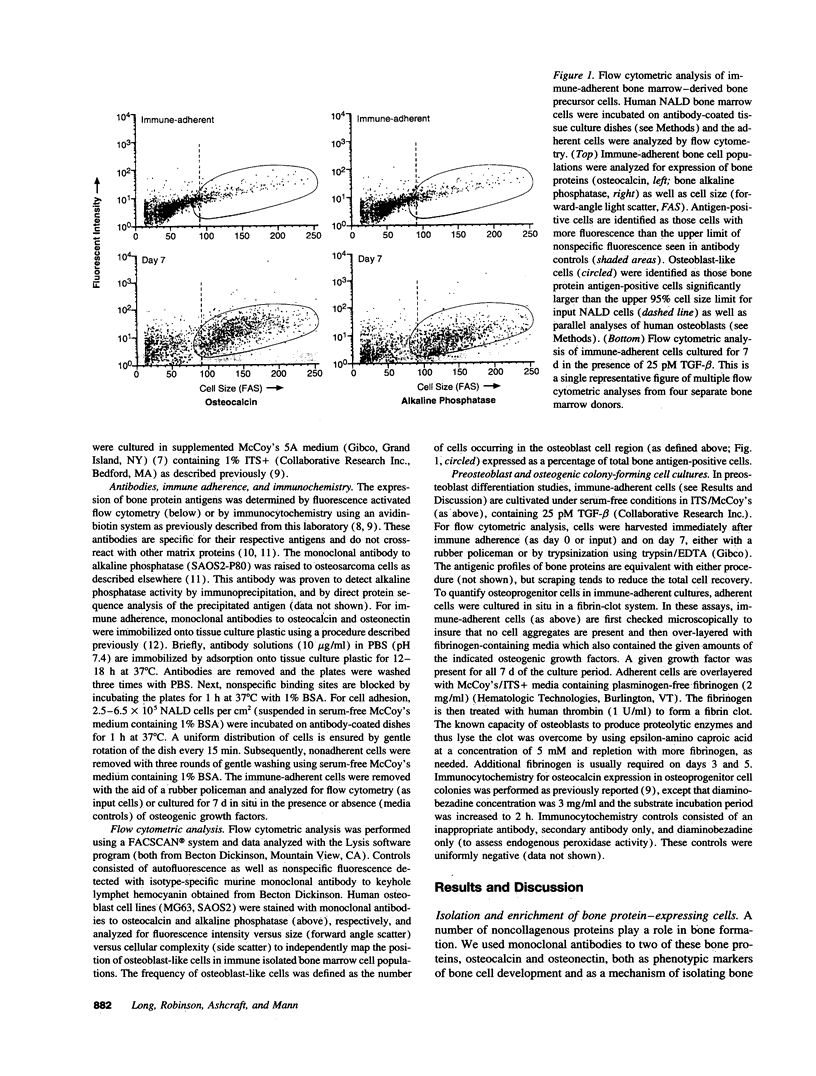
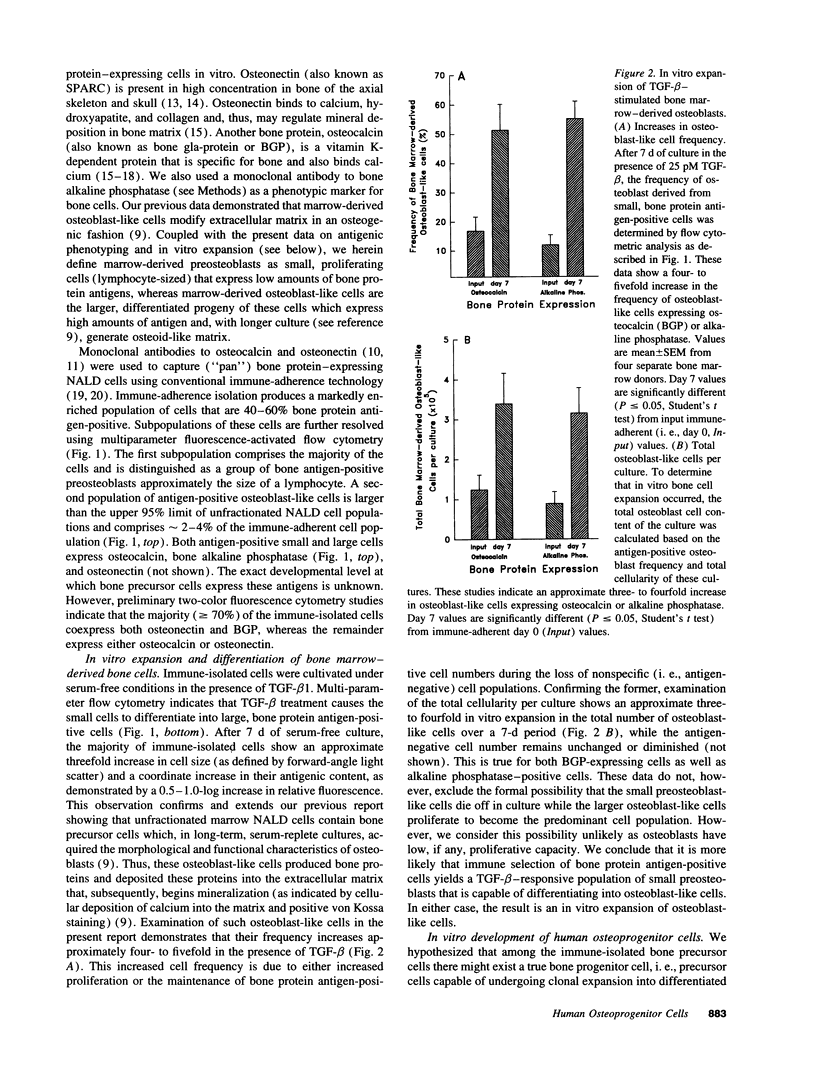
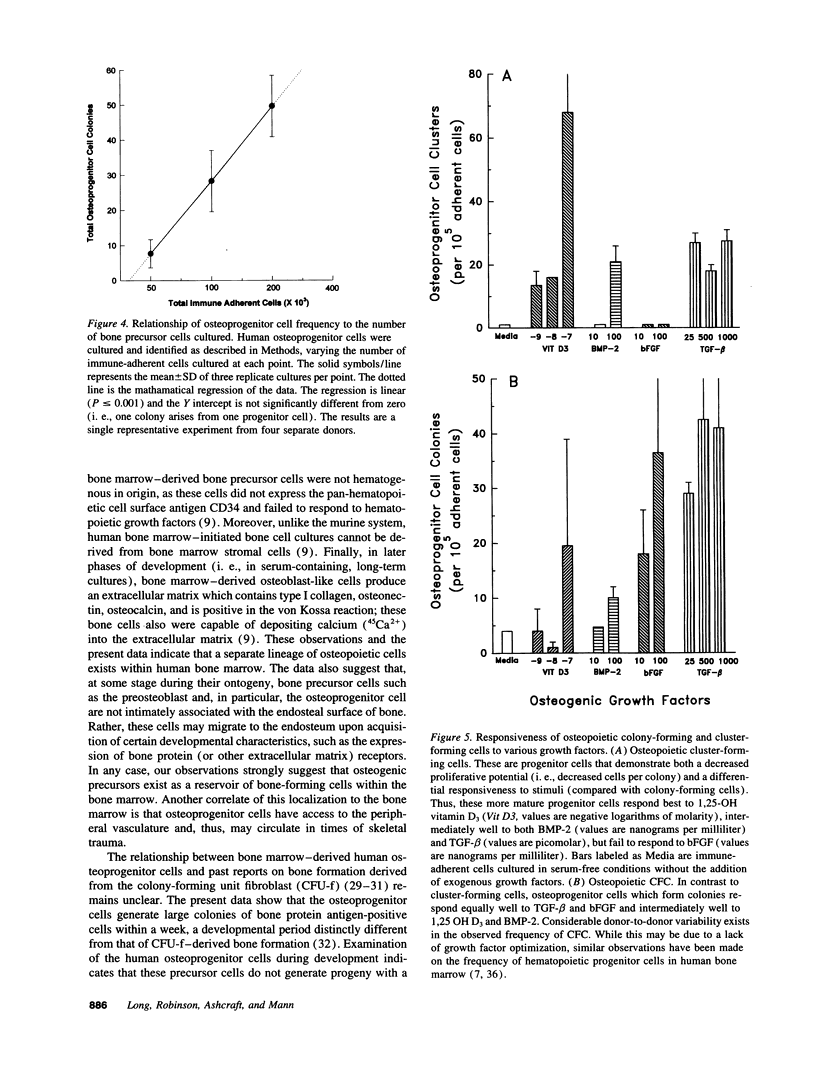
Abstract
Human bone marrow contains a distinct cell population that expresses bone proteins and responds to transforming growth factor beta 1 (TGF-beta), but not to hematopoietic growth factors (Long, M. W., J. L. Williams, and K. G. Mann. 1990. J. Clin. Invest. 86:1387-1395). We now report the isolation, characterization, and growth factor responsiveness of these precursors to human osteoblasts and the identification of a human osteoprogenitor cell. Immunological separation of human bone marrow nonadherent low-density (NALD) cells results in a marked enrichment of cells that express osteocalcin, osteonectin, and bone alkaline phosphatase. Flow cytometric analyses show that distinct cell subpopulations exist among these isolated cells. The majority of the bone antigen-positive cells are approximately the size of a lymphocyte, whereas other, less frequent antibody-separated subpopulations consist of osteoblast-like cells and osteoprogenitor cells. In serum-free cultures, TGF-beta stimulates the small, antigen-positive cells to become osteoblast-like, as these cells both increase in size, and express increased levels of osteocalcin and alkaline phosphatase. Antibody-separated cells also contain a separate population of clonal progenitor cells that form colonies of osteoblast-like cells when cultured in serum-free, semi-solid media. Two types of human osteoprogenitor cells are observed: a colony-forming cell (CFC) that generates several hundred bone antigen-positive cells, and a more mature cluster-forming cell that has a lesser proliferative potential and thus generates clusters of 20-50 antigen-positive cells. Osteopoietic colony-forming cells and cluster-forming cells have an obligate but differential requirement for osteogenic growth factors. The CFCs respond to TGF-beta, basic fibroblast growth factor (bFGF), bone morphogenic protein-2 (BMP-2), and 1, 25-dihydroxy vitamin D3 (1,25-OH D3). In contrast to the colony-forming cells, cluster-forming cells are regulated predominantly by 1,25-OH D3 and TGF-beta, but fail to respond to bFGF. We conclude that human bone marrow contains a nonhematogenous, heterogeneous population of bone precursor cells among which exists a population of proliferating osteoprogenitor cells. Further characterization of these bone precursor cell populations should yield important information on their role in osteogenesis in both health and disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash P., Loutit J. F., Townsend K. M. Osteoclasts derived from haematopoietic stem cells. Nature. 1980 Feb 14;283(5748):669–670. doi: 10.1038/283669a0. [DOI] [PubMed] [Google Scholar]
- Ashton B. A., Eaglesom C. C., Bab I., Owen M. E. Distribution of fibroblastic colony-forming cells in rabbit bone marrow and assay of their osteogenic potential by an in vivo diffusion chamber method. Calcif Tissue Int. 1984 Jan;36(1):83–86. doi: 10.1007/BF02405298. [DOI] [PubMed] [Google Scholar]
- Bab I., Ashton B. A., Gazit D., Marx G., Williamson M. C., Owen M. E. Kinetics and differentiation of marrow stromal cells in diffusion chambers in vivo. J Cell Sci. 1986 Aug;84:139–151. doi: 10.1242/jcs.84.1.139. [DOI] [PubMed] [Google Scholar]
- Briddell R. A., Hoffman R. Cytokine regulation of the human burst-forming unit-megakaryocyte. Blood. 1990 Aug 1;76(3):516–522. [PubMed] [Google Scholar]
- Coccia P. F., Krivit W., Cervenka J., Clawson C., Kersey J. H., Kim T. H., Nesbit M. E., Ramsay N. K., Warkentin P. I., Teitelbaum S. L. Successful bone-marrow transplantation for infantile malignant osteopetrosis. N Engl J Med. 1980 Mar 27;302(13):701–708. doi: 10.1056/NEJM198003273021301. [DOI] [PubMed] [Google Scholar]
- Emerson S. G., Yang Y. C., Clark S. C., Long M. W. Human recombinant granulocyte-macrophage colony stimulating factor and interleukin 3 have overlapping but distinct hematopoietic activities. J Clin Invest. 1988 Oct;82(4):1282–1287. doi: 10.1172/JCI113727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHMAN D. A., HAY E. D. Origin of osteoclasts from mononuclear leucocytes in regenerating newt limbs. Anat Rec. 1962 Aug;143:329–337. doi: 10.1002/ar.1091430402. [DOI] [PubMed] [Google Scholar]
- Falla N., Van Vlasselaer, Bierkens J., Borremans B., Schoeters G., Van Gorp U. Characterization of a 5-fluorouracil-enriched osteoprogenitor population of the murine bone marrow. Blood. 1993 Dec 15;82(12):3580–3591. [PubMed] [Google Scholar]
- Hauschka P. V., Lian J. B., Gallop P. M. Direct identification of the calcium-binding amino acid, gamma-carboxyglutamate, in mineralized tissue. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3925–3929. doi: 10.1073/pnas.72.10.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka P. V., Mavrakos A. E., Iafrati M. D., Doleman S. E., Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986 Sep 25;261(27):12665–12674. [PubMed] [Google Scholar]
- Holland P. W., Harper S. J., McVey J. H., Hogan B. L. In vivo expression of mRNA for the Ca++-binding protein SPARC (osteonectin) revealed by in situ hybridization. J Cell Biol. 1987 Jul;105(1):473–482. doi: 10.1083/jcb.105.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jotereau F. V., Le Douarin N. M. The development relationship between osteocytes and osteoclasts: a study using the quail-chick nuclear marker in endochondral ossification. Dev Biol. 1978 Apr;63(2):253–265. doi: 10.1016/0012-1606(78)90132-x. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. A Feulgen-positive nucleolus. Exp Cell Res. 1973 Mar 15;77(1):459–468. doi: 10.1016/0014-4827(73)90600-9. [DOI] [PubMed] [Google Scholar]
- Lian J. B., Stein G. S. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3(3):269–305. doi: 10.1177/10454411920030030501. [DOI] [PubMed] [Google Scholar]
- Long M. W. Blood cell cytoadhesion molecules. Exp Hematol. 1992 Mar;20(3):288–301. [PubMed] [Google Scholar]
- Long M. W., Briddell R., Walter A. W., Bruno E., Hoffman R. Human hematopoietic stem cell adherence to cytokines and matrix molecules. J Clin Invest. 1992 Jul;90(1):251–255. doi: 10.1172/JCI115844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Heffner C. H. Detection of human megakaryocyte antigens by solid-phase radioimmunoassay. Exp Hematol. 1988 Jan;16(1):62–70. [PubMed] [Google Scholar]
- Long M. W., Hutchinson R. J., Gragowski L. L., Heffner C. H., Emerson S. G. Synergistic regulation of human megakaryocyte development. J Clin Invest. 1988 Nov;82(5):1779–1786. doi: 10.1172/JCI113791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. W., Williams J. L., Mann K. G. Expression of human bone-related proteins in the hematopoietic microenvironment. J Clin Invest. 1990 Nov;86(5):1387–1395. doi: 10.1172/JCI114852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria E. A., Owen M. E., Friedenstein A. J., Morris J. F., Kuznetsow S. A. Bone formation in organ cultures of bone marrow. Cell Tissue Res. 1987 May;248(2):449–454. doi: 10.1007/BF00218212. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983 Aug;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Vogel R. Fibroblast growth factor enhances type beta 1 transforming growth factor gene expression in osteoblast-like cells. J Cell Biol. 1989 Nov;109(5):2529–2535. doi: 10.1083/jcb.109.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S., Wills A. J., Edwards D. R., Heath J. K., Hogan B. L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J Cell Biol. 1988 Feb;106(2):441–450. doi: 10.1083/jcb.106.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price P. A., Lothringer J. W., Baukol S. A., Reddi A. H. Developmental appearance of the vitamin K-dependent protein of bone during calcification. Analysis of mineralizing tissues in human, calf, and rat. J Biol Chem. 1981 Apr 25;256(8):3781–3784. [PubMed] [Google Scholar]
- Price P. A., Otsuka A. A., Poser J. W., Kristaponis J., Raman N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci U S A. 1976 May;73(5):1447–1451. doi: 10.1073/pnas.73.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Yoon K., Rodan G. A. Opposing effects of fibroblast growth factor and pertussis toxin on alkaline phosphatase, osteopontin, osteocalcin, and type I collagen mRNA levels in ROS 17/2.8 cells. J Biol Chem. 1989 Nov 25;264(33):19934–19941. [PubMed] [Google Scholar]
- Shull S., Tracy R. P., Mann K. G. Identification of a vitamin D-responsive protein on the surface of human osteosarcoma cells. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5405–5409. doi: 10.1073/pnas.86.14.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner D. D., Romberg R. W., Tracy R. P., Katzmann J. A., Riggs B. L., Mann K. G. Monoclonal antibodies to native noncollagenous bone-specific proteins. Proc Natl Acad Sci U S A. 1984 May;81(9):2868–2872. doi: 10.1073/pnas.81.9.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Termine J. D., Kleinman H. K., Whitson S. W., Conn K. M., McGarvey M. L., Martin G. R. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981 Oct;26(1 Pt 1):99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- Urist M. R., DeLange R. J., Finerman G. A. Bone cell differentiation and growth factors. Science. 1983 May 13;220(4598):680–686. doi: 10.1126/science.6403986. [DOI] [PubMed] [Google Scholar]
- Urist M. R., Sato K., Brownell A. G., Malinin T. I., Lietze A., Huo Y. K., Prolo D. J., Oklund S., Finerman G. A., DeLange R. J. Human bone morphogenetic protein (hBMP). Proc Soc Exp Biol Med. 1983 Jun;173(2):194–199. doi: 10.3181/00379727-173-41630. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wysocki L. J., Sato V. L. "Panning" for lymphocytes: a method for cell selection. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2844–2848. doi: 10.1073/pnas.75.6.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]