Abstract
By affecting the physiology and structure of plant canopies, increasing atmospheric CO2 and O3 influence the capacity of agroecosystems to capture light and convert that light energy into biomass, ultimately affecting productivity and yield. The objective of this study was to determine if established remote sensing indices could detect the direct and interactive effects of elevated CO2 and elevated O3 on the leaf area, chlorophyll content, and photosynthetic capacity of a soybean canopy growing under field conditions. Large plots of soybean (Glycine max) were exposed to ambient air (∼380 μmol CO2 mol−1), elevated CO2 (∼550 μmol mol−1), elevated O3 (1.2× ambient), and combined elevated CO2 plus elevated O3 at the soybean free air gas concentration enrichment (SoyFACE) experiment. Canopy reflectance was measured weekly and the following indices were calculated from reflectance data: near infrared/red (NIR/red), normalized difference vegetation index (NDVI), canopy chlorophyll content index (chl. index), and photochemical reflectance index (PRI). Leaf area index (LAI) also was measured weekly. NIR/red and LAI were linearly correlated throughout the growing season; however, NDVI and LAI were correlated only up to LAI values of ∼3. Season-wide analysis demonstrated that elevated CO2 significantly increased NIR/red, PRI, and chl. index, indicating a stimulation of LAI and photosynthetic carbon assimilation, as well as delayed senescence; however, analysis of individual dates resolved fewer statistically significant effects of elevated CO2. Exposure to elevated O3 decreased LAI throughout the growing season. Although NIR/red showed the same trend, the effect of O3 on NIR/red was not statistically significant. Season-wide analysis showed significant effects of O3 on PRI; however, analysis of individual dates revealed that this effect was only statistically significant on two dates. Elevated O3 had minimal effects on the total canopy chlorophyll index. PRI appeared to be more sensitive to decreased photosynthetic capacity of the canopy as a whole compared with previously published single leaf gas exchange measurements at SoyFACE, possibly because PRI integrates the reflectance signal of older leaves with accumulated O3 damage and healthy young, upper canopy leaves, enabling detection of significant decreases in photosynthetic carbon assimilation which have not been detected in previous studies which measured gas exchange of upper canopy leaves. When the canopy was exposed to elevated CO2 and O3 simultaneously, the deleterious effects of elevated O3 were diminished. Reflectance data, while less sensitive than direct measurements of physiological/structural parameters, corroborate direct measurements of LAI and photosynthetic gas exchange made during the same season, as well as results from previous years at SoyFACE, demonstrating that these indices accurately represent structural and physiological effects of changing tropospheric chemistry on soybean growing in a field setting.
Keywords: Canopy reflectance, elevated CO2, elevated O3, NIR/red, PRI, soybean (Glycine max), SoyFACE
Introduction
Increases in atmospheric CO2 and O3 concentrations projected to occur by the middle of this century will probably have dramatic and contrasting effects on the productivity of agroecosystems. While elevated atmospheric CO2 may increase leaf-level photosynthesis, leaf area index (leaf area per unit ground area, LAI) and overall productivity of soybean (Ainsworth et al., 2002; Long et al., 2004; Dermody et al., 2006), growth in elevated O3 generally has the opposite effects (Morgan et al., 2003; Long et al., 2005; Dermody et al., 2006), and elevated CO2 may partially mitigate the negative effects of elevated O3 when plants are exposed to elevated CO2 and elevated O3 simultaneously (Cardoso-Vilhena et al., 2004; Booker and Fiscus, 2005; Dermody et al., 2008). As global concentrations of CO2 and O3 continue to increase, it will be necessary to understand their effects on the structure and physiological properties of plant canopies. Remote sensing allows rapid and non-invasive estimation of physiological parameters which are important to determining productivity, such as leaf area and photosynthetic carbon assimilation, and is an excellent tool for large-scale assessment of ecosystem structure and function. It is crucial, however, to verify whether reflectance indices calculated from canopy-level remote sensing measurements have the accuracy and sensitivity to detect the effects of elevated CO2 and O3 on ecosystem structure and function.
The leaf area and structure of a soybean canopy determine the area available for interception of incoming solar radiation and affect canopy photosynthesis and ecosystem productivity. In elevated CO2, lower light compensation points and an extended period for leaf growth contribute to greater LAI (Pearcy, 1983; Hirose et al., 1996; Ferris et al., 2001; Dermody et al., 2006). Exposure to elevated CO2 in growth chambers or open-top chambers caused an 18% stimulation of LAI in soybean (Ainsworth et al., 2002), but effects of this magnitude are not always observed when plants are grown in more realistic field conditions (Long et al., 2004; Ainsworth and Long, 2005). At the soybean free air gas concentration enrichment (SoyFACE) experiment, elevated CO2 increased peak LAI by 9–25% in 2002–2004, but did not affect peak LAI in 2001 (Dermody et al., 2006, 2008). In contrast, O3 is highly reactive and even at moderate concentrations can inhibit photosynthesis, causing accumulated damage in mature leaves late in the growing season, and O3 may accelerate senescence and late-season loss of leaf area in soybean (Sandermann et al., 1998; Morgan et al., 2003, 2004; Dermody et al., 2006, 2008).
Many remote sensing indices correlate with LAI, including the normalized difference vegetation index (NDVI; Table 1) and the ratio of near infrared to red reflectance (NIR/red; Table 1). Although NDVI is a sensitive indicator of canopy structure at low values of LAI, it is relatively insensitive to variation in the structure of canopies with LAI >3 (Gamon et al., 1995; Panferov et al., 2001). More recently, NIR/red has been used to estimate LAI and, in contrast to NDVI, NIR/red is sensitive to variation in canopy structure through high values of LAI (Daughtry et al., 2000). Both NDVI and simple ratios such as NIR/red may be particularly useful for detecting subtle changes in the rate of canopy development and senescence because they utilize the sharp differences between soil and foliage reflectance in the near infrared and visible portions of the spectrum (Peñuelas and Filella, 1998).
Table 1.
Canopy reflectance indices utilized in this experiment, including their acronyms, formulae, the biological parameter estimated by each index, and the reference
| Reflectance index | Acronym | Formula | Estimated biological parameter | References |
| Normalized difference vegetation index | NDVI | (R900–R680)/(R900+R680) | Leaf area index (LAI) | Gamon et al. (1995) |
| Near infrared/red | NIR/red | (R801/R670) | Leaf area index (LAI) | Daughtry et al. (2000) |
| Photochemical reflectance index | PRI | (R531–R570)/(R531+R570) | PSII efficiency, Net CO2 uptake | Gamon et al. (1997) |
| Total canopy chlorophyll content index | chl. index | (∫R840–870/∫R720–730) | Canopy chlorophyll content | Gitelson et al. (2005) |
In addition to the leaf area available for light interception, the duration of the canopy is an important determinant of productivity and, because of their disparate effects on photosynthesis and LAI, elevated CO2 and O3 generally have opposing effects on the timing of canopy senescence (Dermody et al., 2006). By extending the period for leaf development at the end of the growing season, elevated CO2 may delay senescence of soybean canopies (Dermody et al., 2006, 2008). Elevated O3 generally increases the rate of leaf loss deep in the canopy (Morgan et al., 2003; Dermody et al., 2006). The decline in canopy chlorophyll content that accompanies senescence is a reliable indicator of reduced photosynthetic capacity and can be estimated using reflectance indices such as the total canopy chlorophyll content index (chl. index) (Table 1). Numerous other indices have been developed which accurately assess chlorophyll content on a leaf basis in a wide variety of species, for example the chlorophyll normalized difference index (Gitelson and Merzlyak, 1994; Gamon and Surfus, 1999; Richardson et al., 2002). In a previous study, the chl. index accounted for 92% of the variation in canopy chlorophyll contents in maize and soybean, and was influenced by chlorophyll content at the leaf level as well as green LAI (Gitelson et al., 2005), making this index a promising candidate for detecting effects of CO2 and O3 on canopy senescence.
Determining the response of whole canopy photosynthetic carbon assimilation rates to elevated CO2 and O3 throughout the growing season is an important step in predicting crop productivity in a future high CO2 and high O3 atmosphere. Elevated CO2 increases leaf-level photosynthesis of C3 plants such as soybean (Drake et al., 1997; Ainsworth et al., 2002; Ainsworth and Long, 2005). When administered in closed systems such as growth chambers, elevated O3 reduces photosynthesis (Morgan et al., 2003), but, in a FACE system, the effects of O3 on leaf-level photosynthesis were evident only late in the growing season and in mature leaves (Morgan et al., 2004). The photochemical reflectance index (PRI) is correlated with photosystem II (PSII) efficiency and net CO2 assimilation across a range of environmental conditions (Peñuelas et al., 1995; Gamon et al., 1997; Guo et al., 2006), making it a promising tool for assessing whether leaf level responses to elevated CO2 and O3 persist at the canopy level and throughout the growing season.
The purpose of this study was to determine whether reflectance indices capture the effects of elevated CO2 and O3 on the structural and physiological properties of a soybean canopy. The soybean crop was exposed to elevated CO2, elevated O3, and elevated CO2 plus O3 treatments in a field setting using FACE technology at the SoyFACE experiment in Champaign, IL, USA. The SoyFACE facility provided a unique opportunity to determine the sensitivity of canopy reflectance indices to the physiological and structural effects of elevated CO2 and O3 on an intact soybean canopy because plant responses to these treatments at SoyFACE have been well characterized since 2001. It was predicted that elevated CO2, by increasing LAI, leaf area duration, and photosynthetic capacity, would increase NIR/red and PRI throughout the growing season, and would increase total canopy chl. index during late-season measurements. In contrast, it was predicted that elevated O3, by decreasing LAI throughout the season, reducing photosynthetic carbon assimilation, and increasing the rate of leaf loss to senescence, would lead to lower values of NIR/red, PRI, and chl. index, especially during late-season measurements.
Materials and methods
Site description
This research was conducted at the SoyFACE facility at the University of Illinois in Champaign, Illinois (40°02'N, 88°14'W, 228 m above sea level; http://www.soyface.uiuc.edu). SoyFACE consists of 16 octagonal experimental plots, 20 m in diameter and circumscribed by pipes that release CO2 and O3 through 300 μm pores at a supersonic velocity above the soybean canopy (Miglietta et al., 2001). The experiment is arranged in four randomized blocks, each containing four plots. Within each block, plots were exposed to elevated CO2, elevated O3, elevated CO2 plus O3, or ambient conditions. Each plot was divided in two, with one half planted with Glycine max L. cv. Pioneer 93B15 while the other half was subdivided into 23 rectangular subplots (3×2 m) each containing a different soybean cultivar.
Daytime CO2 concentrations in ambient plots averaged 387 μl l−1 at SoyFACE in 2005. The target concentration for elevated CO2 plots was 550 μl l−1 and, during the 2005 growing season, the average CO2 concentration in elevated plots was 552 μl l−1. The target concentration for elevated O3 plots was 1.5× ambient concentrations, but, because plants were not fumigated when leaf surfaces were wet, the actual treatment was ∼1.2× ambient concentration. Average ambient O3 concentration between 10:00 and 18:00 CST (central standard time) was 49.7 nl l−1, and the average O3 concentration in elevated plots was 58.7 nl l−1.
Wind speed and direction were measured continuously to adjust the rate and position of gas release to maintain target concentrations within experimental plots. Plants were fumigated during daylight hours, and 1 min average CO2 and O3 were ±20% of the target for >90% of the time during the 2005 growing season (E Ainsworth, personal communication). Experimental plots were separated by at least 100 m to avoid cross-contamination of CO2 and O3 (Nagy et al., 1994).
Leaf area index measurement
LAI was measured at weekly intervals during the 2005 growing season with a plant canopy analyser (LAI-2000, Li-Cor, Lincoln, NE, USA), as described by Dermody et al. (2006). Briefly, LAI was measured when the sun was completely obscured by cloud cover or within 1 h of sunset to ensure that incident light was diffuse. Measurements were made at six random locations in each plot and at each location; one measurement was made above the canopy and four measurements were made below the canopy. The entire hemispherical view was used to calculate LAI, and the operator was excluded from measurements by a view cap. LAI data were used to calculate leaf area duration (LAD), or the area under the curve of LAI plotted against time (Dermody et al., 2006). For each experimental plot, the relationship between LAI and time was fitted using the trapezoidal rule (Sigmaplot ver. 10, Systat Software Inc., Point Richmond, CA, USA).
Canopy reflectance estimation
Upwelling irradiance from the soybean canopy was measured from 300 nm to 1100 nm with a portable spectroradiometer (UniSpec Spectral Analysis System, PP Systems, Haverhill, MA, USA) on 12 dates from mid June to mid September, 2005. The spectroradiometer was equipped with a visible/near infrared detector with a Raleigh resolution of <10 nm and a bin size of 3.3 nm. On each sampling date, 16 readings were taken in the plot containing the Pioneer 93B15 cultivar, and these 16 readings were then averaged to produce one reading for each plot (n=4). Measurements were taken under clear skies and in full sunlight; to minimize errors associated with changes in solar angle (J Gamon, personal communication) measurements were taken between the hours of 10:00 and 14:00 CST. Measurements were taken from a height of ∼1 m above the canopy with the fibreoptic probe pointed downward to measure upwelling irradiance of the soybean canopy. With an acceptance angle of 45 º the area of canopy sampled by the probe when held at a height of 1 m was ∼0.54 m2. To standardize for differing levels of irradiance on different sampling dates, a photographic grey card of ∼18% reflectance was used as a field standard. For each measurement, upwelling canopy radiance was divided by the upwelling radiance of the grey card and multiplied by a correction factor to yield reflectance values. The correction factor was calculated as 100×(Rgrey card/Rreference), where Rgrey card is the reflectance of the grey card, and Rreference is the reflectance of a white reference standard (PP Systems, Haverhill, MA, USA), measured under uniform light conditions in the laboratory.
Calculation of reflectance indices
To quantify the effects of elevated CO2 and elevated O3 on LAI, canopy chlorophyll content, and net CO2 assimilation, four indices were calculated from canopy reflectance measurements. The NDVI is a widely used index which probes changes in LAI, and was calculated as the normalized ratio of reflectance at 900 nm and 680 nm, (R900–R680)/(R900+R680), where Rx indicates canopy reflectance at x wavelength in nanometres (Table 1; Gamon et al., 1995). To obtain a more accurate assessment of the effects of CO2 and O3 when LAI was >3, the ratio of reflectance in the NIR/red also was calculated, as the non-normalized ratio of reflectance at 801 nm and 670 nm, (R801/R670; Table 1; Daughtry et al., 2000). The PRI was calculated as the normalized ratio of reflectance at 531 nm and 570 nm (R531–R570)/(R531+R570) (Table 1; Peñuelas et al., 1995; Gamon et al., 1997). PRI is responsive to PSII efficiency because conversion of xanthophyll cycle pigments involved in dissipation of excess light energy can be detected through reflectance at 531 nm, with reflectance at 570 nm serving as a reference (Gamon et al., 1997). Canopy chlorophyll content was estimated using the canopy chl. index, calculated as the ratio of the area under the curve in the 840–870 nm region and the 720–730 nm region (∫R840–870/∫R720–730; Table 1; Gitelson et al., 2005). The chl. index is sensitive to variation in chlorophyll content because it incorporates reflectance at or near the red edge, the point of maximum slope in reflectance of vegetation. This is the point in the reflectance spectrum of vegetation where reflectance changes from low in the visible, chlorophyll absorption region to high in the near infrared region due to leaf and canopy scattering (Filella and Peñuelas, 1994).
Statistical analysis
After verifying that the data satisfied the assumptions of analysis of variance (ANOVA), a repeated measures ANOVA (PROC MIXED: SAS ver. 8.1 SAS Institute, Cary, NC, USA) was used to test for treatment effects on LAI, NDVI, NIR/red, PRI, and chl. index. Analyses were performed on plot means. For LAI data, blocks and blocks×CO2×O3 were included as random components, and for reflectance data blocks were included as the only random component because the blocks×CO2×O3 interaction term was not significant. All comparisons were made between treatments and control. To avoid type II errors, significant differences from control were defined as P <0.1. Slice statements were utilized to compare treatments within individual days.
Results
Leaf area index
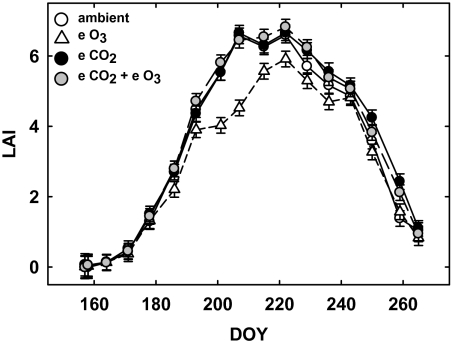
LAI increased to a maximum of ∼6.5 in ambient plots on DOY (day of year) 222 (Fig. 1). Averaged across the growing season, LAI was greater in elevated relative to ambient CO2 plots (Table 2; Fig. 1). However, a significant DOY×CO2×O3 interaction revealed that LAI was significantly greater in elevated CO2 relative to ambient plots only at the end of the growing season, on DOY 250 and 259, and had no effect on peak LAI (Table 2; Fig. 1). LAI was significantly lower in elevated relative to ambient O3 plots when averaged across the growing season (Table 2; Fig. 1). A significant DOY×CO2×O3 interaction revealed that this difference was significant during mid-season measurements (DOY 193–222; Table 2; Fig. 1). When elevated CO2 and elevated O3 were applied in combination, elevated CO2 ameliorated the negative effects of elevated O3, making LAI in combination plots indistinguishable from that in ambient plots, except on DOY 229 and 259, where LAI was higher in combination plots relative to ambient plots (Table 2; Fig. 1).
Fig. 1.
Leaf area index (LAI) estimates of soybean grown in ambient air (open circles), elevated CO2 (filled circles), elevated O3 (open triangles), and combined elevated CO2 and elevated O3 (grey circles). Each point represents the least squared mean ±SE (n=4). Dates of measurement are indicated on the x-axis as day of year (DOY).
Table 2.
Three-way ANOVA of the effects of date, elevated CO2 treatment, elevated O3 treatment, and two- and three-way interactions on LAD, LAI, NIR/red, PRI, and the chl. index of soybean. Significant effects are shown in bold.
| Effect | Numerator DF | LAD |
LAI |
NIR/red |
PRI |
Chl. index |
||||||
| F-stat | P-value | F-stat | P-value | F-stat | P-value | F-stat | P-value | F-stat | P-value | |||
| Date | 11 (LAI=16) | – | – | 358.34 | <0.0001 | 85.24 | <0.0001 | 112.57 | <0.0001 | 36.48 | <0.0001 | |
| CO2 | 1 | 10.40 | 0.0104 | 22.1 | 0.0012 | 9.68 | 0.0040 | 6.27 | 0.0181 | 3.67 | 0.0656 | |
| Date×CO2 | 11 (LAI=16) | – | – | 1.93 | 0.0216 | 0.76 | 0.6794 | 0.60 | 0.8210 | 0.97 | 0.4797 | |
| O3 | 1 | 4.39 | 0.0655 | 7.41 | 0.0237 | 1.48 | 0.2334 | 4.83 | 0.0360 | 0.35 | 0.5575 | |
| Date×O3 | 11 (LAI=16) | – | – | 1.55 | 0.0903 | 0.55 | 0.8640 | 1.27 | 0.2526 | 0.82 | 0.6241 | |
| CO2×O3 | 1 | 8.85 | 0.0156 | 6.59 | 0.0306 | 1.01 | 0.3236 | 0.01 | 0.9123 | 0.16 | 0.6929 | |
| Date×CO2×O3 | 11 (LAI=16) | – | – | 1.85 | 0.0290 | 0.75 | 0.6857 | 0.38 | 0.9626 | 1.95 | 0.0432 | |
When administered at ambient O3, growth under elevated CO2 had no effect on LAD [ambient mean, 398.1±17.2 (SE; d m−2 m−2), elevated CO2 mean: 402.90±17.2]. There was, however, a substantial reduction in LAD for plants grown under elevated O3 (elevated O3 mean: 301.2±17.2) that was eliminated when plants were exposed to elevated O3 and elevated CO2 simultaneously (combined elevated CO2+elevated O3 mean: 419.7±17.2; Table 2).
Relationship between leaf area index and reflectance indices
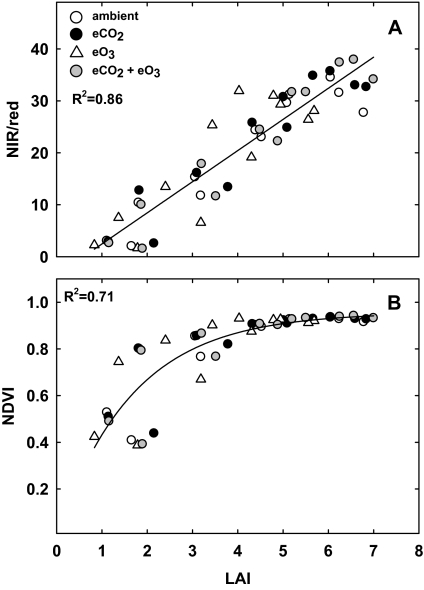
To determine which index was more sensitive to variation in LAI caused by seasonal changes in canopy cover and by atmospheric CO2 and O3 treatments, the values for NDVI and NIR/red were regressed against those obtained from weekly measurements of LAI. NIR/red was linearly related to LAI over its entire range (Fig. 2; R2=0.86). The relationship between NDVI and LAI was non-linear and, above LAI values of ∼3, NDVI tended to saturate and became less sensitive to variation in LAI (Fig. 2b). The relationship between NIR/red and NDVI with LAI was not affected by elevated CO2 or elevated O3, and the regressions shown are for all treatments.
Fig. 2.
NIR/red (a) and NDVI (b) plotted against LAI of soybean measured on the same dates in ambient air (open circles), elevated CO2 (filled circles), elevated O3 (open triangles), and combined elevated CO2 and elevated O3 (grey circles) treatments, including a linear regression for (a) and an exponential regression for (b) (R2=0.86 and 0.71, respectively).
Near infrared/red
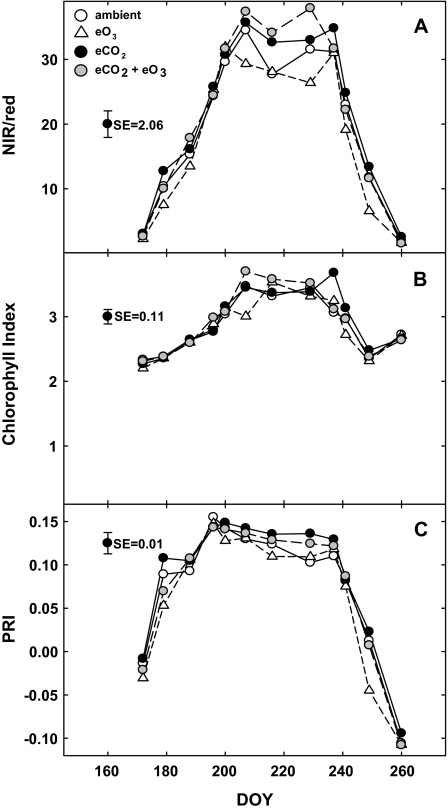
NIR/red was a sensitive indicator of seasonal variation in canopy development and the effects of atmospheric treatments on LAI (Fig. 3a; Table 2). As LAI increased to mid-season peak values, there was a concurrent increase in NIR/red. NIR/red increased from ∼2 early in the season (DOY 172) to 30–35 during peak LAI (DOY 207–216), and decreased to ∼2 late in the season (DOY 260; Fig. 3a).
Fig. 3.
NIR/red (a), chl. index (b), and PRI (c) measured throughout the growing season in soybean grown in ambient air (open circles), elevated CO2 (filled circles), elevated O3 (open triangles), and combined elevated CO2 and elevated O3 (grey circles). Each point represents the least squared mean, and date of measurement is indicated on the x-axis as day of year (DOY). The size of the SE bars is represented on the left side of each panel (n=4).
When averaged across the growing season, NIR/red was 8.5% higher in elevated relative to ambient CO2 plots (Table 2; Fig. 3a). However, when CO2×O3×DOY effects were analysed for each day separately using slice statements, NIR/red in elevated CO2 plots was not significantly different from NIR/red in ambient plots on any measurement date, suggesting that the significant main effect of elevated CO2 was due to amelioration of O3 damage in combination plots, rather than direct stimulation of leaf area by elevated CO2 (Fig. 3a).
The effect of O3 was not significant (P <0.1), but there was a consistent trend for lower NIR/red in elevated O3 relative to ambient plots (Fig. 3a; Table 2). CO2×O3×DOY effects analysed using slice statements revealed that NIR/red was significantly lower in elevated O3 relative to ambient plots on DOY 207 and 249 (P <0.1; Fig. 3a). Elevated O3 treatment resulted in a 9% depression of NIR/red when averaged across the growing season (Fig. 3a).
Although a significant interaction between CO2 and O3 on NIR/red (P <0.1) was not detected, there was a trend for exposure to elevated CO2 to reduce the negative effect of elevated O3 when both gases were applied simultaneously (Fig. 3a; Table 2). When averaged across the season, NIR/red was 7.6% higher in combination relative to ambient plots (Fig. 3a). This is a considerable stimulation relative to the 9% depression of NIR/red that was observed when plots were exposed to elevated O3 alone (Fig. 3a).
Chlorophyll index
There was significant seasonal variation in the chl. index, increasing from ∼2.3 early in the growing season (DOY 172) to a maximum of ∼3.5 during peak LAI (DOY 216) and decreasing to ∼2.7 late in canopy senescence (DOY 260; Fig. 3b; Table 2).
When averaged across the growing season, the chl. index increased by ∼2.3% in elevated CO2 compared with ambient plots (Fig. 3b). A significant CO2×O3×DOY interaction revealed that this significant effect of CO2 was primarily caused by the increase in chl. index by elevated CO2 on one date (DOY 237, P=0.007, Fig. 3b).
When data were averaged across the season, there were no significant direct effects of O3 on chl. index (Table 2). However, a significant CO2×O3×DOY interaction revealed that the chl. index was significantly lower in elevated O3 relative to ambient plots on only one date (DOY 207, P=0.004; Fig. 3b).
Photochemical reflectance index
PRI varied significantly throughout the growing season, increasing from negative values before canopy closure (DOY 172) to a maximum of ∼0.15 on DOY 196, and decreasing to negative values as the canopy senesced (DOY 249–260; Fig. 3c). There was an average 14% stimulation of PRI in elevated CO2 relative to ambient plots across the growing season (Fig. 3c).
When data were averaged across the season, PRI was significantly depressed in elevated O3 plots relative to ambient plots (Fig. 3c; Table 2). When data for each day were analysed separately using slice statements, PRI in elevated O3 plots was significantly less than in ambient plots on one early season date and one late season date (DOY 179 and 249; Fig. 3c).
There was a trend for elevated CO2 to ameliorate the negative effects of elevated O3 on PRI when plots were exposed to elevated CO2 and elevated O3 in combination (Fig. 3c). When averaged across the growing season, PRI in combination plots was ∼2% higher than PRI in ambient plots (Fig. 3c). This is a noteworthy increase relative to the 14% depression of PRI that was observed when plots were exposed to elevated O3 in combination with ambient CO2; however, this interaction was not significant at the P <0.1 level (Table 2).
Discussion
The remote sensing indices used in this experiment are promising tools for monitoring canopy structure and function because they are non-destructive and have the potential for application at many spatial scales, including leaf-, canopy-, aircraft-, and satellite-based measurements. NIR/red, to a greater extent than NDVI, accurately estimated LAI throughout the growing season. NIR/red was, however, less sensitive than direct measurement of LAI in detecting the negative effects of elevated O3 during mid-season measurements and delayed senescence observed in elevated CO2 plots. The chl. index showed little response to CO2 and O3 treatments. PRI was sensitive to variation in photosynthetic CO2 uptake by the soybean canopy throughout the season, demonstrating increased CO2 uptake in elevated CO2 treatment and decreased CO2 uptake in elevated O3 treatment. Although remote sensing was, in some cases, less sensitive than direct measurement of physiological parameters, NIR/red and PRI revealed similar treatment effects to those that direct measurement of leaf area and leaf level gas exchange have revealed in previous studies at SoyFACE (Bernacchi et al., 2006; Dermody et al., 2006, 2008, Leakey et al., 2009). This study is novel in its successful application of established remote sensing indices to detect effects of elevated atmospheric CO2 and O3 on an intact soybean canopy in a field setting.
A linear relationship was found between NIR/red and LAI through a maximum LAI of 6, and a non-linear relationship between NDVI and LAI, where NDVI became insensitive to variation in LAI at values >3 or 4 (Fig. 2). Similarly, NDVI was a sensitive indicator of canopy structure only at LAI values <2 in grassland, chaparral shrub, and oak woodland ecosystems (Gamon et al., 1995). Daughtry and colleagues (2000) used simulated data to demonstrate that LAI accounted for 99.4% of the variation in NIR/red and a slightly smaller percentage (98%) of the variation in NDVI. These authors concluded that NIR/red could reliably estimate LAI, but recognized that this needed to be confirmed using real rather than simulated data (Daughtry et al., 2000). The present data demonstrate that the correlation between NIR/red and LAI is conserved in field-grown soybean at elevated CO2 or O3 and that NIR/red appears to be a better index than NDVI for estimating LAI via canopy reflectance measurements.
Although ANOVA indicated a significant direct effect of elevated CO2 on LAI, CO2×O3×DOY interactive effects demonstrated that elevated CO2 increased LAI only at the end of the season (DOY 250 and 259; Fig.1), and did not affect peak LAI in 2005 (Fig. 1). Correspondingly, ANOVA indicated a significant direct effect of CO2 on NIR/red, but no significant effects of CO2 on specific dates were found using slice statements. In the case of LAI and NIR/red, the compensatory effect of elevated CO2 on the negative effects of elevated O3 probably contributed to significant main effects of CO2 on both parameters (Figs 1, 3a).
Large CO2 stimulation of leaf area of soybean has been measured in growth chamber studies (Ainsworth et al., 2002); however, data from free air CO2 enrichment experiments suggest that this is not always the case (Long et al., 2004; Ainsworth and Long, 2005). At SoyFACE, elevated CO2 did not affect peak LAI in 2001, but stimulated peak LAI by 10% in 2002 (Dermody et al., 2006). Canopy senescence was delayed by elevated CO2 in both years, associated with an extended period for addition of new leaves (Dermody et al., 2006). Similarly in 2003 and 2004, exposure to elevated CO2 increased maximum LAI by 9–25%, and this difference persisted as the canopy senesced (Dermody et al., 2008). The present data demonstrate that elevated CO2 did not affect peak LAI in 2005, and this absence of a treatment effect was accurately captured by the NIR/red reflectance index (Figs 1, 3). As seen in 2001–2004 at SoyFACE (Dermody et al., 2006, 2008), elevated CO2 delayed canopy senescence in 2005, as demonstrated by significantly higher LAI in elevated CO2 on DOY 250 and 259 (Fig. 1). Despite a significant season-wide stimulation of NIR/red by elevated CO2, there were no significant CO2 effects on individual dates, indicating that NIR/red used at the present replication level may not be sensitive enough to detect delayed senescence caused by elevated CO2.
NIR/red in elevated O3 plots consistently was lower (though non-significantly) than in ambient plots, and this effect was of a similar magnitude to that seen in direct measurement of LAI. In a meta-analysis of studies that used enclosures to examine the effects of elevated O3 on soybean, Morgan and colleagues (2003) demonstrated that elevated O3 decreased green leaf area by 32% and total leaf area by ∼10% compared with charcoal-filtered air. At SoyFACE in 2002–2004, peak LAI of soybean in elevated O3 was not different from LAI in ambient plots, but elevated O3 accelerated senescence and contributed to significantly lower LAI late in the growing season (Dermody et al., 2006, 2008). In contrast to previous years, peak LAI in elevated O3 plots was ∼10% lower than in ambient plots in 2005 (Fig. 1).
Significant interactive effects of CO2 and O3 on LAI were detected in 2005 where elevated O3 decreased LAI, but when plots were exposed to elevated CO2 in combination with elevated O3, elevated CO2 stimulated LAI, compensating for the negative effects of O3 (Fig. 1); however, this interaction was not detected at the P <0.1 level using NIR/red (Fig. 3a). Elevated CO2 also offset negative effects of elevated O3 on LAI at SoyFACE in 2003–2004 (Dermody et al., 2008).
The chl. index was marginally higher in elevated relative to ambient CO2 plots in 2005, an effect that was mainly driven by one measurement date (DOY 237; Fig. 3b), suggesting that effects of atmospheric CO2 on canopy chlorophyll content of soybean were minimal in this study, corroborating previous experimental results at this and other field sites. During the 2004 growing season at SoyFACE, elevated CO2 did not affect leaf chlorophyll content (Ainsworth et al., 2007). Additionally, a meta-analysis of 12 FACE experiments found no change in leaf chlorophyll content in plants grown under elevated CO2 (Ainsworth and Long, 2005).
Significant effects of elevated O3 on the chl. index were observed on only one day during the 2005 growing season (DOY 207; Fig. 3b), suggesting minimal effects of O3 on canopy chlorophyll content. Decreased leaf chlorophyll content caused by elevated O3 has not been observed at SoyFACE (Christ et al., 2006). Because decreases in leaf chlorophyll content have not previously been measured at SoyFACE and the chl. index is influenced by leaf area as well as canopy chlorophyll content, it is likely that the significant effect of O3 on DOY 207 was caused by a depression in LAI (Fig. 1), rather than a difference in chlorophyll content.
The present data demonstrate that PRI was sensitive to the effects of elevated CO2 and O3 on photosynthetic rates as well as variation in photosynthetic rates across the growing season. Elevated CO2 stimulated PRI by 14% on average, relative to ambient air (Fig. 3c). Similarly, elevated CO2 stimulated midday photosynthetic carbon assimilation (A) by 20% on average during the 2005 growing season (Leakey et al., 2009). This is consistent with previously observed stimulation of midday photosynthesis at SoyFACE; elevated CO2 stimulated A by 24% in 2002, 31% in 2003, and 23% in 2004 (Bernacchi et al., 2006). The results build on previous findings that the relationship between PRI and radiation use efficiency was conserved in a wide variety of tree saplings grown under elevated CO2 in enclosures (Guo and Trotter, 2006).
The sensitivity of PRI to the negative effects of elevated O3 on photosynthetic carbon assimilation at SoyFACE is particularly interesting because Morgan and colleagues (2004) found that elevated O3 did not affect light-saturated photosynthesis, carboxylation capacity of Rubisco, or maximum electron transport in the youngest fully expanded leaf during any stage of crop development at SoyFACE in 2002. These authors did, however, find that in soybean leaves formed during reproductive growth, elevated O3 resulted in faster loss of photosynthetic capacity as leaves aged, indicating accumulated damage from prolonged exposure to elevated O3 (Morgan et al., 2004). Similarly, Bernacchi and colleagues (2006) found no effect of elevated O3 on photosynthesis of young, recently expanded leaves during the 2002, 2003, or 2004 growing seasons at SoyFACE. These observations, coupled with the depression of PRI that was measured in elevated O3 at the beginning and end of the growing season, suggest that estimating photosynthetic CO2 uptake via canopy reflectance rather than gas exchange measurement of young leaves in the upper canopy may increase our power to detect loss of photosynthetic capacity caused by elevated O3, as this estimation incorporates the reflectance signal from young, upper canopy leaves as well as older leaves deeper in the canopy, which have experienced a larger cumulative dose of O3 and are more likely to exhibit O3 damage.
It was demonstrated previously that PRI was sensitive to elevated O3 at the leaf level in soybeans grown in open-top chambers (Campbell et al., 2007). Additionally, when white clover (Trifolium repens) was exposed to elevated O3 in growth chambers, canopy PRI decreased coincident with decreased photosynthetic carbon assimilation and decreased light use efficiency (Meroni et al., 2008). The present data build on previous work in controlled environments and demonstrate that canopy PRI was sensitive to the negative effects of elevated O3 on photosynthesis of field-grown soybean, and may give a more realistic estimate of canopy loss of photosynthetic capacity compared with leaf-level measurements of gas exchange.
In this study, a consistent trend for elevated CO2 to ameliorate the negative effects of elevated O3 on PRI was observed when plots were exposed to elevated CO2 plus elevated O3 in combination (Fig. 3c; Table 2). Averaged across the 2003–2004 growing seasons at SoyFACE, elevated CO2 alone increased photosynthesis by 27% while elevated CO2 in combination with elevated O3 increased photosynthesis by 19% (Bernacchi et al., 2006). Elevated CO2 often compensates for the negative effects of elevated O3 on productivity by decreasing stomatal conductance and reducing O3 diffusion into the leaf (Fiscus et al., 1997; Reid and Fiscus, 1998; Cardoso-Vilhena et al., 2004; Booker and Fiscus, 2005).
Gamon et al. (1997) demonstrated that PRI changed rapidly with irradiance levels, and that PRI showed a strong linear relationship with radiation use efficiency only at light levels >500 μmol m−2 s−1. It is possible that changes in irradiance may contribute to seasonal variation in PRI. However, because the present measurements were collected during full sunlight at midday, it is unlikely that irradiance levels affected differences in PRI between treatments.
Conclusions
The canopy reflectance indices utilized in this study have been used previously at the leaf or canopy levels, often in controlled environments, to estimate the effects of environmental perturbations, such as changing atmospheric composition, on canopy structure and function. This study demonstrates that the sensitivity of NIR/red and PRI and, to a lesser extent, chl. index to changes in LAI, photosynthetic carbon assimilation, and canopy chlorophyll content, respectively, were conserved when soybean was grown in a field setting under elevated CO2 and elevated O3. Additionally, CO2 and O3 effects on LAI and photosynthetic carbon assimilation estimated from remote sensing indices were similar to treatment effects reported in previous studies where LAI and gas exchange were measured directly at SoyFACE (Morgan et al., 2004; Dermody et al., 2006, 2008; Bernacchi et al., 2006; Leakey et al., 2009). Elevated CO2 increased leaf area and carbon assimilation and delayed senescence of the soybean canopy, as demonstrated by LAI, NIR/red, PRI, and the chl. index in this study. In contrast, elevated O3 decreased leaf area of the soybean canopy, as indicated by the effects of O3 on LAI and NIR/red. Elevated O3 also decreased PRI on two measurement dates, indicating reduced photosynthetic carbon assimilation. Projected increases in atmospheric CO2 and O3 concentrations in this century will probably have opposing effects on canopy structure, photosynthesis, and ultimately the productivity of the soybean agroecosystem. These data demonstrate that, although not as sensitive as direct measurements of physiological or canopy structural parameters, canopy reflectance indices show great promise for measuring the effects of increasing atmospheric CO2 and O3 on soybean canopies. With higher replication, which would allow greater statistical power, these indices may be useful for rapid screening of multiple cultivars of soybean for differential responsiveness to elevated CO2 or O3.
Acknowledgments
The authors thank Andrew Leakey and members of the Leakey laboratory for their thoughtful and constructive comments on an earlier version of this manuscript. We also thank Donald Bullock for advice on statistical analysis. SG was supported by the Undergraduate Mentoring in Environmental Biology Fellowship from the National Science Foundation. This research was supported in part by grants from the Office of Science (BER), US Department of Energy (no. DE-FG02-04ER63489), the National Research Initiative of USDA Cooperative State Research, Education and Extension Service (2002-02723), and the National Science Foundation (IBN 0236053). The SoyFACE experiment is supported by the Illinois Council for Food and Agricultural Research (C-FAR), Archer Daniels Midland Co., and USDA-ARS.
References
- Ainsworth EA, Davey PA, Bernacchi CJ, et al. A meta-analysis of elevated CO2 effects on soybean (Glycine max) physiology, growth and yield. Global Change Biology. 2002;8:695–709. [Google Scholar]
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Leakey ADB, Heady LE, Gibon Y, Stitt M, Schurr U. Does elevated atmospheric [CO2] alter diurnal C uptake and the balance of C and N metabolites in growing and fully expanded soybean leaves? Journal of Experimental Botany. 2007;58:579–591. doi: 10.1093/jxb/erl233. [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Leakey ADB, Heady LE, et al. Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant, Cell and Environment. 2006;29:2077–2090. doi: 10.1111/j.1365-3040.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Booker FL, Fiscus EL. The role of ozone flux and antioxidants in the suppression of ozone injury by elevated CO2 in soybean. Journal of Experimental Botany. 2005;56:2139–2151. doi: 10.1093/jxb/eri214. [DOI] [PubMed] [Google Scholar]
- Campbell PKE, Middleton EM, McMurtrey JE, Corp LA, Chappelle EW. Assessment of vegetation stress using reflectance or fluorescence measurements. Journal of Environmental Quality. 2007;36:832–845. doi: 10.2134/jeq2005.0396. [DOI] [PubMed] [Google Scholar]
- Cardoso-Vilhena J, Balaguer L, Eamus D, Ollerenshaw J, Barnes J. Mechanisms underlying the amelioration of O3 induced damage by elevated atmospheric concentrations of CO2. Journal of Experimental Botany. 2004;55:771–781. doi: 10.1093/jxb/erh080. [DOI] [PubMed] [Google Scholar]
- Christ MM, Ainsworth EA, Nelson R, Schurr U, Walter A. Anticipated yield loss in field-grown soybean under elevated ozone can be avoided at the expense of leaf growth during early reproductive growth stages in favourable environmental conditions. Journal of Experimental Botany. 2006;57:2267–2275. doi: 10.1093/jxb/erj199. [DOI] [PubMed] [Google Scholar]
- Daughtry CST, Walthall CL, Kim MS, de Colstoun EB, McMurtrey JE. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sensing of Environment. 2000;74:229–239. [Google Scholar]
- Dermody O, Long SP, DeLucia EH. How does elevated CO2 or ozone affect the leaf-area index of soybean when applied independently? New Phytologist. 2006;169:145–155. doi: 10.1111/j.1469-8137.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- Dermody O, Long SP, McConnaughay K, DeLucia EH. How do elevated CO2 and O3 affect the interception and utilization of radiation by a soybean canopy? Global Change Biology. 2008;14:556–564. [Google Scholar]
- Drake BG, Gonzalez-Meler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2. Annual Review of Plant Physiology. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Ferris R, Sabatti M, Miglietta F, Mills RF, Taylor G. Leaf area is stimulated in Populus by free air CO2 enrichment (POPFACE), through increased cell expansion and production. Plant, Cell and Environment. 2001;24:305–315. [Google Scholar]
- Filella I, Peñuelas J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. International Journal of Remote Sensing. 1994;15:1459–1470. [Google Scholar]
- Fiscus EL, Reid CD, Miller JE, Heagle AS. Elevated CO2 reduces O3 flux and O3 induced yield losses in soybeans: possible implications for elevated CO2 studies. Journal of Experimental Botany. 1997;48:307–313. [Google Scholar]
- Gamon JA, Field CB, Goulden ML, Griffin KL, Hartley AE, Joel G, Peñuelas J, Valentini R. Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecological Applications. 1995;5:28–41. [Google Scholar]
- Gamon JA, Serrano L, Surfus JS. The photochemical reflectance index: an optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia. 1997;112:492–501. doi: 10.1007/s004420050337. [DOI] [PubMed] [Google Scholar]
- Gamon JA, Surfus JS. Assessing leaf pigment content and activity with a reflectometer. New Phytologist. 1999;143:105–117. [Google Scholar]
- Gitelson A, Merzlyak MN. Spectral reflectance changes associated with autumn senescence of Aesculus hippocastanum L. and Acer platanoidesL. leaves: spectral features and relation to chlorophyll estimation. Journal of Plant Physiology. 1994;143:286–292. [Google Scholar]
- Gitelson AA, Viña A, Ciganda V, Rundquist DC, Arkebauer TJ. Remote estimation of canopy chlorophyll content in crops. Geophysical Research Letters. 2005;32 L08403. [Google Scholar]
- Guo J, Trotter CM. Estimating photosynthetic light-use efficiency using the photochemical reflectance index: the effects of short-term exposure to elevated CO2 and low temperature. International Journal of Remote Sensing. 2006;27:4677–4684. [Google Scholar]
- Guo J, Trotter CM, Newton PCD. Initial observations of increased requirements for light-energy dissipation in ryegrass (Lolium perenne) when source/sink ratios become high at a naturally grazed Free Air CO2 Enrichment (FACE) site. Functional Plant Biology. 2006;33:1045–1053. doi: 10.1071/FP06168. [DOI] [PubMed] [Google Scholar]
- Hirose T, Ackerly DD, Traw MB, Bazzaz FA. Effects of CO2 elevation on canopy development in the stands of two co-occurring annuals. Oecologia. 1996;108:215–223. doi: 10.1007/BF00334644. [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Xu F, Gillespie KM, McGrath JM, Ainsworth EA, Ort DR. Genomic basis for stimulated respiration by plants growing under elevated carbon dioxide. Proceedings of the National Academy of Sciences, USA. 2009;106:3597–3602. doi: 10.1073/pnas.0810955106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants FACE the future. Annual Review of Plant Biology. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Morgan PB. Global food insecurity: treatment of major food crops with elevated carbon dioxide or ozone under large-scale fully open-air conditions suggests recent models may have overestimated future yields. Philosophical Transactions of the Royal Society B: Biological Sciences. 2005;360:2011–2020. doi: 10.1098/rstb.2005.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni M, Rossini M, Picchi V, Panigada C, Cogliati S, Nali C, Colombo R. Assessing steady-state fluorescence and PRI from hyperspectral proximal sensing as early indicators of plant stress: the case of ozone exposure. Sensors. 2008;8:1740–1754. doi: 10.3390/s8031740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglietta F, Peressotti A, Vaccari FP, Zaldei A, deAngelis P, Scarascia-Mugnozza G. Free-air CO2 enrichment (FACE) of a poplar plantation: the POPFACE fumigation system. New Phytologist. 2001;150:465–476. [Google Scholar]
- Morgan PB, Ainsworth EA, Long SP. How does elevated ozone impact soybean? A meta-analysis of photosynthesis, growth and yield. Plant, Cell and Environment. 2003;26:1317–1328. [Google Scholar]
- Morgan PB, Bernacchi CJ, Ort DR, Long SP. An In vivo analysis of the effect of season-long open-air elevation of ozone to anticipated 2050 levels on photosynthesis in soybean. Plant Physiology. 2004;135:2348–2357. doi: 10.1104/pp.104.043968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy J, Lewin KF, Hendrey G, Hassinger R, Lamorte E. FACE facility CO2 concentration control and CO2 use in 1990 and 1991. Agricultural and Forest Meteorology. 1994;70:31–48. [Google Scholar]
- Panferov O, Knyazikhin Y, Myneni RB, Sazarzynski J, Engwald S, Schnitzler KG, Gravenhorst G. The role of canopy structure in the spectral variation of transmission and absorption of solar radiation in vegetation canopies. IEEE Transactions on Geoscience and Remote Sensing. 2001;39:241–253. [Google Scholar]
- Pearcy RW. The light environment and growth of C3 and C4 tree species in the understory of a Hawaiian forest. Oecologia. 1983;58:19–25. doi: 10.1007/BF00384537. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends in Plant Science. 1998;3:151–156. [Google Scholar]
- Peñuelas J, Filella I, Gamon JA. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytologist. 1995;131:291–296. [Google Scholar]
- Reid CD, Fiscus EL. Effects of elevated [CO2] and/or ozone on limitations to CO2 assimilation in soybean (Glycine max) Journal of Experimental Botany. 1998;49:885–895. [Google Scholar]
- Richardson AD, Duigan SP, Berlyn GP. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytologist. 2002;153:185–194. [Google Scholar]
- Sandermann JH, Ernst D, Heller W, Langebartels C. Ozone: an abiotic elicitor of plant defense reactions. Trends in Plant Science. 1998;3:47–50. [Google Scholar]