo OJ, Liu JR. A large population of small chloroplasts in tobacco leaf cells allows more effective chloroplast movement than a few enlarged chloroplasts. Plant Physiology. 2002;129:112–121. doi: 10.1104/pp.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota A, Sato Y, Wada M. Intracellular chloroplast photorelocation in the moss Physcomitrella patens is mediated by phytochrome as well as by a blue-light receptor. Planta. 2000;210:932–937. doi: 10.1007/s004250050700. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Kasahara M, Abe T, Yoshida S, Wada M. Function analysis of phototropin2 using fern mutants deficient in blue light-induced chloroplast avoidance movement. Plant and Cell Physiology. 2004;45:416–426. doi: 10.1093/pcp/pch045. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- Kagawa T, Wada M. Phytochrome and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum. Planta. 1996;107:389–398. [Google Scholar]
- Kagawa T, Wada M. Chloroplast-avoidance response induced by high-fluence blue light in prothallial cells of the fern Adiantum capillus-veneris as analyzed by microbeam irradiation. Plant Physiology. 1999;119:917–923. doi: 10.1104/pp.119.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa T, Wada M. Blue light-induced chloroplast relocation in Arabidopsis thaliana as analyzed by microbeam irradiation. Plant and Cell Physiology. 2000;41:84–93. doi: 10.1093/pcp/41.1.84. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Kagawa T, Oikawa K, Suetsugu N, Wada M. Chloroplast avoidance movement reduces photodamage in plants. Nature. 2002;420:829–832. doi: 10.1038/nature01213. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kagawa T. Blue light-induced chloroplast avoidance and phototropic responses exhibit distinct dose dependency of PHOTOTROPIN2 in Arabidopsis thaliana. Photochemistry and Photobiology. 2009;85:1260–1264. doi: 10.1111/j.1751-1097.2009.00564.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K-i. phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- Kodama Y, Tsuboi H, Kagawa T, Wada M. Low temperature-induced chloroplast relocation mediated by a blue light receptor, phototropin 2, in fern gametophytes. Journal of Plant Research. 2008;121:441–448. doi: 10.1007/s10265-008-0165-9. [DOI] [PubMed] [Google Scholar]
- Koniger M, Delamaide JA, Marlow ED, Harris GC. Arabidopsis thaliana leaves with altered chloroplast numbers and chloroplast movement exhibit impaired adjustments to both low and high light. Journal of Experimental Botany. 2008;59:2285–2297. doi: 10.1093/jxb/ern099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosei I, Tatsuya S, Shingo T. Blue light-dependent nuclear positioning in Arabidopsis thaliana leaf cells. Plant and Cell Physiology. 2007;48:1291–1291. doi: 10.1093/pcp/pcm095. [DOI] [PubMed] [Google Scholar]
- Kraml M, Herrmann H. Red blue-interaction in Mesotaenium chloroplast movement: blue seems to stabilize the transient memory of the phytochrome signal. Photochemistry and Photobiology. 1991;53:255–259. [Google Scholar]
- Lechowski Z. Chloroplast arrangement as a factor of photosynthesis in multilayered leaves. Acta Societatis Botanicorum Poloniae. 1974;63:531–540. [Google Scholar]
- Liscum E, Briggs WR. Mutations in the nph1 locus of Arabidopsis disrupt the perception of phototropic stimuli. The Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luesse DR, DeBlasio SL, Hangarter RP. Plastid movement impaired 2, a new gene involved in normal blue-light-induced chloroplast movements in arabidopsis. Plant Physiology. 2006;141:1328–1337. doi: 10.1104/pp.106.080333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malec P. Kinetic modeling of chloroplast phototranslocations in Lemna trisulca L. 2. Rate-limiting components. Journal of Theoretical Biology. 1994;169:189–195. [Google Scholar]
- Melis A. Photosystem-II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends in Plant Science. 1999;4:130–135. doi: 10.1016/s1360-1385(99)01387-4. [DOI] [PubMed] [Google Scholar]
- Nozue K, Kanegae T, Imaizumi T, Fukuda S, Okamoto H, Yeh KC, Lagarias JC, Wada M. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proceedings of the National Academy of Sciences, USA. 1998;95:15826–15830. doi: 10.1073/pnas.95.26.15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Kagawa T, Kasahara M, Swartz TE, Christie JM, Briggs WR, Wada M, Okada K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proceedings of the national Academy of Sciences, USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Wada T, Ishiguro S, Okada K. RPT2: A signal transducer of the phototropic response in Arabidopsis. The Plant Cell. 2000;12:225–236. doi: 10.1105/tpc.12.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiology. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van der Straeten D. Reaching out of the shade. Current Opinion in Plant Biology. 2005;8:462–468. doi: 10.1016/j.pbi.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Wagner G, Haupt W, Laux A. Reversible inhibition of chloroplast movement by cytochalasin B in the green alga Mougeotia. Science. 1972;176:808–809. doi: 10.1126/science.176.4036.808. [DOI] [PubMed] [Google Scholar]
- Walczak T, Gabrýs H. New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica. 1980;14:65–72. [Google Scholar]
- Whippo CW, Hangarter RP. Phytochrome modulation of blue-light-induced phototropism. Plant, Cell and Environment. 2004;27:1223–1228. [Google Scholar]
- Yatsuhashi H, Wada M, Hashimoto T. Dichroic orientation of phytochrome and blue-light photoreceptor in Adiantum protonemata as determined by chloroplast movement. Acta Physiologiae Plantarum. 1987;9:163–173. [Google Scholar]
- Zurzycki J. Chloroplast arrangement as a factor in photosynthesis. Acta Societatis Botanicorum Poloniae. 1955;24:27–63. [Google Scholar]
- Zurzycki J. The influence of chloroplast displacements on the optical properties of leaves. Acta Societatis Botanicorum Poloniae. 1961;30:503–527. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.