Abstract
Loss of bladder function is an important consequence of a spinal cord injury (SCI) but is rarely assessed in animal studies of SCI. Here, we use a simple outcome measure (volume of retained urine) to assess bladder dysfunction over time following moderate contusion injuries at 3 different thoracic levels (T1, T4, or T9) and complete crush injuries (T1 vs T9). The volume of urine retained in the bladder was measured daily for fourteen days post-injury by anesthetizing the animals with isoflurane, expressing the bladder, and weighing the urine. To compare bladder deficits with the degree of impairment of hindlimb motor function, locomotion was assessed using the BBB open field rating scale. Rats with contusions at T4 and T9 exhibited bladder impairments reflected by increased urine retention from 1-12 days post injury. In contrast, rats with contusions at T1 exhibited minimal deficits (smaller volumes of retained urine). Lesion size and overall functional impairment was comparable between groups based on quantitative assessments of lesion area at the epicenter and BBB locomotor scores. Moreover, a sector analysis of sparing of different portions of the white matter revealed no differences in sparing of different funiculi between the groups. Injections of Fluorogold into lumbar segments led to retrograde labeling of a larger number of neurons in the pontine micturition center (PMC) following T1 injury when compared to T4 or T9. Thus, moderate contusion lesions at T1 spare a critical descending pathway able to mediate at least reflex voiding in rats.
Keywords: Spinal cord injury, Bladder, Contusion, Crush, Fluorogold
Introduction
The dual functions of the urinary bladder to store and periodically eliminate urine (de Groat and Yoshimura, 2006) are mediated by pathways that span the neuraxis. The circuitry involved includes afferent and efferent pathways in the periphery that include elements of the viscerosensory, sympathetic, parasympathetic, and somatic motor systems, local circuitry in the lumbosacral spinal cord, ascending projections to the brainstem, and descending pathways back down to the lumbosacral spinal cord (de Groat and Yoshimura, 2006; Shefchyk, 2002; Sugaya et al., 2005). Voluntary voiding also involves pathways from the cortex to the brainstem, although these are less well-defined than the pathways to and from the brainstem and spinal cord.
Depending on the level and severity, spinal cord injuries can disrupt either the ascending or descending tracts or the local circuitry at the segmental levels that are important for bladder function. In humans, spinal cord injuries (SCI) in the lumbosacral region disrupt local reflex circuitry causing bladder areflexia (Abdel-Azim et al., 1991; Kaplan et al., 1991). Lesions above the level of the lumbosacral spinal cord disrupt ascending and descending pathways causing characteristic symptoms that evolve over time. At early post-lesion intervals, reflex contraction of the bladder detrusor muscle is impaired. The result of this is urinary retention which, left untreated, can be life-threatening (Grundy and Russell, 1986). Over time, changes occur in circuitry mediating bladder reflexes that lead to other functional alterations including detrusor sphincter dyssynergia, in which bladder contractions occur at the same time that lack of detrusor activation blocks urine outflow (de Groat et al., 1990). This produces pathological intra-bladder pressures that induce bladder hypertrophy and can damage the urinary tract. Under the best of circumstances, the bladder of an individual with a spinal cord injury rarely empties completely creating conditions that foster the development of urinary tract infections (UTIs). Indeed, prior to the development of penicillin, UTIs greatly shortened the life expectancy of individuals with SCI. For this reason, individuals with SCI regard the recovery of bladder function as one of their highest priorities (Anderson, 2004; Estores, 2003; Rosenzweig and McDonald, 2004).
Experimental models investigating the effects of both partial and complete SCI at the thoracic level have documented the same phenomenon of urinary retention in both rats and mice (Engesser-Cesar et al., 2005; Keirstead et al., 2005; Pikov and Wrathall, 2001; Zinck et al., 2007). Interestingly, however, recent studies reporting on the development of new models of SCI at the cervical level in rodents have reported minimal if any bladder deficits (Anderson et al., 2007). This is in contrast to the situation in humans with incomplete cervical injuries where bladder dysfunction is a major concern (Kaplan et al., 1991; Weld and Dmochowski, 2000).
Although these previous studies hint at possible differences in the extent of bladder function following cervical vs. thoracic level injuries, there have been no direct comparisons using similar lesion models and methods of bladder assessment. Accordingly, the primary goal of the present study was to directly assess whether there were differences in bladder dysfunction following comparable partial contusion injuries at different spinal levels. We report here that histologically similar lesions at T4 or T9 produce impairments in bladder function whereas lesions at T1 produce minimal deficits.
One explanation for the relative lack of bladder dysfunction following contusion lesions at the cervical or high thoracic level is that the lesion spares different white matter regions that contain projections important for bladder control. Thus, the second goal of the present study was to assess the degree sparing of different portions of the white matter following lesions at T4 and T9 that produce substantial impairment of bladder function, vs. lesions at T1 that produce minimal impairment. In addition, we use retrograde labeling techniques to assess the degree of sparing of projections from the pontine micturition center following lesions at different levels to assess whether sparing of descending projections was related to sparing of bladder function.
Materials and Methods
Experimental groups
Adult female Sprague-Dawley rats weighing 190-230 grams were used. Rats were group-housed (five per cage) in a room with controlled temperature, humidity, and light cycle. Rats had access to food and water ad libitum. All animals were handled for one week prior to injury.
In six separate experiments, a total of 81 rats received a spinal cord injury at one of three different thoracic levels: T1, T4, or T9. In the first three experiments (labeled as experiments 1, 2, and 3), a total of 30 rats received moderate contusion injuries at T1, T4, or T9. A follow-up experiment with 16 rats (experiment 4) then repeated a direct comparison between groups with contusion injuries at T1 vs. T4 that were run at the same time (8 rats per group). An additional experiment with 28 rats (experiment 5) directly compared bladder function following lesions at T1, T4, or T9, and then injected the retrograde tracer Fluorogold into the lumbar enlargement (T12) at day 15 after injury (7 rats per group) to retrogradely label any surviving pathways from the bladder control centers in the brainstem. This experiment included a group of seven control rats that received no injury, but underwent identical animal care, behavioral moinitoring, and Fluorogold injection. Finally, an experiment with 14 rats (experiment 6) directly compared groups of rats that received crush injuries at T1 and T9. These rats also received Fluorogold injections into the lumbar enlargement 15 days after injury (7 rats per group).
Surgical Procedures: Contusion injury
Rats were anesthetized with an intraperitoneal injection of Ketamine and Xylazine (100 mg/kg and 10 mg/kg, respectively). Laminectomies were performed at T1, T4, or T9. Moderate contusion injuries were produced using the Infinite Horizon impact device (Precision Systems & Instrumentation [PSI], Lexington, KY). Contusion force was 200 kDynes; dwell time was 0 seconds. The average displacement was 1252 μm; the average velocity was 119 mm/second.
Postoperatively, rats were housed in cages with Alpha-Dri bedding and were placed on a water-jacketed heating pad at 37 °C for the first night following injury. Bladders were expressed every 12 hours following injury until animals were killed humanely 2 weeks later. Additionally, rats received subcutaneous injections of Baytril (0.5 mg/kg), Buprenorphine (0.01 mg/kg), and lactated ringers (10 ml) following the morning bladder expression. The rats were maintained on the Baytril and buprenorphine for the first 10 days post injury (dpi), and received saline for the entire 14 day survival interval.
Surgical Procedures: Crush Injury
Rats were anesthetized as above and received laminectomies at either T1 or T9. Injuries were produced by crushing the spinal cord with #5 Dumont forceps for 3 seconds. Post-operative care was as described above.
Retrograde labeling
The 42 rats from the final two experiments were anesthetized as above and received laminectomies at T12. Fluorogold (4% w/v in saline, Fluorochrome, LLC) was injected into the exposed spinal cord with a 10 μl Hamilton syringe with a pulled glass tip. Injections were delivered stereotactically at three sites in the spinal cord: at midline (depth: 0.6 mm) and 1.1 mm lateral to midline (depth 1.1 mm), bilaterally. All animals received 0.3 μl per site resulting in a total volume of 0.9 μl injected into the spinal cord.
Assessment of bladder function
Urine was collected and weighed during the morning bladder expression. For this purpose, each rat was individually anesthetized with isoflurane gas (1.5 L/min at a concentration of 3.0% in oxygen) for 5 minutes. Previous studies have demonstrated that the amount of urine manually expressed under anesthesia is more highly correlated with the actual amount of urine in the bladder, as detected using ultrasonography, than when bladders are expressed in un-anesthetized rats (Keirstead et al., 2005).
The amount of retained urine was determined by collecting the expressed urine and weighing it. A grid was placed under the animal in the anesthesia induction chamber to collect urine that was voided while the rat was being anesthetized (this was usually a small amount). The urine collected during induction of anesthesia was combined with the urine collected during manual expression. Rats recovered from anesthesia within 2 minutes following bladder expression.
Assessment of hindlimb locomotor function
All rats were assessed with the Basso, Beattie, Bresnahan Locomotor Rating Scale (BBB) (Basso et al., 1995) on days 2 and 13 after injury in order to relate bladder dysfunction with locomotor ability. The BBB is a 21-point scale designed to assess hind limb locomotor recovery following thoracic spinal cord injury. A BBB score of 0 indicates no hindlimb movement. A BBB score of 1 through 8 indicates joint movement, but no weight support. A BBB score of 9 through 20 indicates an ability to support weight and use the limb for locomotion but with some degree of abnormality. A BBB score of 21 corresponds to the locomotion of a normal rat. Bladders were expressed 10 minutes prior to locomotor testing.
Histology
At 14 days post-injury, rats were killed humanely with an overdose of sodium pentobarbital and were transcardially perfused with 4% paraformaldehyde. Spinal cords were removed from the vertebral column and post-fixed overnight at 4°C before being cryoprotected in 27% sucrose at room temperature. A 20 mm block of spinal cord centered at the injury site was embedded in Tissue-Tek OCT compound, frozen, and stored at −80 °C until sectioning.
Sections of spinal cord were cut transversely at 30 μm on a cryostat. Sections were thaw-mounted directly onto slides that were divided into 5 sets in which each set contained a 1-in-5 series of serial sections. The distance between each consecutive section in a given set was 150 μm. One set of slides was stained with Hematoxylin & Eosin (H & E).
Rats that received Fluorogold were killed humanely 7 days following Fluorogold injections with an overdose of sodium pentobarbital and were transcardially perfused with 4% paraformaldehyde. Their brains were dissected out and post-fixed overnight at 4°C before being cryoprotected in 27% sucrose at room temperature. Brainstems were embedded in OCT, frozen, and stored at −80 °C until sectioning. Spinal cords were dissected and sectioned as above. Sections of the brainstem were cut transversely at 30μm on a cryostat and serial sections were thaw-mounted directly onto slides.
Assessment of lesion size and tissue sparing
Images of H&E stained cross sections were captured on an Olympus AX-80 microscope using MagnaFire SP 2.1B software. Both the total cross-sectional area of the spinal cord and the area of the lesion were outlined and calculated using ImageJ software (Rasband, 1997-2007). In both cases, the region of interest was outlined three consecutive times. The values from the three measurements were then averaged. The amount of spared tissue in each section was calculated as the difference between the total spinal cord area and the lesion area in a given section. The epicenter of the lesion is defined as the section in which the percentage of spared tissue was the least.
A sector analysis was performed on each epicenter image by superimposing a template that divided the spinal cord into 6 regions: dorsal column, ventral column, dorsolateral funiculi (2), and ventrolateral funiculi (2). The total spinal cord area and the lesioned spinal cord area were outlined and calculated as above. The percentage of the total spinal cord area occupied by the lesion was determined for each of the six regions separately.
The rostral and caudal borders of the lesion were defined as the last sections in the series that contain abnormal tissue in either direction along the spinal cord. The longitudinal extent of the lesion was calculated by determining the distance from the rostral-most and caudal-most section with abnormal histology.
Assessment of Fluorogold labeling
In order to count retrogradely-labeled neurons in the pontine micturition center (PMC), every section was collected and divided into 5 sets. Sections through the brainstem were visualized with a filter for DAPI excitation (360-370 nm). Images were captured on an Olympus AX-80 microscope using MagnaFire SP 2.1B software. The PMC is located in the pons just ventral to the fourth ventricle, subjacent to the locus coeruleus (Paxinos and Watson, 1998). As such, a 1 mm × 1 mm “sampling box” was placed 1.0 mm lateral to the center of the fourth ventricle and between 0.5 and 1.0 mm ventral to the lateral-most edge of the fourth ventricle. All Fluorogold labeled cell bodies within the sampling box were counted in each section on both sides of the brain. The rostral border of the PMC was determined by the absence of the Sylvian aqueduct and appearance of the 4th ventricle. The caudal border of the PMC was determined by the 4th ventricle reaching ventrally into the medial longitudinal fasciculus. A physical dissector was used by employing Adobe Photoshop software to overlay consecutive sections, to obtain an unbiased estimate of cell numbers.
Statistical analysis
Data were analyzed using SPSS 12.0 for Windows. One-way ANOVA or repeated measures ANOVA were the primary tests, using a p < 0.05 significance level. Throughout the text, figures, and tables, the mean value ± SEM notations are used in describing the results.
Results
Effect of injury level on bladder function: experiments 1-3
As reported in previous studies, rats with contusion injuries at T9 retained significant amounts of urine as documented by high volumes of expressible urine (Figure 1a). Between 3-5 days post injury, the amount of expressible urine decreased somewhat, indicating a partial recovery of bladder function reaching a plateau at about 7 days post-injury that was still substantially higher than uninjured controls. Rats with injuries at T4 exhibited the same sort of urine retention as rats with injuries at T9. In contrast, the rats with injuries at T1 demonstrated almost no urine retention even at the earliest stages following the injury. The amount of expressible urine was negligible beginning on day 2 and remained so for the duration of the study, significantly different from the T9 group (Figure 1a). Statistical analysis by ANOVA revealed that there were no significant differences between the values from the T4 and T9 groups, but both differed from the T1 group [T1 vs. T4 (p<0.001), T1 vs. T9 (p<0.01), T4 vs. T9 (p>0.1)].
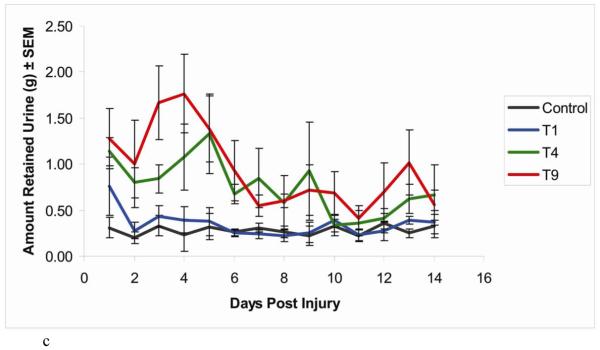
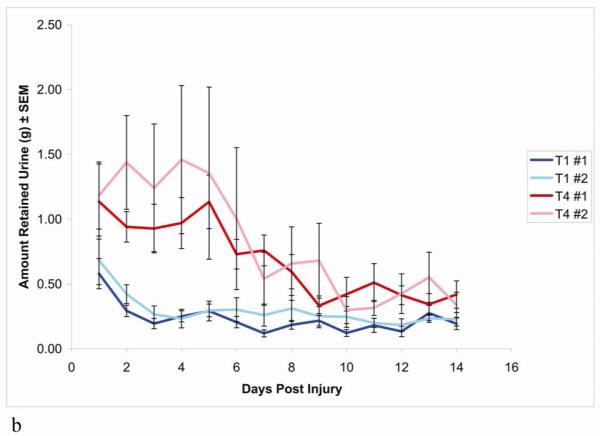
Figure 1.
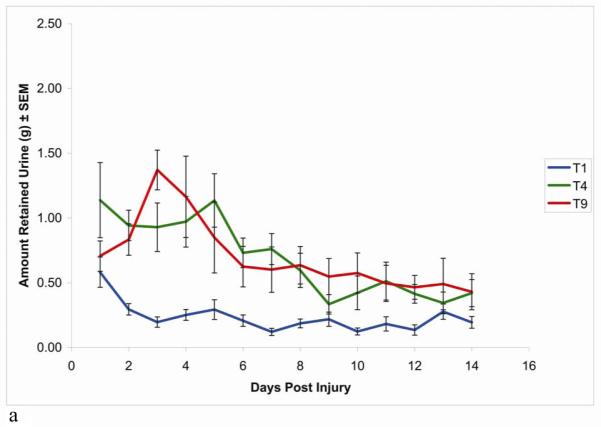
Time course of recovery of spontaneous voiding following SCI at T1, T4, or T9. Urinary bladders were expressed every 12 hours; the urine from the morning expression was then collected and weighed. All animals had progressively less urine with time, reaching a plateau by 7 days post injury. (a) Compared with T4 and T9 SCI animals, the T1 SCI animals had little to no expressible urine. n = 10 rats in each group.
(b) Compared with both groups of T4 SCI animals, the two groups of T1 SCI animals had little to no expressible urine. n = 10 rats each in groups (T1 #1 & T4 #1), n = 8 rats each in second groups (T1 #2 & T4 #2).
(c) Compared with T4 and T9 SCI animals, the control and T1 SCI animals had little to no expressible urine. n = 7 rats in each group.
Data are expressed as mean ± SEM
Effect of injury level on bladder function: experiments 4-5
When animals from the T1 and T4 injury groups were compared directly in experiment 4, the observed bladder impairment was consistent with the data from groups 1-3 of T1 and T4 injured animals (Figure 1b). ANOVA showed no difference between the two T1 groups or the two T4 groups, whereas the difference between T1 and T4 was again significant [T1 vs. T1 (p>0.1), T4 vs. T4 (p>0.1), T1 vs. T4 (p<0.001)].
Rats from the fifth experiment showed the same pattern of bladder impairment as in the first three experiments (Figure 1c). Rats that received contusions at T4 or T9 again had bladder impairment whereas those with T1 injuries demonstrated almost no urine retention, comparable to control uninjured rats [T1 vs. T4 (p<0.001), T1 vs. T9 (p<0.001), T4 vs. T9 (p>0.1), Control vs. T1 (p>0.1), Control vs. T4 (p<0.001), Control vs. T9 (p<0.001)].
Assessment of bladder function following complete crush injuries at different levels: experiment 6
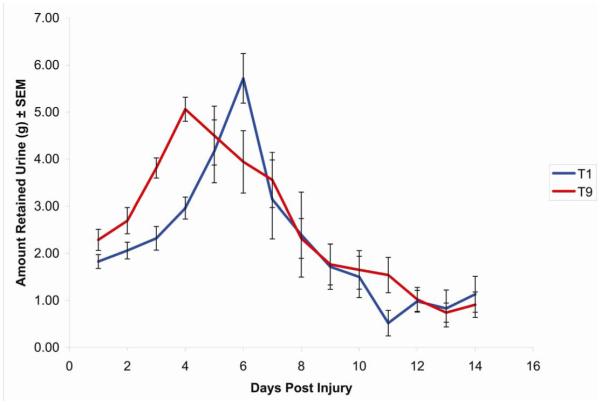
One possible explanation of the preservation of bladder function after lesions at T1 is that circuitry present at lower levels is sufficient to mediate at least reflex bladder emptying. In this case, lesions at T1 would be expected to always produce less severe deficits in bladder function regardless of lesion severity. Alternatively, if contusion injuries at T1 spare more descending axons than lesions at lower levels, then complete crush injuries at different levels, which damage all descending axons, should produce comparable deficits. To assess this, Experiment 6 assessed bladder function after complete crush injuries at T1 vs. T9. Crush injuries at both levels produced more severe impairments in bladder function than following contusion injuries (Figure 2). The maximal amount of retained urine following a T9 contusion was 1.37 ± 0.15 grams, whereas it was 5.06 ± 0.25 grams following a T9 crush. This demonstrates that the outcome following a complete injury to the lower thoracic spinal cord, where there is no connectivity to the PMC, is far worse than the outcome following an incomplete injury, where at least the opportunity for some connectivity to the PMC still exists. Moreover, the deficits following lesions at T1 were as severe as those following lesions at T9, although peak urine retention was delayed by about 2 days. Even at 12-14 days post-injury, when reflex bladder emptying had recovered, retention was higher than following contusion injuries (an average of 0.94 ± 0.29 ml in the case of crush injuries vs. about 0.33 ± 0.10 ml in the case of contusion injuries).
Figure 2.
ime course of recovery of spontaneous voiding following spinal crush injury at T1 or T9. Urinary bladders were expressed every 12 hours; the urine from the morning expression was then collected and weighed.
Data are expressed as mean ± SEM, n = 7 rats in each group.
Severity of spinal cord injury
One possible explanation for the differences in bladder function following contusion injuries at T1 vs. lower thoracic levels is that the severity of the lesion is different. We assessed this in three ways: 1) by comparing the actual force delivered by the IH device during the production of the lesion; 2) by assessing hindlimb locomotor function in the different groups; and 3) by measuring lesion size.
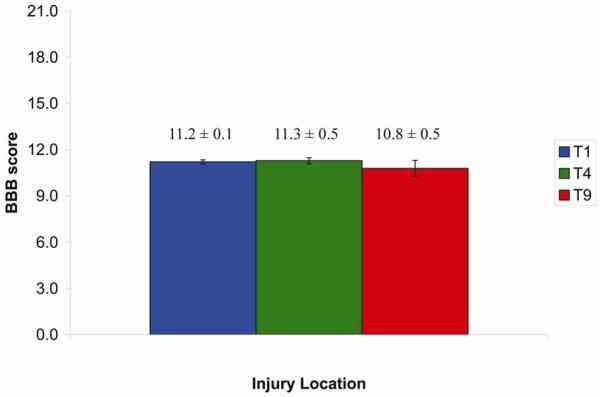
The IH impactor allows the user to select the desired force, in kilodynes, and also calculates the “actual” force that was delivered. Table 1 reveals that there was no significant difference (p > 0.1) in actual force delivered between treatment groups in any of the experiments. Indeed, mean forces were almost identical. Similarly, assessment of hindlimb motor function with the BBB locomotor scale also revealed no significant differences between the groups in any of the experiments (Figure 3). Indeed, mean scores were almost identical.
Table 1. Degree of contusive SCI and resulting bladder weights from to all three thoracic injury locations.
Amount of contusive force (kilodynes) that was delivered to the spinal cord was recorded from the force sensor immediately following contusion.
The bladder was dissected out immediately following animal sacrifice and was weighed after blot-drying.
Data are expressed as mean ± SEM, n = 10 rats in each group.
| SCI group | T1 (n=10) | T4 (n=10) | T9 (n=10) |
|---|---|---|---|
| Injury Force (kd) | 203.0 ± 1.4 | 206.7 ± 2.4 | 203.7 ± 1.7 |
| Bladder weight (g) | 0.174 ± 0.014 | 0.252 ± 0.030 | 0.266 ± 0.025 |
Figure 3.
Consistent degree of SCI was produced by IH Impactor at T1, T4, and T9 thoracic levels. The hindlimb behavioral test done 13 days following contusion injury indicates similar level of somatic sensorimotor recovery as quantified by BBB test.
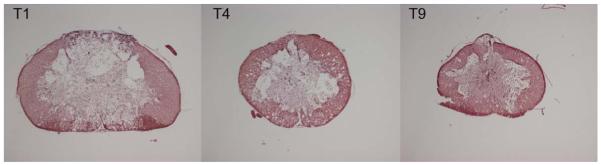
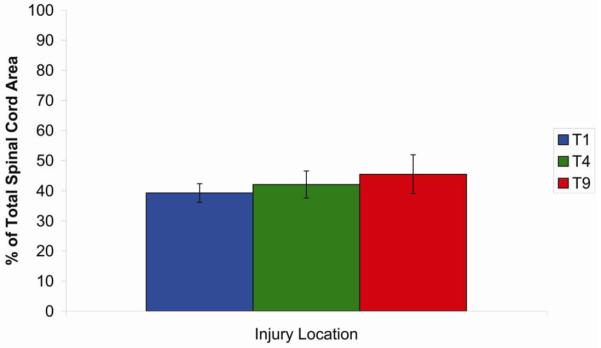
To assess lesion size, cross-sections from each of the groups in experiments 1-3 were stained for H & E (Figure 4). The percentage of spared tissue at the lesion epicenter of each animal was calculated and averaged across groups (Figure 5). There was no significant difference (p > 0.1) between treatment groups for either analysis. In all groups, percent sparing was approximately 40%. Together these analyses fail to support the hypothesis that differences in bladder function are due to differences in overall lesion severity.
Figure 4.
Epicenter of SCI following moderate contusion injury at T1, T4, or T9.
Photomicrographs of representative sections through the lesion epicenter from the three SCI groups T1, T4, or T9, stained with Hematoxylin & Eosin.
Figure 5.
Proportion of spared tissue at the epicenter following moderate contusion injury at T1, T4, or T9.
The percentage of spared tissue at the lesion epicenter of each animal was calculatednd averaged across groups
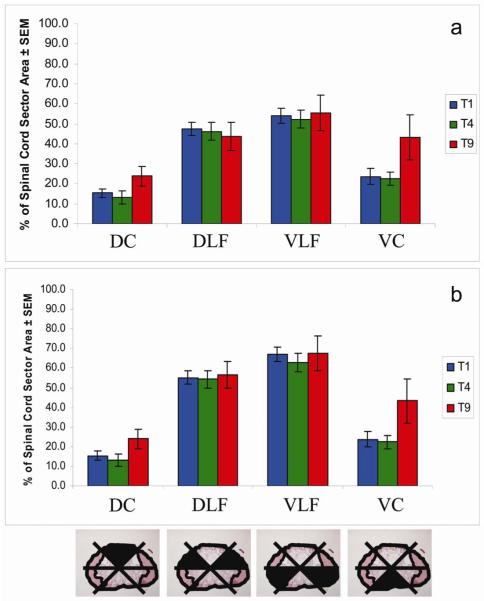
Another possible explanation for the differences in bladder function following lesions at T1 vs. lower thoracic levels is that the lesion at T1 may spare a critical portion of the white matter that contains descending axons that mediate bladder function and that lesions at lower thoracic levels damage these axons. Descending tracts are found in characteristic locations in the white matter, and so this hypothesis would predict differences in the degree of sparing of different white matter regions. To assess whether the lesions at different levels spared different portions of the white matter, we assessed sparing of different sectors of the white matter as described in the Methods. Figure 6a shows the percentage of sparing of each spinal cord sector separately, with the values for the dorsolateral and ventrolateral funiculi on the two sides averaged together. The sector containing the dorsal column was the most severely damaged in all three groups, averaging between 10 and 25 % sparing. The sector containing the ventral columns was the next most seriously damaged, averaging 25% sparing in rats injured at T1 or T4. The average amount of tissue sparing in the ventral columns was closer to 45% in rats injured at T9. The greatest amount of sparing existed in the sectors containing the dorsolateral and ventrolateral funiculi, averaging between 45 and 56% spared. There was no statistically significant difference between the groups of experiments 1-3 in the degree of sparing of any of the sectors.
Figure 6.
Sector analysis of proportion of spared tissue at the epicenter, following moderate contusion injury at T1, T4, or T9.
The spinal cord was divided into sectors at the epicenter. The amount of spared area was converted into a percentage of the spared area for each sector. (a) The values for the dorsolateral funiculi and ventrolateral funiculi on the two sides were averaged together.
(b) The value for the individual dorsolateral funiculus and ventrolateral funiculus that had the greatest degree of sparing was plotted.
Central pathways that control bladder function are bilateral; thus unilateral preservation of critical pathways could be sufficient to maintain bladder function. Accordingly, we also plotted the data from the sector analysis using the values from the side with the greatest degree of sparing (Figure 6b). The values for the dorsal and ventral column sectors remained the same, but the values for the both the dorsolateral and ventrolateral funiculi sectors increased by 8 to 14%. Again, statistical analysis revealed no significant difference between lesions at different levels.
Retrograde labeling of neurons in the pontine micturition center
Rats from the final two experiments (5 and 6) received spinal injections of Fluorogold at the 12th thoracic vertebra in order to detect neurons in the brainstem whose connectivity to the sacral micturition center remained uninterrupted following thoracic spinal cord injury at T1, T4, or T9. The PMC is an ovoid cluster of neurons ventromedial to the locus coeruleus that extends for approximately 750 μm in the rostro-caudal axis. Although the PMC is a bilateral structure, some animals had asymmetrical labeling. In those rats with asymmetrical labeling, there was no consistency as to which side had the larger number of labeled neurons.
When retrogradely labeled, the cell bodies of the pontine micturition center are filled with Fluorogold. To quantify the number of retrogradely labeled neurons in the PMC, a sampling box whose borders extended slightly beyond those of the PMC was overlaid bilaterally on all figures to ensure that all labeled cells from the PMC were counted. The number of labeled cells in the left and right sides of the PMC were counted individually and then summed.
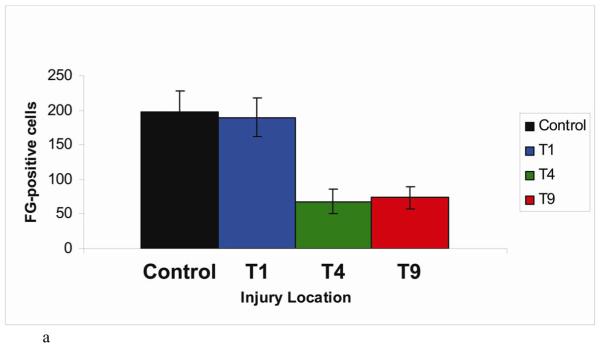
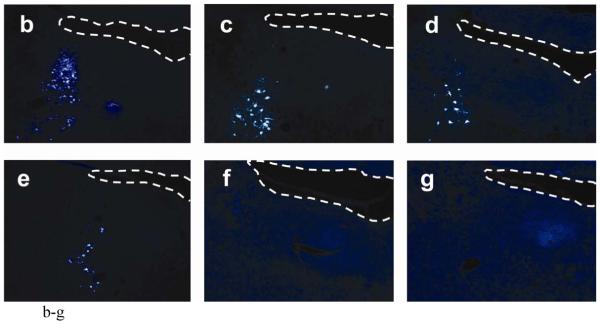
In experiment 5, the number of Fluorogold-labeled cells in the pontine micturition center following T1 contusion was similar to that seen in uninjured control animals. Fewer retrogradely labeled neurons were seen in rats with T4 or T9 contusions (T1: 190 ± 27 cells, T4: 68 ± 17 cells, T9: 73 ± 15 cells) (Figure 7a). Representative sections from each injury group are shown (Figure 7b-e). The pattern of Fluorogold labeling in both control and T1 contusion animals is very similar, with labeled cells seen up to 2 mm ventral to the fourth ventricle, but concentrated within the sampling box. The pattern of Fluorogold labeling following T4 and T9 contusions however was much more sparse, with labeled cells seen almost exclusively within the sampling box and at a much lower density.
Figure 7.
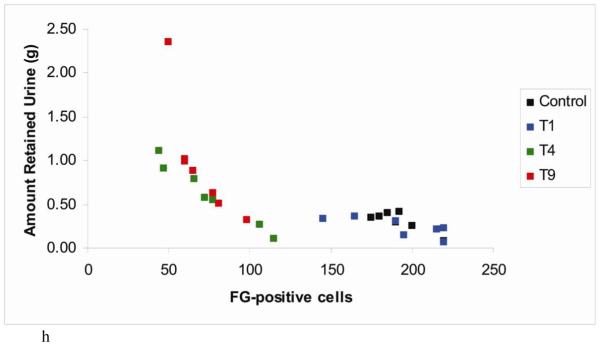
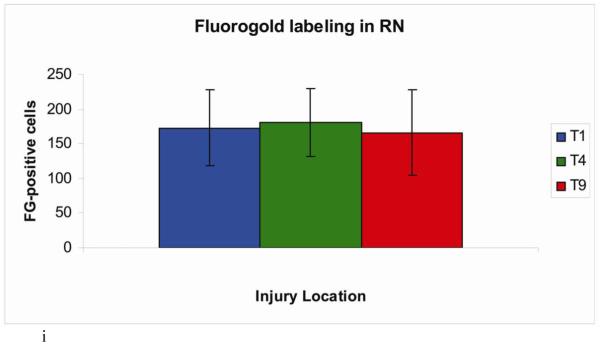
Demonstration of retrograde labeling in the pontine micturition center following SCI. (a) The number of Fluorogold positive cells in the PMC after injury. (b-e) Representative images of FG-labeled cells in a control animal and following contusion injury at T1, T4, or T9. (f-g) Representative images of FG-labeled cells following crush injury at T1 or T9. (h) Correlation plot showing number of FG-positive cells vs. amount of retained urine on the day of FG injection. (i) The number of Fluorogold positive cells in the red nucleus after contusion injury.
Note: Although FG counts were bilateral, only one side of PMC is shown in figure.
Following complete crush injuries at either T1 or T9, injections of FG into the lumbar enlargement did not lead to retrograde labeling of cells in the pontine micturition center or anywhere else in the brainstem (Figure 7f-g).
It was of interest to determine whether individual differences in the extent of bladder impairment were related to the degree of sparing of projections from the PMC. To assess this, we plotted the relationship between the amount of retained urine and the number of retrogradely-labeled neurons in the PMC. The amount of retained urine from each individual animal 14 days following injury (one day prior to Fluorogold injection) was plotted because it is the most representative of the connectivity at the time of Fluorogold injection. The resulting scatter plot revealed an inverse relationship in rats with T4 and T9 lesions (Figure 7h); the rats with the greatest amount of retained urine (indicative of worse bladder impairment) had the fewest FG labeled cells in the PMC. In rats with T1 lesions, the relationship was flat as would be expected because urine retention in these rats was comparable to control uninjured rats. These results suggest that the degree of bladder impairment/sparing following lesions is directly related to the degree of damage/sparing of descending projections from the PMC.
The fact that hindlimb locomotor function was similarly impaired following lesions at the 3 levels suggests that other descending systems are not differentially affected. Consistent with this, counts of retrogradely-labeled neurons in the red nucleus were similar across groups (T1: 173 ± 55 cells, T4: 181 ± 49 cells, T9: 166 ± 62 cells) (Figure 7i). Taken together, our results indicate that lesions at T1 vs. lower levels differentially spare descending projections that are critical for bladder function although other pathways are affected to a similar degree.
Discussion
Our results demonstrate that moderate contusion injuries at T4 or T9 disrupt bladder function of rats, whereas similar lesions at T1 do not. These results indicate that lesions at T1, but not T4 or below, spare circuitry that is sufficient to mediate at least reflex bladder function. The differences in bladder function are not due to differences in lesion size or to differential sparing of different portions of the white matter. Nevertheless, retrograde tracing studies reveal that lesions at T1 spare descending projections from the brainstem pontine micturition center to lumbosacral levels to a greater extent than lesions at T4 or T9. These results suggest that the differential consequences of contusion injuries at different levels are due to differential sparing of descending pathways that mediate bladder control. We cannot exclude the possibility, however, that sparing of function is due to preservation of a circuit below T1 that is sufficient to mediate reflex bladder function. In what follows, we consider these two possibilities in more detail.
Differences in bladder function after lesions at different levels are not due to differences in lesion size or white matter sparing
Lesions at all levels of the spinal cord could disrupt ascending pathways carrying sensory information regarding bladder distention and descending pathways from the PMC that mediate bladder emptying. In addition, it is possible that lesions at lower thoracic levels (T4 and T9) could disrupt preganglionic sympathetic neurons that play a role in bladder function by relaxing the bladder detrusor muscle to allow bladder filling. Disruption of sympathetic outflow would not be expected to impede bladder emptying, and so the urine retention seen after SCI at thoracic levels is thought to be due to interruption of ascending and descending pathways that control parasympathetic function and the somatic control of the external urethral sphincter.
Quantitative assessments revealed no differences in lesion size or overall white matter sparing following moderate contusions at T1, T4, and T9. Moreover, the sector analysis revealed no differences in the sparing of particular segments of the white matter. The only trend observed in the sector analysis (not significant) was that there was a slightly greater sparing of the ventral column following lesions at T9. This cannot account for the results, however, because the prediction would be that lesions at T9 should cause less sparing than lesions at T1, whereas the opposite was seen.
The second possible explanation would be that the same amount of white matter is spared at all thoracic regions following injury, but that the fibers subserving bladder function travel through different regions at T1 then they do at T4 or T9. The idea of a pathway shifting as it travels caudally down the spinal cord was proposed by Fedirchuk and Shefchyk in the case of the pathways involved in bladder function in cats (Fedirchuk and Shefchyk, 1991). However, this would only make sense if there was a region of spinal cord that was preferentially spared following T1 injury. Again the sector analysis suggests this is not the case.
Retrograde tracing reveals differential sparing of descending projections from the PMC
Analysis of retrograde labeling with Fluorogold revealed a larger number of retrogradely labeled neurons in the PMC following moderate contusion injuries at T1 in comparison to injuries at T4 or T9. These results suggest that differential sparing of bladder function is due to differential sparing of the critical descending projections from the PMC to the lumbosacral levels. At the same time, there was no difference in the extent of retrograde labeling of neurons in the red nucleus, supporting the conclusion that overall lesion severity was similar across groups.
Although sparing of ability to empty the bladder with lesions at T1 does seem to be related to sparing of descending projections from the PMC, other explanations cannot be excluded. One possibility, for example, is that spared bladder function is actually due to the sparing of ascending pathways conveying information about bladder distention. It is also possible that between T1 and T4, there exists a circuit that is able to facilitate voiding. This “subpontine micturition center” would have to be located between T1 and T4. Consistent with this idea, neurons at T3-T4 level respond to bladder distention (Qin et al., 2003). This could be the result of a descending supraspinal pathway responding to bladder pressure, however.
The idea of a reflex pathway that is not used under normal conditions is not novel. Both human and rodent “infants” are born with one bladder reflex, and then develop a different bladder reflex as they age. It has been argued that the C-fiber spinal reflex that appears several weeks after a complete spinal transection in cats is merely the same reflex from birth being unmasked. If this is the reflex that accounts for bladder function after lesions at T1, it must be immediately unmasked after T1 lesions in contrast to the delay seen after complete transection in cats.
The main argument against the idea of a circuit between T1 and T4 that can mediate bladder emptying is the profound loss of bladder function after crush injuries at T1. This, together with the evidence from retrograde tracing make it most likely that T1 lesions spare bladder function because descending projections from the PMC are relatively more spared. The explanation for this differential sparing remains unclear.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Abdel-Azim M, Sullivan M, Yalla SV. Disorders of bladder function in spinal cord disease. Neurol Clin. 1991;9(3):727–740. [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2007;206(2):318–331. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Auton Nerv Syst. 1990;30(Suppl):S71–77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary wheel running improves recovery from a moderate spinal cord injury. J Neurotrauma. 2005;22(1):157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Estores IM. The consumer’s perspective and the professional literature: what do persons with spinal cord injury want? J Rehabil Res Dev. 2003;40(4 Suppl 1):93–98. doi: 10.1682/jrrd.2003.08.0093. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Shefchyk SJ. Effects of electrical stimulation of the thoracic spinal cord on bladder and external urethral sphincter activity in the decerebrate cat. Exp Brain Res. 1991;84(3):635–642. doi: 10.1007/BF00230976. [DOI] [PubMed] [Google Scholar]
- Grundy D, Russell J. ABC of spinal cord injury. Urological management. Br Med J (Clin Res Ed) 1986;292(6515):249–253. doi: 10.1136/bmj.292.6515.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan SA, Chancellor MB, Blaivas JG. Bladder and sphincter behavior in patients with spinal cord lesions. J Urol. 1991;146(1):113–117. doi: 10.1016/s0022-5347(17)37727-3. [DOI] [PubMed] [Google Scholar]
- Keirstead HS, Fedulov V, Cloutier F, Steward O, Duel BP. A noninvasive ultrasonographic method to evaluate bladder function recovery in spinal cord injured rats. Exp Neurol. 2005;194(1):120–127. doi: 10.1016/j.expneurol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pikov V, Wrathall JR. Coordination of the bladder detrusor and the external urethral sphincter in a rat model of spinal cord injury: effect of injury severity. J Neurosci. 2001;21(2):559–569. doi: 10.1523/JNEUROSCI.21-02-00559.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Chandler MJ, Foreman RD. Effects of urinary bladder distension on activity of T3-T4 spinal neurons receiving cardiac and somatic noxious inputs in rats. Brain Res. 2003;971(2):210–220. doi: 10.1016/s0006-8993(03)02362-x. [DOI] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Version 1.38x. National Institutes of Health; Bethesda, Maryland, USA: 1997-2007. [Google Scholar]
- Rosenzweig ES, McDonald JW. Rodent models for treatment of spinal cord injury: research trends and progress toward useful repair. Curr Opin Neurol. 2004;17(2):121–131. doi: 10.1097/00019052-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Spinal cord neural organization controlling the urinary bladder and striated sphincter. Prog Brain Res. 2002;137:71–82. doi: 10.1016/s0079-6123(02)37008-0. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Nishijima S, Miyazato M, Ogawa Y. Central nervous control of micturition and urine storage. J Smooth Muscle Res. 2005;41(3):117–132. doi: 10.1540/jsmr.41.117. [DOI] [PubMed] [Google Scholar]
- Weld KJ, Dmochowski RR. Association of level of injury and bladder behavior in patients with post-traumatic spinal cord injury. Urology. 2000;55(4):490–494. doi: 10.1016/s0090-4295(99)00553-1. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Rafuse VF, Downie JW. Sprouting of CGRP primary afferents in lumbosacral spinal cord precedes emergence of bladder activity after spinal injury. Exp Neurol. 2007;204(2):777–790. doi: 10.1016/j.expneurol.2007.01.011. [DOI] [PubMed] [Google Scholar]