Abstract
Many chronic pain conditions including complex regional pain syndrome are exacerbated by sympathetic activity. In animal models, sympathetic fibers sprout into the dorsal root ganglia (DRG) after peripheral nerve injury, forming abnormal connections with sensory neurons. However, functional studies of sympathetic-sensory connections have been limited largely to in vivo studies. This study describes a new method for studying sympathetic-sensory connections in an isolated whole DRG preparation in the rat spinal nerve ligation (SNL) model. Three days after ligation of the ventral ramus of the spinal nerve (SNL), sympathetic fibers sprouting into the DRG were observed to originate largely in the intact dorsal ramus of the spinal nerve, which at the lumbar level is a small branch of the spinal nerve separating from the ventral ramus near the intervertebral foramen. In whole DRG isolated 3 days after SNL, microelectrode recordings of sensory neurons showed that repeated stimulation of the dorsal ramus enhanced spontaneous activity in large and medium diameter neurons, and reduced rheobase in large neurons. These effects, which were slow and long-lasting, were attributed to stimulation of the sympathetic sprouts because: stimulation had no effect in uninjured DRG; and effects could be reduced or eliminated by a “cocktail” of antagonists of norepinephrine and ATP receptors, by pretreatment with the sympathetic release blocker bretylium, or by pre-cutting the grey ramus through which sympathetic fibers coursed to the ligated DRG. The latter treatment, a relatively minimal form of sympathectomy, was also highly effective in reducing mechanical pain ipsilateral to the SNL.
Keywords: Sympathetic fibers, complex regional pain syndrome, neuropathy, sprouting, nerve injury, dorsal root ganglion
INTRODUCTION
Normally, the sensory neurons in the dorsal root ganglia (DRG) are not directly innervated by the sympathetic nervous system. In normal DRG sympathetic fibers are associated only with blood vessels. McLachlan et al. first described abnormal sprouting of sympathetic fibers into the DRG after sciatic nerve transection[34]. Subsequent studies have shown that such sprouting occurs in many animal pain models [13; 42; 29; 38]. This may include formation of dramatic “basket” formations in which sympathetic fibers form a dense plexus around individual somas (particularly of large diameter cells), and/or an increase in overall sympathetic fiber density in the cellular region of the DRG. Basket structures have also been observed in DRG from human neuropathic pain patients[46]. Sympathetic fibers in the DRG originate in the grey ramus, which enters the spinal nerve close to each DRG[42; 46; 12]. Sprouting may occur from the fibers that are already present in the DRG and normally innervate the blood vessels, or as newly ingrowing collateral fibers from other more distal sympathetic fibers. Sprouting occurs around both intact and axotomized cells[32], and can also occur rapidly in the absence of any axotomy, if the DRG is locally inflamed[58] or compressed[11].
The discovery of sympathetic sprouting in the DRG generated much excitement because it provided a possible explanation for clinical syndromes of sympathetically maintained pain. Many chronic pain conditions such as complex regional pain syndrome (CRPS) have long been known to be maintained or exacerbated by sympathetic activity in some patients, especially at earlier stages, and to respond to various methods of reducing sympathetic input[45; 2]. However, preclinical studies in this field have yielded sometimes conflicting results on the behavioral importance of the sympathetic-sensory connections. Many but not all behavioral studies have shown that various forms of sympathectomy reduce or eliminate mechanical or thermal pain behaviors in rodents (see table I, ref.[38]).
TABLE 1.
Distribution of capacitance values in spontaneously active vs. not spontaneously active cells
| Spontaneously Active | Quiescent | ||||
|---|---|---|---|---|---|
| Mean CM (pF) | SD | Mean CM (pF) | SD | p | |
| Large cells | |||||
| Normal | N/A | 139.0 ± 4.3 | 41.0 ± 2.1 | N/A | |
| SNL POD3 | 91.6 ± 2.0 | 17.7 ± 2.1 | 119.4 ± 2.4 | 30.6 ± 2.5 | p<0.0001 |
| SNL POD3 plus stimulation | 101.5 ± 1.8 | 21.0 ± 1.8 | 111.6 ± 2.3 | 27.3 ± 2.3 | p=0.0103 |
| Medium cells | |||||
| Normal | N/A | 61.9 ± 0.9 | 15.1 ± 0.9 | N/A | |
| SNL POD3 | 71.4 ± 0.2 | 4.1 ± 0.2 | 67.1 ± 2.1 | 18.8 ± 2.2 | p<0.0001 |
| SNL POD3 plus stimulation | 71.7 ± 0.9 | 5.5 ± 0.9 | 72.6 ± 2.1 | 12.6 ± 2.1 | p=0.2146 |
Values for mean and SD are average ± SEM of best fit to Gaussian curve to the capacitance histograms. Capacitance values from cells shown in Fig. 3.
P value = probability that same parameters are best fit for both SA and non SA groups
There were not enough SA cells in normal DRG to fit a distribution
Few functional studies of abnormal sympathetic-sensory neuron connections have been reported; these used fiber recording methods to measure increased rates of spontaneous activity after addition of alpha agonists or preganglionic stimulation in vivo [10; 34; 18]. These studies were not designed to detect increased incidence of spontaneous activity.
Here, we describe a new method for stimulating the sprouted sympathetic fibers in an isolated, whole DRG preparation and observing effects in sensory neurons using intracellular microelectrodes. This allows measurement not only of sympathetic effects on spontaneous activity, including recruitment of newly spontaneously active neurons, but also of effects such as action potential broadening or reduced threshold. Pharmacological manipulations are more readily accomplished, without the systemic effects that may occur in in vivo preparations. We have used this advantage to investigate possible roles of the sympathetic co-transmitter ATP in sympathetic-sensory neuron interactions. Many previous studies in the field have focused solely on norepinephrine (NE). In light of the clinical importance of sympathetically enhanced chronic pain states, and the relative dearth of functional studies available, we were interested in developing and characterizing this new method of studying functional interactions between sympathetic sprouts and sensory neurons.
METHODS
Animals
Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) were used for all behavioral experiments. Rats were housed one or two per cage under a controlled diurnal cycle of 12 h light and 12 h dark with free access to water and food. The ambient environment was maintained at constant temperature (22 ± 0.5°C) and relative humidity (60–70%). All the surgical procedures and the experimental protocol were approved by the institutional animal care and use committee of the University of Cincinnati (Cincinnati, OH).
Surgical procedure for spinal nerve ligation (SNL) and grey ramus transection
For behavioral experiments, male rats weighing 200–250 g received a unilateral ligation of the ventral ramus of the L5 spinal nerve following the original description by Kim and Chung [24]. Rats were anesthetized by isoflurane. The skin on the back was cut along the spine from S1 to L4 (DRG level). After clearly exposing L5 and L4 transverse processes by separating the back spine paraspinal muscles covering them, the L5 spinal nerve (ventral ramus) could be visualized in between these two transverse processes and freed from surrounding tissue. Part of the L5 ventral ramus is covered by L5 transverse process before it enters into spinal canal through the intervertebral foramen. The dorsal ramus separates from ventral ramus immediately distal from the point at which the spinal nerve exits the spinal canal and is not covered by bone. In some rats the space between L5 transverse process and intervertebral foramen was too narrow to free the L5 spinal nerve from surrounding tissue, in which case a very small round piece of bone (around 0.5 mm diameter) was cut and removed from L5 transverse process to further expose L5 ventral ramus for ligation. Following exposure, the L5 spinal nerve was isolated and tightly ligated with 6-0 silk approximately 10 mm distal to the ganglion. A similar procedure was used for spinal nerve ligation in rats used for electrophysiological and histological experiments, except that female rats weighing 80 – 100 g were used, and the ventral ramus ligation and subsequent experiments were performed in the L4 DRG instead of the L5. In some experiments, as indicated, the grey ramus located in close proximity to the DRG was isolated and cut, either at the time of, or three weeks before, the spinal nerve ligation surgery. In this procedure the gray ramus was identified on the ventral side of the spinal nerve at the position very close to the intervertebral foramen. At this site, where the gray ramus merges into the spinal nerve just across from the juncture where the dorsal ramus diverges from the ventral ramus; see Fig. 1), the grey ramus was cut and disconnected from spinal nerve. Around 1 mm of gray ramus nerve tissue was further removed to make a gap and prevent regeneration. In some experiments the dorsal ramus was cut in addition to the ventral ramus, as indicated. Otherwise, great care was taken to avoid damaging the dorsal ramus during the surgeries; any rats in which the dorsal ramus was inadvertently damaged were excluded from the study. The incision was closed in layers after these surgeries.
Figure 1.

Anatomy in region of normal L4 and L5 DRGs. . Top panel: Schematic diagram showing the normal DRG anatomy (not to scale). See text for explanation. Sensory neurons (blue); Sympathetic postganglionic neurons (red); motor neuron (green). The connections between sympathetic ganglia and the various pathways of preganglionic fibers from more rostral regions of the spinal cord are not shown; however, at this level no white rami are found in rats[3]. SNL refers to ligation and transection of the ventral ramus. Diagram modified from ref: [45]. Bottom: composite low power image of a normal DRG. Neurons are stained with NeuN (green), and tyrosine hydroxylase in red. Note the high proportion of TH-positive fibers in the dorsal ramus, and the much larger diameter of the ventral ramus compared to the dorsal ramus at this spinal level. The dorsal ramus has been folded back from the angle it would have in vivo, which would be coming up from the plane of the figure. Scale bar: 500 μm
Mechanical sensitivity testing
Animals were inspected and tested every other day for 3 trials prior to SNL surgery (baseline), and after surgery as indicated. As described in our previous publications[48; 59], the rat was placed in a closed Plexiglas box with a mesh floor through which stimulus probes were applied. After 15 min of acclimation, each von Frey filament containing the same 100-μm cylindrical tip was applied from underneath the cage floor to the plantar surface of the foot. Each filament was applied to 6 different spots on the ventral surface of the paw spaced across nearly the entire extent of the paw. The duration of each stimulus was 1 s, and the interstimulus interval approximately 10–15 s. The filaments were applied in order of bending force of 20, 40, 60, 80, 100, 120, and 160 mN, with a given filament delivered to each spot alternatively from one paw to the other in sequence from the first to the sixth spot. Since the percent withdrawal responses plotted as a function of the stimulus magnitudes (bending forces) were not linear, the Hill Equation , was used to estimate the S50 and the slope factor (γ) as this provides the best non-linear, least-square curve-fit (Levenberg-Marquardt algorithm of χ2 minimization) to the data. The parameters in the equation are the percent response (P), maximal response or 100% withdrawal response (Pmax), stimulus magnitude (S), stimulus magnitude or the force associated with 50% response of foot withdraw (S50), and slope factor (γ), known as the Hill coefficient. Data was fit to the Hill equation using a curve-fitting program (Microcal Origin 7.0, OriginLab Corp., Northampton, MA, USA); the derived S50 value is presented as a measure of 50% response force of the withdrawal threshold. The mean withdrawal thresholds (baseline) of each hind paw before surgery were obtained from an average of three testing sessions (plotted as day 0 value).
Immunostaining of TH and NeuN in whole DRG
Tyrosine hydroxylase (TH) was used as the marker for sympathetic fibers. For whole mount DRG staining of TH, the unsectioned whole DRGs were fixed in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.4) for 2 h prior to incubation in primary and secondary antibodies. After blocking with 10% normal goat serum in PBS for 60 min, DRGs were incubated in antibodies to TH (1:1000, from Pel-Freeze, Rogers, AR, USA) and monoclonal mouse anti-NeuN conjugated to Alexa 488 (1:100, from Chemicon/Milipore, Billerica, MA, USA) for 24 h at 4°C, followed by reaction with goat anti-rabbit Alexa 594 conjugated secondary antibody. The TH antibody is an affinity-purified polyclonal rabbit antibody; the antigen is purified and denatured rat TH isolated from pheochromocytoma cells. Specificity is demonstrated by ability to stain the noradrenergic and dopamine systems in rat brain with low background. Triton-X (0.3%) was used in all reaction solutions to enhance antibody penetration. Whole-mount DRGs were placed on slides coverslipped with anti-fade mounting medium for florescent light-microscopic observation.
In vitro microelectrode intracellular recording from isolated whole DRG
For electrophysiological experiments, the spinal nerve ligation and recording were performed on the L4 DRG. On postoperative day (POD) 3 or as indicated, intracellular recording was performed on sensory neurons from an isolated DRG nerve-ganglion preparation: Rats were anesthetized with pentobarbital sodium (40 mg/kg, i.p.). A laminectomy was performed at the level of L3-L6, and the ipsilateral L4 DRG identified. Oxygenated artificial cerebro-spinal fluid (ACSF; in mM: NaCl 130, KCl 3.5, NaH2PO4 1.25, NaHCO3 24, Dextrose 10, MgCl2 1.2, CaCl2 1.2, pH = 7.3, bubbled with 95% O2/5% CO2) was dripped onto the surface of the ganglion periodically during the surgical procedure to prevent drying and hypoxia. The ganglion and the attached peripheral nerve including the dorsal ramus were carefully and quickly removed from the rat and placed in a 100-mm Petri dish filled with oxygenated ACSF. Under the dissecting microscope, as the suture used for ligation of the ventral ramus was cut off; the perineurium and epineurium that form the ganglionic capsule were carefully removed; the ventral root was carefully separated from dorsal root and teased off from the DRG; and the dorsal root beyond the DRG was carefully trimmed. The whole preparation was kept at room temperature (23–24°C in oxygenated ACSF for 30 min before electrophysiological recording. The ganglion, with attached dorsal ramus, was placed in the recording chamber and mounted on the stage of an upright microscope (BX70-WI, Olympus, Melville, NY, USA). A U-shaped stainless steel rod with 2 pieces of fine silver wire crossed from one side to the other was used to gently hold the ganglion in place within the recording chamber. The dorsal ramus was pulled into a suction electrode for electrical stimulation. The DRG was continuously perfused with oxygenated ACSF at a rate of 2 ml/min throughout the recording. The temperature was maintained at 36±1°C by a temperature controller (TC-344A, Warner Instrument Corporation, Hamden, CT, USA). Intracellular recordings were made from individual sensory neurons near the dorsal surface of the DRG using microelectrodes filled with 3M KCl (pH=7.0) and coated with Sigmacote (Sigma, St. Louis, USA). Satisfactory recordings were obtained with electrodes of 40–60 MΩ. DRG soma were classified according to the capacitance and the shapes of action potentials[36; 53]. Any spontaneous activity observed after impalement of the cell was recorded before measurement of the other excitability parameters began, and re-confirmed at the end of the recording period. To insure that observed spontaneous activity was not due to impalement-induced injury, spontaneous activity was observed for 1 – 2 minutes and/or re-observed after recording other parameters in that cell as 3–4 minutes later (it usually took 3–4 min to finish recording parameters of each neuron). Spontaneous activity could be distinguished from injury responses because the latter tended to decline in amplitude within a few seconds. We only rarely observed even a brief period of firing due to impalement injury (this kind of firing was very brief, less than 5 spikes and lasted less than 1 second); in most cells impalement was indicated only by the appearance of a resting potential and membrane time constant. The low incidence of spontaneous activity in normal DRG, and increased incidence spontaneous activity in various pain models including SNL, have been widely observed with either fiber recording methods or with the method used here, of sampling many cells in the isolated DRG by microelectrode recording (e.g., see refs [16; 30; 56; 60]. After successful impalement, and recording of spontaneous activity (if present), the whole-cell input resistance for each cell was obtained from hyperpolarizing currents, 100-ms duration, delivered in steps of 0.1 nA from 0.2 to -2 nA. Depolarizing currents of 0.1nA - 4 nA 80 ms duration were then delivered in increments of 0.1 nA until an action potential (AP) was evoked. The threshold current was defined as the minimum current required to evoke an AP. The AP voltage threshold was defined as the first point on the rising phase of the spike at which the change in voltage exceeded 50 mV/ms. Duration of the AP was measured at the threshold voltage. Afterhyperpolarization (AHP) amplitude was measured as the difference between resting potential and lowest point in the afterhyperpolarization. In experiments in which dorsal ramus stimulation was used, a single stimulation pulse was applied to the dorsal ramus at the end of the recording protocol, to determine whether the recorded cell was activated by the stimulating electrode on the dorsal ramus. Data were collected and analyzed with the use of Clampex (Molecular Devices, Sunnyvale CA, USA) acquisition software on a personal computer.
Data analysis
For electrophysiological data, experimental groups were compared using Students’ t-test, Rank Sum Test or Kruskal-Wallis One Way Analysis of Variance on ranks, followed by Dunn’s post test, as appropriate. Chi square test was used to test significance of differences in spontaneous activity incidence. Large, medium, and small diameter cells were analyzed separately except as indicated. For behavioral data, groups were compared using two-way repeated measures ANOVA with Bonferroni post test. Significance was ascribed for p values < 0.05.
RESULTS
The dorsal ramus of the spinal nerve is a major source of sympathetic sprouts into the DRG at early times in the SNL model
In both humans and rodents, each spinal nerve divides into a ventral ramus and dorsal ramus just after exiting the intervertebral foramen, with the ventral rami innervating the extremities and the dorsal rami innervating the posterior structures[47]. At the lumbar level, the dorsal ramus is quite small and forms a nearly 90 degree angle with the larger ventral ramus. Although this division of the spinal nerve into dorsal and ventral rami is not always mentioned in publications, the term L5 spinal nerve ligation actually refers to ligation of the ventral ramus of the L5 spinal nerve (e.g. see[7]). In our work with transgenic mice in which the sympathetic neurons express EGFP [57], we noticed when viewing whole DRG preparations that the dorsal ramus has a high percentage of postganglionic sympathetic fibers. This branch also appeared to be a major source of sympathetic fibers sprouting into the DRG after SNL. As shown in Fig. 1 to 3, these observations are also true for rat. Fig. 1(bottom) shows a composite of a dorsal view of a normal L4 DRG from a whole mount preparation. TH, a marker for sympathetic neurons, is shown in red; neurons stained for NeuN are in green. The small size of the dorsal ramus relative to the ventral, and the relatively high percentage of sympathetic fibers in the dorsal ramus, are evident in these pictures at a gross anatomical level. Previous studies using DRG sections and higher magnification have demonstrated that sympathetic fibers within the normal DRG are sparse and associated primarily with blood vessels [46], but examining the normal DRG in whole mount shows that there are also fibers sparsely distributed across the surface. As previously reported, there are small TH-positive neurons in the DRG which are thought to be dopaminergic [41; 40; 52]. The anatomical pathways for the sympathetic fibers near the normal lumbar DGG are summarized in the diagram in Fig. 1 (top), based on published studies [6; 51]. Sensory neurons (blue) send one axon to the dorsal horn of the spinal cord and the other to the periphery in normal animals. At the L4/L5 level, most sensory axons enter the ventral ramus and innervate portions of the hind limb and paw. A small number enter the dorsal ramus and innervate regions near the spine. The dorsal ramus in this region is very small and separates from the ventral ramus shortly after the spinal nerve exits the intervertebral foramen (IVF). Grey rami connect the sympathetic postganglionic ganglia (red) with the next most caudal DRG (though some of the fibers in the grey rami may have their cell bodies in a more distant sympathetic ganglion). Here, a few sympathetic axons turn and enter the DRG, primarily innervating blood vessels. Some course through the dorsal ramus; others reach the periphery via the ventral ramus. The connections between sympathetic ganglia and the various pathways of preganglionic fibers from more rostral regions of the spinal cord are not shown; however, at this level no white rami are found in rats[6]. Additional whole mount views of the normal DRG, in which the ventral root and grey ramus entry zone were left intact, can be viewed as an online supplement.
Figure 3.

Sprouting early after SNL is reduced by cutting either the grey ramus or the dorsal ramus: A. Whole mount DRG 3 days after ventral ramus ligation and 24 days after sectioning of the grey ramus to that spinal nerve, showing the great reduction in TH-positive sprouting on the DRG surface (compare to figure 2A). The arrow indicates the dorsal ramus. Scale bar 500 μm. B and C. Higher magnification view (scale bar 100 μm applies to both panels) of whole mount DRGs in the region near where the spinal nerve meets the DRG. B is another example of a DRG 3 days after ventral ramus ligation, showing TH-positive fiber sprouting over this region of the DRG. Panel C shows an analogous region of a DRG on POD3 after both ventral and dorsal rami were ligated. Note the great reduction in TH-positive fibers over the surface of the DRG if the dorsal ramus is also sectioned.
SNL refers to ligation and transection of the ventral ramus; in this condition we see fibers apparently coming from the dorsal ramus sprout into the DRG. A composite picture of a whole mount DRG 3 days after ligation of its ventral ramus is shown in Fig. 2. The sprouting of the sympathetic fibers over the surface of the DRG, especially the more distal half, is evident at this time. This is consistent with previous studies using high magnification DRG sections that have also shown sprouting in the DRG can be extensive even by POD3 in the SNL model [14]. As seen by examining the whole DRG, many of these fibers appear to come from the dorsal ramus at this time. A more magnified image of the region where the dorsal ramus fibers course towards the DRG shows this more clearly (Fig. 2B). In the distal portion of the DRG, where sprouting is extensive (Fig. 2C), further magnification (Fig. 2D) reveals some perineuronal basket structures around individual neurons (* symbols) and TH-positive growth cones (arrows), though these have been more extensively described in previous publications using thin sections instead of whole mount (e.g., refs [13] [30]). In the region where the fibers from the dorsal ramus course towards the DRG (Fig. 2E), higher magnification reveals some branching and bifurcating TH-positive fibers (Fig. 2F). Also note the disappearance of the TH-positive neurons on POD3 (Fig. 2A), as previously reported in nodose and petrosal ganglia after peripheral axotomy [22; 21]. A diagrammatic summary of the DRG region after SNL is shown in the top of Fig. 2. A collateral fiber sprouting into the DRG from an intact sympathetic fiber in the dorsal ramus is shown; fibers in the ligated ventral ramus are shown degenerating. The collateral sprout from the dorsal ramus is based on the observations in whole mount tissue, as well as on the observation that cutting these fibers greatly reduces sprouting (see below), and that stimulating the dorsal ramus causes electrophysiological changes consistent with sympathetic transmitter release (see below). The possibility that the collateral fiber originates much further up the dorsal ramus than shown in the simple diagram, and doubles back towards the DRG, would also be consistent with our data.
Figure 2.

The dorsal ramus is a primary source of sympathetic sprouts in the DRG early after SNL. A: Composite whole mount DRG picture 3 days after (ventral ramus) spinal nerve ligation: showing extensive TH-fiber sprouting onto the surface of the DRG. Many of the sprouting fibers originate in the dorsal ramus onto the dorsal surface of the DRG. Scale bar 500 μm. B. Magnification of the boxed region in A, showing TH-positive fibers coursing from the dorsal ramus towards the DRG. Scale bar 100 μm. C. Whole mount image from another DRG on POD3, in the region on the edge of the DRG near the spinal nerve. TH-positive fibers are shown on the surface of the DRG originating primarily from the dorsal ramus. Scale bar 100 μm. At higher magnification (panel D; scale bar 20 μm), fibers surrounding individual neurons can be observed (* symbols), as previously described in sectioned DRG. Arrows indicate varicosities and growth cone-like structures. E. Image from more distal region of the same DRG on POD3, showing TH-positive fibers in the region of the dorsal ramus. Scale bar 100 μm. At higher magnification (F; scale bar 20 μm) examples of bifurcating or branching fibers can be observed (arrows). G. Schematic showing the proposed origin of sympathetic sprouts in the dorsal ramus after ventral ramus ligation (SNL). Neuron color scheme is as in Fig. 1. The dotted line represents a newly sprouted sympathetic fiber entering the DRG as a collateral from an intact sympathetic axon in the dorsal ramus. Wavy lines indicate fibers in the ventral ramus that have been severed by the SNL.
Additional whole mount views of the DRG 3 days after SNL, in which the ventral root and grey ramus entry zone were left intact, can be viewed as an online supplement.
As expected, cutting the grey ramus to the DRG 3 weeks prior to SNL greatly reduces the TH-positive fibers in the spinal nerve rami and reduces the sprouting onto the DRG (Fig. 3A). Further evidence that the dorsal ramus is a primary source of sympathetic fibers sprouting into the DRG at early times after spinal nerve ligation is shown in Fig. 3C. In this experiment, the dorsal ramus was sectioned at the same time that the ventral ramus was ligated. This greatly reduced the sympathetic fiber density on the distal surface of the DRG on POD 3 (for comparison, this region of a DRG that received only the usual ventral ramus ligation, rather than ventral plus dorsal ramus ligation, is shown in Fig. 3B). Similar results were obtained in at least 4 animals per experimental condition for the results shown in Figs. 1 – 3.
Stimulation of the dorsal ramus enhances spontaneous activity and excitability of large and medium diameter sensory neurons in isolated DRG after ventral ramus spinal nerve ligation
Based on these anatomical observations, we decided to test whether stimulation of this small dorsal ramus in the whole DRG prep might provide a mechanism to electrically stimulate sprouting sympathetic fibers (induced by ventral ramus SNL) and observe their functional effects on excitability of DRG neurons in an isolated DRG preparation, as measured with microelectrodes. Preliminary experiments indicated that dorsal ramus stimulation had relatively slow and long-lasting effects on DRG neuron excitability (see below), so for most experiments we adopted an experimental design in which cells from DRGs without dorsal ramus stimulation were compared with cells from other DRGs in which dorsal ramus stimulation was applied intermittently throughout the recording session. In these experiments we excised the whole DRG from rats (control, or 3 days after ligation of the ventral ramus of the spinal nerve to the L4 DRG from which recordings were made), and attached a suction stimulating electrode to the dorsal ramus. The dorsal ramus was electrically stimulated with a train of spikes at 1 train per second for 1 min. Each train consisted of five 750 μs 0.5 mA pulses at 50 Hz. Five to six neurons were recorded in the next 10 – 15 minutes, then the stimulation protocol was repeated. A single 750 μs 0.5 mA pulse was applied to the dorsal ramus at the end of each cellular recording, to determine whether or not the stimulation protocol would generate an action potential in the recorded cell. This alternation of stimulating then recording continued throughout the recording session (up to 3 hours). For recording, individual DRG neurons near the surface of the DRG were impaled with a microelectrode, spontaneous activity (when present) was recorded, and excitability parameters were measured under current clamp as described in Methods. Cells were classified as large, medium, or small diameter as described in Methods, and these classes were analyzed separately. Each experimental group included at least 5 different animals.
As previously reported by our group[30] and others, in large diameter cells, SNL per se caused a significant increase in the incidence of spontaneous activity observed on POD 3, as well as significantly reducing rheobase (Fig. 4, left). As also shown in Fig. 4, in large diameter cells, stimulation of the dorsal ramus in the L5 DRG isolated 3 days after SNL led to a dramatic further increase in the percentage of spontaneously active cells, as well as significant further reductions in rheobase. The increase in spontaneous activity after dorsal ramus stimulation reflected increases in all three patterns of activity: bursting (from 4.9% to 8.4%), tonic (from 2.0% to 12.0%), and irregular firing (from 5.4% to 8.9%). Effects of dorsal ramus stimulation on spontaneous activity were not seen in large cells in DRG from normal rats, but were only observed in rats that had undergone (ventral ramus) SNL, as might be expected since postganglionic sympathetic neurons sprout into the DRG in this model whereas normal DRG have sympathetic innervation that is very sparse and not closely associated with the neurons. In normal uninjured DRG, stimulation of the dorsal ramus also has no significant effect on other excitability parameters in large cells such as rheobase (Fig. 4, left), the size of afterhyperpolarization (p = 0.63), or the input resistance (p = 0.25).
Figure 4.
Dorsal ramus stimulation increases excitability in large and medium diameter cells only after SNL. “Stim”, dorsal ramus stimulation applied periodically throughout experiment. *, p<0.05; **, p<0.01; ***; p<0.001. SA, spontaneous activity. N = 191 – 261 large cells and 45 – 82 medium cells per group, 5 animals per group.
Some other electrophysiological parameters measured in large diameter cells were affected by SNL but not further affected by dorsal ramus stimulation to any significant degree. These include action potential width, which increased from 0.72 ± 0.02 msec in normal DRG to 1.11 ± 0.04 msec (p<0.001) in SNL DRG; afterhyperpolarization amplitude, which was reduced from 12.4 ± 0.41 mV to 7.1 ± 0.39 mV (p<0.001); and membrane specific conductance, which was reduced from 0.75 ± 0.03 nS/pF to 0.58 ± 0.02 nS/pF (p=0.002) after SNL but not further changed by dorsal ramus stimulation in (0.58 ± 0.02 in the SNL + stimulation group; p = 0.89). Input resistance (without normalization for membrane area) was significantly altered by SNL, increasing from 14.6 ± 0.58 to 19.4 ± 0.85 MΩ, p<0.0001), but dorsal ramus stimulation did not cause further significant changes in input resistance in SNL DRG (19.37 ± 0.83, p = 0.67).
Dorsal ramus stimulation and SNL had similar effects in medium diameter cells. Spontaneous activity was increased by SNL, and a further modest but significant increase was observed after dorsal ramus stimulation (Fig. 4, right), comprised of increases in all 3 types of spontaneous activity: bursting (from 12.2% to 21.8%), tonic (from 7.3% to 10.3%), and irregular (from 11.0% to 14.1%). SNL also decreased rheobase and lowered the action potential threshold (Fig. 4, right); these changes were further enhanced after dorsal ramus stimulation but these effects of stimulation did not reach significance, unlike in large cells. As observed for large diameter cells, in uninjured DRG, dorsal ramus stimulation had no effect on spontaneous activity, threshold, or rheobase in medium cells (Fig 4, right), or on action potential width (p = 0.5), afterhyperpolarization (p = 0.5), or input resistance (p = 0.06). Action potential width in medium diameter cells was increased after SNL from 1.69 ± 0.07 to 1.95 ± 0.09 msec, but this was not a significant difference, and this parameter was not further affected by dorsal ramus stimulation (p = 0.09). Afterhyperpolarization was reduced after SNL from 12.9 ± 1.3 mV to 8.3 ± 0.6 mV (p <0.001), but not further affected by stimulation (p = 0.6). Specific conductance in medium cells from normal DRG was 0.90 ± 0.22 nS/pF; after SNL the value was 0.60 ± 0.04, a non-significant change (p = 0.15), and no further significant changes were observed after stimulation (0.59 ± 0.03 in SNL DRG with stimulation, p = 0.79). Input resistance changes (not normalized by capacitance) showed no significant effects of SNL in medium diameter cells (32.7 ± 1.9 vs. 33.7 ± 2.15 MΩ, p = 0.72) and no changes were observed after stimulation of dorsal ramus in SNL ganglia (30.8 ± 1.67 MΩ, p = 0.28). Hence in both medium and large diameter cells, the primary parameter affected by dorsal ramus stimulation in axotomized DRG was the incidence of spontaneous activity; rheobase and threshold were also affected by stimulation in large cells. Other membrane parameters were affected by SNL but not further affected by stimulation.
In small diameter cells, spontaneous activity was rare under any of the experimental conditions described in this study. Although we observed effects of SNL on threshold (−28.4 ± 1.5 mV on POD 3 vs. −20.7 ± 2.5 mV in control, p=0.01), we did not observe any significant further effects of dorsal ramus stimulation on threshold, spontaneous activity, or rheobase in small cells in any experimental groups described in this study (N = 24 – 26 cells per group). Hence in the figures only data from medium and large diameter cells are presented.
Spontaneously active cells have a distinct size distribution and noninflected action potentials
We observed that spontaneously active cells, both before and after dorsal ramus stimulation, tended to have sizes near the boundary between medium and large diameter cells. Very large cells did not often show spontaneous activity. This is shown in figure 5, in which the combined distribution of cell capacitance values from medium and large diameter cells from the experiments shown in Fig. 4 is summarized. For reference an “equivalent diameter” is shown below the x axis, derived from membrane capacitance assuming a sphere with 1 uF/cm2 specific capacitance. It can be seen that spontaneously active cells form a much tighter distribution than non spontaneously active cells. This is shown more quantitatively in Table 1, in which it is evident that fitting the histograms of cell capacitance values with a Gaussian curve gives smaller values for the standard deviation in spontaneously active cells.
Figure 5.
Histogram of membrane capacitance values for the cells in Fig. 4. All non-spontaneously active (quiescent) cells have been combined into a single distribution (black). Spontaneously active cells after SNL (blue), or after SNL plus dorsal ramus stimulation (red), had a narrower distribution; in general the largest cells were not spontaneously active in any condition. Bins with capacitance values from 250 to 350 are not shown (total frequency of 0.005, non SA cells only). For clarity, SA cells from normal or normal plus stim groups are not shown; the number of cells in these groups was small. Bin size 12.5 pF. See also Table 1.
As another possible source of information about what types of cells are spontaneously active after dorsal ramus stimulation, we tried classifying cells according to whether they had action potentials with inflections on the falling phase, which in several species including rat is characteristic of high threshold/nociceptor cells [27; 28]. In some studies, this trait has proved to be preserved under various models of pathological pain [15; 20; 44], but not in other studies[25; 49; 31]. We have observed that the percentage of large and medium cells with an inflected action potential did not significantly change between DRG from naïve vs. SNL (day 3) animals (p = 0.12); nor was it affected by another pain model[58], locally inflaming the DRG with zymosan (day 3; p = 0.20). However, in DRG from SNL animals, stimulation of the dorsal ramus caused a significant decrease in the percentage of medium and large cells with inflected action potentials (from 31% to 20%; p <0.0001). This suggested that some cells may have had inflected action potentials before the sympathetic stimulation but not afterwards, making it difficult to use this criterion to classify which sensory neurons were affected by the stimulation. We observed that virtually all spontaneously active cells (97%) had non-inflected action potentials.
Effects of dorsal ramus stimulation are relatively slow and long-lasting
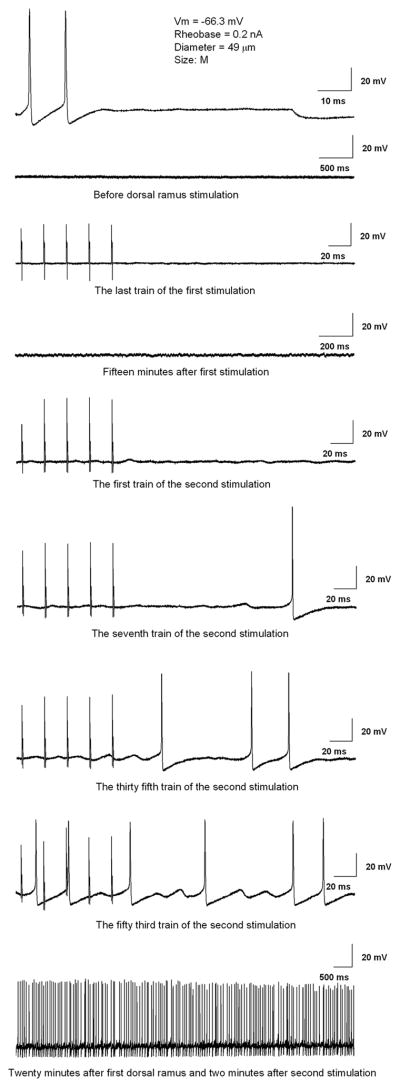
In the above experiments, enhanced excitability was observed in sensory neurons impaled up to several minutes after the stimulation of the dorsal ramus. This showed that the excitatory effects must have been relatively long lasting; our experimental protocol would have precluded observing effects that lasted only 10s of seconds or less. The protocol of repeated stimulation was used because, in preliminary experiments, we found that no significant changes in excitability could be observed using a single round of dorsal ramus stimulation instead of repeated stimulation over the course of the 3 hour recording sessions (n = 308 cells, 5 DRG isolated on POD3 after SNL). We attempted to record from individual neurons before, during, and after multiple stimulations of the dorsal ramus, but found it was often difficult to maintain a stable recording for this length of time while applying the stimulation protocol; in addition this experimental protocol was impractical because it could only yield one cell per DRG. An example of the small number of successful recordings in which a single cell was monitored through several rounds of dorsal ramus stimulation is shown in Fig 6. When recorded before dorsal ramus stimulation, this cell had a rheobase of 0.2 nA, and no spontaneous activity. As for most cells, stimulation of the dorsal ramus did not directly evoke action potentials (see stimulation artifacts in third trace). The first round of dorsal ramus stimulation did not evoke spontaneous activity, but beginning during the second round of dorsal ramus stimulation, subthreshold oscillations and then spontaneous firing could be observed. Spontaneous activity continued after dorsal ramus stimulation ended (bottom trace). As predicted from the data presented in Fig. 4, which were collected from cells up to 15 minutes after a round of dorsal ramus stimulation, the response in this cell is slower and more long lasting than the excitatory responses observed in fiber recording experiments following sympathetic stimulation (see Discussion). Similar results were obtained in 6 other cells recorded throughout several rounds of dorsal ramus stimulation; an additional 18 cells were recorded that did not respond with spontaneous activity.
Figure 6.
Effects of dorsal ramus stimulation are relatively slow and long lasting. Example of a cell recorded before any dorsal ramus stimulation, and during and after two rounds of dorsal ramus stimulation. Top: initially, 0.2 nA current injection could evoke an action potential, but there was no spontaneous activity (second trace shows membrane potential on a slower time base; V = −64 mV). Stimulation of the dorsal ramus (third trace) shows stimulation artifacts but does not evoke an action potential or spontaneous activity (V = −63 mV). Membrane potential oscillations and spontaneous firing appeared during a second round of dorsal ramus stimulation, and spontaneous activity continued after the end of the stimulation protocol.
In addition, we conducted experiments in which a small number of cells were identified, and were re-impaled and re-recorded several times through the course of the experimental protocol. A total of 25 medium and large cells in 4 DRG (SNL POD3) were recorded 2 or 3 times, before and after rounds of dorsal ramus stimulation. Four of these were spontaneously active before any dorsal ramus stimulation, and of these 2 were still active when recorded again 4 or 15 minutes after one round of dorsal ramus stimulation. Of the 21 cells that were not spontaneously active before dorsal ramus stimulation, 11 showed spontaneous activity when re-impaled after 1 (n = 7 of 14 recordings), 2 (n = 3 of 8 recordings) and/or 3 (n = 3 of 5 recordings) rounds of dorsal ramus stimulation. These instances of spontaneous activity were observed from 2 to 35 minutes (average 11.3 min) after the previous stimulation. In some cases the observed spontaneous activity was recorded continuously for up to 25 minutes. The spontaneous activity was not induced by re-impaling the same cell several times; in control DRG no spontaneous activity was observed by using a similar protocol of dorsal ramus stimulation and re-impalement of identified cells. These control experiments were done in 15 cells, including 1 that was recorded 3 times (before, and after 1st and 2nd stimulations) and 7 that were recorded 4 times each (3 stimulations). These results show that, as implied by the results presented previously, the changes in excitability observed after dorsal ramus stimulation are relatively slow to develop and long-lasting.
In these re-impalement and prolonged recording experiments we deliberately selected cells in the size range most likely to be spontaneously active, which may account for the relatively high incidence of spontaneous activity observed. In all other experiments we systematically recorded from cells of all sizes encountered (except that some small cells were skipped over, once it became evident that these were not affected by stimulation), moving from one side of the DRG to the other.
Evidence that effects of dorsal ramus stimulation are due to stimulation of sympathetic sprouts in the DRG but not to stimulation of the sensory afferents
The finding that dorsal ramus stimulation affects excitability only after SNL, when sympathetic fibers have sprouted from that structure onto the DRG, suggests that the enhanced excitability may be due to stimulation of the sympathetic sprouts. Further evidence for this hypothesis was obtained by using several different methods to block sympathetic sprouting, release, or transmitter effects. These experiments are summarized in Fig. 7. Experiments were conducted in axotomized DRG (SNL, POD 3) using the dorsal ramus stimulation protocol described above. Three different methods of blocking or removing inputs from sympathetic fiber sprouts in the DRG were used:
Figure 7.
Several forms of sympathetic blockade reduce effects of dorsal ramus stimulation in large diameter cells (left) and medium diameter cells (right). See text for explanation of the different experimental groups. Values of the variables after SNL without stimulation, or in control cells (dashed lines) are taken from Fig. 4; values of SNL + Stim group are replotted from Figure 4. N = 191 – 249 large cells and 78 – 104 medium cells per group; 5 animals per group.
In one set of experiments, the DRG were exposed throughout the recording session to a cocktail of known sympathetic transmitters ATP and NE (1 μM phentolamine, alpha adrenergic blocker; and blockers of several ATP receptors known to be expressed in DRG: 1 μM A438079, P2X7receptor blocker; and 25 nM TNP-ATP, blocker of P2X1, PX3, and PX2/3 receptors). This experimental group is labeled “SNL with stim + symp blockers” in the figures.
In another set of experiments (“SNL with stim + bretylium”) the DRG were pre-treated with 3 μM bretylium for one hour prior to the recording. On a short time scale such as used, bretylium specifically blocks release (of both NE and ATP) from sympathetic terminals; specificity arises from the fact that the drug is taken into the neurons via the NE transporter[9; 8].
In the third set of experiments, the grey ramus to the L4 DRG was transected at the time of the L4 SNL surgery, to prevent formation of sympathetic sprouts. (By “grey ramus to the L4 DRG” we refer to the grey ramus from the L3 sympathetic chain ganglion that enters the L4 spinal nerve just adjacent to the L4 DRG, containing post-ganglionic sympathetic fibers, see Fig. 1 and ref [6].). This group is labeled “SNL with stim + cut grey ramus.
As shown in Figure 7 (left), in large cells, all three methods of sympathetic blockade reduced the effects of dorsal ramus stimulation on spontaneous activity, threshold, and rheobase. The values of spontaneous activity, rheobase, and threshold were restored to values not significantly different from those seen in axotomized DRG in the absence of dorsal ramus stimulation.
The experiments in which the grey ramus was cut to prevent sympathetic sprouting, or bretylium was used to block sympathetic terminals, also indicate that the effects of stimulation reported in Fig. 4 cannot be attributed to action potentials being stimulated in the relatively small number of DRG neurons that project into the dorsal ramus of the spinal nerve, nor are they due to excitation of other DRG neurons by current flow escaping from the stimulating electrode. Another possible mechanism by which dorsal ramus stimulation might affect excitability is cross-excitation, i.e. stimulation of sensory neurons with axons in the dorsal ramus might cause excitatory effects on their neighboring neurons, as described in isolated DRG preparations following stimulation of sciatic nerve or dorsal root inputs [4; 5]. One would have to further postulate that the different methods of sympathetic blockade shown in Fig. 7 also reduced cross excitation, either by nonspecific effects on neuronal excitability (blocker cocktail; bretylium), or by surgically damaging the dorsal ramus (during grey ramus removal). We deemed this unlikely because the overall percentage of large and medium cells in which an action potential could be evoked by a single stimulus to the dorsal ramus was quite small, ranging from 0.7 to 2.6% for the 4 experimental groups shown in Fig. 7 and the “normal plus stimulation” and “normal plus stimulation plus sympathetic blockers” groups (Fig. 4). In addition none of these percentages was significantly changed by either SNL or by any of the sympathetic blocking methods (p values ranging from 0.23 to 1.00, Fisher’s exact test), arguing against any of these procedures altering the number of neurons providing any potential cross-excitatory stimulus to their neighbors. The time course for cross-excitation (10s of seconds[3; 4]) is also much shorter than the dorsal ramus stimulation effects (see above); and cross-excitation is associated with increases in input resistance which we did not observe in the dorsal ramus stimulation experiments (see above). In additional experiments in which we stimulated half of the dorsal root, instead of the dorsal ramus, using stimulation protocols previously used to evoke cross-excitation [4], we were unable to evoke either cross-excitation or spontaneous activity in cells (n = 8) not directly activated by the dorsal root stimulation, despite presumably stimulating a much greater fraction of neighboring sensory neurons. The lack of cross-excitation in these experiments may be due to differences in the recording conditions; in particular, we used a different recording solution with lower Ca2+ concentration than published studies of cross-excitation.
As shown in Fig. 7 (right), similar effects of the different methods of sympathetic blockade were observed in medium diameter cells, except that the effects on threshold did not reach significance. An important difference was that the incidence of spontaneous activity in medium cells was reduced even below that seen in un-stimulated SNL DRG, approaching the value seen in normal, non-axotomized DRG. This suggested that, for medium cells, some of the effects of sympathetic sprouts did not require dorsal ramus stimulation, as might be the case if some of these fibers released transmitter spontaneously in the absence of stimulation.
Further evidence for effects of stimulus-independent effects of sympathetic transmitters on medium diameter cells but not in large diameter cells is shown in Fig. 8. In medium cells (right), after SNL, the combination of NE and ATP blockers, or the removal of sympathetic innervation by previous sectioning of the grey ramus, has significant effects on spontaneous activity even in the absence of stimulation. In normal DRG these manipulations have no effect. This is in contrast to the situation in large cells, in which, in the absence of dorsal ramus stimulation, no significant effects on the measured parameters were observed after grey ramus removal or sympathetic blockade in axotomized DRG (with the exception of small effects on rheobase). These sympathetic blockade methods also had no effect on large diameter cells in normal DRG, with the exception of a small increase in rheobase following grey ramus removal. These results are consistent with the idea that some spontaneous activity of medium cells after SNL is promoted by sympathetic transmitter release in the absence of dorsal ramus stimulation. However, spontaneous activity is very sensitive to small changes in membrane properties, so another interpretation is that small long-lasting changes in excitability induced indirectly by either removing the grey ramus or blocking ATP/NE receptors, caused the decrease in spontaneous activity in medium cells even though other measured parameters of excitability were unaffected.
Figure 8.
Effects of the various forms of sympathetic blockade in large cells (left) and medium cells (right), in the absence of dorsal ramus stimulation. See text for explanation of experimental groups. Normal, SNL groups replotted from Fig. 4. N = 261 – 336 large cells and 66 – 111 medium cells per group; 5 – 6 animals per group.
Cutting the grey ramus to the L5 DRG reduces mechanical sensitivity in the SNL model
Because surgical sympathectomy is a very difficult procedure to accomplish in the rat, we were interested in determining whether simply sectioning the grey ramus near the L5 DRG would be sufficient to reduce pain behaviors in the SNL model. This outcome might be expected if the excitatory effects described above are occurring in vivo. In these experiments, the grey ramus to the ipsilateral L5 DRG was cut 21 days before the ventral ramus of the same DRG was ligated, which as shown in Fig. 3 greatly reduced sprouting into the injured DRG on POD 3. This procedure alone had no effect on mechanical behavior prior to spinal nerve ligation, as indicated by the lack of a significant difference between the two experimental groups in the baseline behavior point before the spinal nerve ligation. (This baseline is the average of measurements taken on 3 different days.) As shown in Fig. 9, cutting the grey ramus reduced the mechanical pain sensitivity induced by SNL by approximately 40%, an effect that was maintained for 7 weeks (at which time the SNL-induced pain behavior was beginning to resolve). The much smaller increase in contralateral sensitivity induced by SNL was unaffected by cutting the grey ramus. When both the L4 and L5 grey rami were cut prior to the L5 spinal nerve ligation (Fig 9, middle), the mechanical sensitivity was reduced by approximately 85%; however, on most days (except POD 1,14, and 43) the mechanical sensitivity did not differ significantly from that measured after precutting only the L5 grey ramus. Cutting the L5 grey ramus at the time of spinal nerve ligation (instead of 21 days before) also reduced mechanical pain (Fig. 9, bottom); however, in this case the effects occurred with a delay, not reaching significance until after POD 3.
Figure 9.
Effects of cutting grey rami to the DRG on mechanical pain behavior. Mechanical withdrawal threshold was determined with the von Frey method (see Methods). Ligation of the ventral ramus of the L5 spinal nerve (SNL) was performed on day 0. Baseline behavior plotted on day -1 is average of measurements on three separate preceding days. Average ipsilateral baseline is indicated by the dashed line. Top: cutting the grey ramus to the L5 DRG, 3 weeks before the SNL surgery (n = 8 animals), significantly reduced the mechanical pain sensitivity induced by SNL (n = 13) on the ipsilateral side (left panels) but had no effect on the contralateral side (right panels). Differences between the two ipsilateral groups were significant at all points except baseline and POD 14. Middle: cutting both the L4 and L5 grey rami 3 weeks before the SNL surgery gave a similar effect (n = 15). SNL data is replotted from top figure. Bottom: cutting the grey ramus to the L5 DRG at the time of surgery also reduces mechanical pain behavior, with a delay (n = 5). SNL data is replotted from top figure.
DISCUSSION
We found that the dorsal ramus of the spinal nerve is a primary source of sympathetic sprouts into the DRG early after ligation of the corresponding ventral ramus of the spinal nerve (SNL). Since the dorsal ramus is preserved in SNL, this suggests that sprouting in the DRG occurs primarily as collaterals from intact sympathetic fibers that run through the dorsal ramus, not from transected sympathetic fibers in the ventral ramus. Assuming that only intact sympathetic fibers sprout into the DRG is the simplest way to explain the finding that cutting the dorsal ramus along with the ventral ramus dramatically reduces sprouting. This is qualitatively consistent with the results of McLachlan et al. [34], who showed, using a more distal injury, that severed sympathetic neurons degenerated rather than regenerating to produce sprouts. Sprouting from intact sympathetic fibers that normally innervate DRG blood vessels has been demonstrated in the SNL model, but occurs only later time points (POD 7) than studied here [12]. The same study showed that sprouting did not occur via the doubling back of severed sympathetic fibers regenerating from the ligated ventral ramus, but as collateral fibers coming from “the more proximal part of the ligated nerve”. In this study, and others[43] that concluded that sprouts originated from axotomized sympathetic fibers, there is no mention of the dorsal ramus as a possible source of intact sympathetic fibers in “the spinal nerve”. Failure to consider this structure may account for discrepancies between this and previous studies.
A more direct approach to stimulating sympathetic sprouts in vitro might seem to be stimulating the grey ramus near the DRG, but we found that this structure was too short and delicate to make such experiments practical; in addition it could not be preserved during the desheathing of the DRG required for microelectrode access and visualization.
Previously most functional studies of sympathetic sprouts have been conducted using extracellular recording methods, often in vivo where pharmacological studies are difficult. Using this in vitro preparation, we found that stimulation of the dorsal ramus enhances the incidence of spontaneous activity of large and medium diameter sensory neurons in DRG isolated 3 days after ventral ramus ligation, when extensive sympathetic sprouting onto the DRG is already observed. Stimulation had little effect in normal DRG (that lack sympathetic sprouts), or in DRG subjected to any of three distinct methods of sympathetic blockade. In large cells, dorsal ramus stimulation also reduced rheobase. Other membrane properties were strongly affected by spinal nerve ligation but not further affected by dorsal ramus stimulation.
Some discrepancies in the literature about the relevance of sympathetic-sensory interactions to pain may be due to a focus on NE, excluding known sympathetic co-transmitters such as ATP. In some reports, effects described as “sympathetic stimulation” were actually effects of noradrenergic agonists; or, the reported lack of effect of sympathetic blockade was actually a lack of effect of adrenergic blockade (e.g.[23] ). Many researchers have reported increased sensitivity of DRG neurons to NE in nerve injured animals but the doses in these studies are often at μM or mM levels (e.g., [39; 1; 33] ). ATP and NE may have synergistic effects on sensory neurons[35], especially in pain models[37; 33]. We found that a cocktail of NE and ATP blockers was effective in blocking effects of dorsal ramus stimulation, consistent with the idea that ATP may play a role. In preliminary experiments we find that including the ATP-hydrolyzing enzyme apyrase (1 unit/mL) in the extracellular perfusate blocked the increase in spontaneous activity in large cells caused by dorsal ramus stimulation, while using only the NE blocker phentolamine without ATP antagonists was ineffective.
Previous functional studies (extracellular fiber recordings) of the abnormal sympathetic-sensory neuron connections formed in various pain models have generally showed excitatory effects of sympathetic stimulation, as reported here. Direct comparison of these studies is difficult because they examined effects of sympathetic stimulation on fibers selected to have ongoing spontaneous activity (e.g. refs[10; 18] ), and were not designed to detect a previously silent fiber that becomes active after sympathetic stimulation. Also, excitatory effects such as reduction in threshold cannot be measured with fiber recording. In their original paper describing sympathetic sprouting after sciatic nerve transection, McLachlan et al.[34] showed that a short (10 – 30 sec) burst of activity in sensory axons could be recorded (extracellularly) upon stimulation of preganglionic sympathetic fibers in vivo. The excitatory effects reported here were more long-lasting. Our protocol alternating stimulation and recording would not have detected the brief effects that McLachlan et al. reported. It is unknown if this is due to differences in the model used, or in the methodology, particularly their stimulation of preganglionic fibers vs. postganglionic in this study.
We found that cutting the grey ramus to the L5 DRG could profoundly reduce mechanical pain sensitivity in the SNL model. This effect lasted as long as the SNL-induced mechanical sensitivity (7 weeks). A similar result was reported by Kinnman and Levine[26]. Because surgical sympathectomy is extremely difficult to accomplish, and chemical sympathectomy has widespread systemic effects, the method of cutting the L5 grey ramus provides a much less invasive way to study the behavioral relevance of the sympathetic sprouts in DRG.
Sympathetic sprouting and abnormal sympathetic-sensory neuron interactions can occur in the periphery as well as in the DRG. However, our behavioral data suggest that in this model the sympathetic-sensory interactions at the L5 DRG level, not just in the paw region used for behavioral testing, play important roles in pain behaviors. Even though SNL may have caused some sympathetic dennervation and/or subsequent sprouting in or near the peripheral region used for behavior testing [6; 54; 50], no further peripheral sympathetic changes should have occurred by also cutting the grey ramus to L5 – this would involve simply making a second cut to the already severed sympathetic axons that reached the paw region through the L5 ventral ramus. The strong reduction of pain behaviors is most simply attributed to great reduction in sprouting at the DRG level that results from also cutting the grey ramus. Also, we found little further behavioral effect of also cutting the grey ramus to the L4 DRG in addition to the L5, even though this procedure should have had greater effect on sympathetic innervation of the hindpaw; and Kinnman et al. found no behavioral effects of cutting only the L4 grey ramus. These data suggest that sympathetic sprouting into the L5 DRG plays an important role in mechanical pain behavior though we cannot exclude some additional peripheral effects.
We found no effects of dorsal ramus stimulation in small diameter (primarily nociceptive) cells. The most striking effects of stimulation were on incidence of spontaneous activity in large diameter cells, which primarily though not exclusively transmit signals from low-threshold, non-nociceptive receptors. Proposed mechanisms by which spontaneous activity in large diameter low-threshold neurons might contribute through indirect pathways to the mechanical pain observed in the SNL model, even though these neurons do not normally transmit pain signals, include [17]: enhancement of (polysynaptic) pathways transmitting normally innocuous signals from deeper dorsal horn regions into pain-transmitting superficial layers; change in low threshold afferents to release neuromodulators normally associated with nociception or central sensitization; and transmission of spontaneous activity into nucleus gracillus, a pathway associated with innocuous touch and vibration sense, that has also been shown to have major effects on tactile allodynia. Alternatively, the main increase in spontaneous activity may have occurred in myelinated nociceptors. A substantial minority of myelinated afferents have nociceptive thresholds and response properties, and project into lamina I/II of the dorsal horn [19; 55]. In rat this population is estimated at 20% of Aα/β cells [19]; for comparison we found that spontaneous activity in large diameter cells was 12% after SNL, increasing to 29% after dorsal ramus stimulation. This possibility would also be consistent with the narrower size distribution we observed for spontaneously active cells. In medium diameter cells, a higher percentage of which are nociceptive, we found that inhibiting sprouting by cutting the grey ramus to the injured DRG, or inhibiting sympathetic transmitters, effectively reduced spontaneous activity to levels close to those seen in normal DRG. This provides another possible mechanism by which our in vitro electrophysiological results may be causally connected to the observed strong anti-nociceptive behavioral effects of cutting the L5 grey ramus.
In summary, this study presented evidence that the dorsal ramus of the spinal nerve is a source of sympathetic fibers that sprout into the DRG during early phases of the commonly used spinal nerve ligation model. This allows functional studies of the abnormal sympathetic-sensory neuron interaction, because the dorsal ramus can be electrically stimulated in the isolated DRG preparation, and neuronal properties measured with intracellular recording methods. Pre-cutting the grey ramus to the L5 DRG, a much less invasive procedure than surgical or chemical sympathectomy, markedly reduces the mechanical pain induced by spinal nerve ligation. More relevant to clinical situations, cutting the grey ramus also reduced mechanical pain (albeit with a delay) when done at the time of spinal nerve ligation.
Acknowledgments
Supported in part by NIH grants NS55860 and NS45594, and the University of Cincinnati Millennium Fund.
Footnotes
None of the authors has any conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdulla FA, Smith PA. Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J Neurosci. 1997;17:1633–1641. doi: 10.1523/JNEUROSCI.17-05-01633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.AbuRahma AF, Robinson PA, Powell M, Bastug D, Boland JP. Sympathectomy for reflex sympathetic dystrophy: factors affecting outcome. Ann Vasc Surg. 1994;8:372–379. doi: 10.1007/BF02133000. [DOI] [PubMed] [Google Scholar]
- 3.Amir R. Axonal cross-excitation in nerve-end neuromas: comparison of A- and C-fibers. J Neurophysiol. 1992;68:1160–1166. doi: 10.1152/jn.1992.68.4.1160. [DOI] [PubMed] [Google Scholar]
- 4.Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amir R, Devor M. Functional cross-excitation between afferent A- and C-neurons in dorsal root ganglia. Neuroscience. 2000;95:189–195. doi: 10.1016/s0306-4522(99)00388-7. [DOI] [PubMed] [Google Scholar]
- 6.Baron R, Janig W, Kollmann W. Sympathetic and afferent somata projecting in hindlimb nerves and the anatomical organization of the lumbar sympathetic nervous system of the rat. J Comp Neurol. 1988;275:460–468. doi: 10.1002/cne.902750310. [DOI] [PubMed] [Google Scholar]
- 7.Blenk KH, Habler HJ, Janig W. Neomycin and gadolinium applied to an L5 spinal nerve lesion prevent mechanical allodynia-like behaviour in rats. Pain. 1997;70:155–165. doi: 10.1016/s0304-3959(96)03314-3. [DOI] [PubMed] [Google Scholar]
- 8.Broadley KJ. Automonic Pharmacology. London: Taylor and Francis; 1996. [Google Scholar]
- 9.Brock JA, Cunnane TC. Studies on the mode of action of bretylium and guanethidine in post-ganglionic sympathetic nerve fibres. Naunyn Schmiedebergs Arch Pharmacol. 1988;338:504–509. doi: 10.1007/BF00179321. [DOI] [PubMed] [Google Scholar]
- 10.Burchiel KJ. Spontaneous impulse generation in normal and denervated dorsal root ganglia: sensitivity to alpha-adrenergic stimulation and hypoxia. Experimental Neurology. 1984;85:257–272. doi: 10.1016/0014-4886(84)90139-0. [DOI] [PubMed] [Google Scholar]
- 11.Chien SQ, Li C-L, Li H, Xie W, Zhang J-M. Sympathetic fiber sprouting in chronically compressed dorsal root ganglia without peripheral axotomy. J Neuropathic Pain & Symptom Palliation. 2005;1:19–23. doi: 10.1300/J426v01n01_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung K, Chung JM. Sympathetic sprouting in the dorsal root ganglion after spinal nerve ligation: evidence of regenerative collateral sprouting. Brain Res. 2001;895:204–212. doi: 10.1016/s0006-8993(01)02092-3. [DOI] [PubMed] [Google Scholar]
- 13.Chung K, Kim HJ, Na HS, Park MJ, Chung JM. Abnormalities of sympathetic innervation in the area of an injured peripheral nerve in a rat model of neuropathic pain. Neurosic Lett. 1993;162:85–88. doi: 10.1016/0304-3940(93)90566-4. [DOI] [PubMed] [Google Scholar]
- 14.Chung K, Lee BH, Yoon YW, Chung JM. Sympathetic sprouting in the dorsal root ganglia of the injured peripheral nerve in a rat neuropathic pain model. Journal of Comparative Neurology. 1996;376:241–252. doi: 10.1002/(SICI)1096-9861(19961209)376:2<241::AID-CNE6>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Czeh G, Kudo N, Kuno M. Membrane properties and conduction velocity in sensory neurones following central or peripheral axotomy. J Physiol, Lond. 1977;170:165–180. doi: 10.1113/jphysiol.1977.sp011944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devor M. In: Response of nerves to injury in relation to neuropathic pain. McMahon S, Koltzenburg M, editors. |. Book Title|, Vol. Volume|. City|: Publisher|, Year|. p.^pp. Pages|. [Google Scholar]
- 17.Devor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Exp Brain Res. 2009;196:115–128. doi: 10.1007/s00221-009-1724-6. [DOI] [PubMed] [Google Scholar]
- 18.Devor M, Janig W, Michaelis M. Modulation of activity in dorsal root ganglion neurons by sympathetic activation in nerve-injured rats. J Neurophysiol. 1994;71:38–47. doi: 10.1152/jn.1994.71.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev. 2004;46:131–145. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 20.Gurtu S, Smith PA. Electrophysiological characteristics of hamster dorsal root ganglion cells and their response to axotomy. J Neurophysiol. 1988;59:408–423. doi: 10.1152/jn.1988.59.2.408. [DOI] [PubMed] [Google Scholar]
- 21.Helke CJ, Rabchevsky A. Axotomy alters putative neurotransmitters in visceral sensory neurons of the nodose and petrosal ganglia. Brain Res. 1991;551:44–51. doi: 10.1016/0006-8993(91)90911-e. [DOI] [PubMed] [Google Scholar]
- 22.Katz DM. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proceedings of the National Academy of Sciences of the United States of America. 1983;80:3526–3530. doi: 10.1073/pnas.80.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Na HS, Sung B, Hong SK. Amount of sympathetic sprouting in the dorsal root ganglia is not correlated to the level of sympathetic dependence of neuropathic pain in a rat model. Neurosci Lett. 1998;245:21–24. doi: 10.1016/s0304-3940(98)00167-0. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 25.Kim YI, Na HS, Kim SH, Han HC, Yoon YW, Sung B, Nam HJ, Shin SL, Hong SK. Cell type-specific changes of the membrane properties of peripherally-axotomized dorsal root ganglion neurons in a rat model of neuropathic pain. Neuroscience. 1998;86:301–309. doi: 10.1016/s0306-4522(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 26.Kinnman E, Levine JD. Sensory and sympathetic contributions to nerve injury-induced sensory abnormalities in the rat. Neuroscience. 1995;64:751–767. doi: 10.1016/0306-4522(94)00435-8. [DOI] [PubMed] [Google Scholar]
- 27.Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- 28.Koerber HR, Woodbury CJ. Comprehensive phenotyping of sensory neurons using an ex vivo somatosensory system. Physiol Behav. 2002;77:589–594. doi: 10.1016/s0031-9384(02)00904-6. [DOI] [PubMed] [Google Scholar]
- 29.Lee BH, Yoon YW, Chung K, Chung JM. Comparison of sympathetic sprouting in sensory ganglia in three animal models of neuropathic pain. Experimental Brain Research. 1998;120:432–438. doi: 10.1007/s002210050416. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Xie W, Strong JA, Zhang J-M. Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology. 2007;107:469–477. doi: 10.1097/01.anes.0000278907.37774.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 32.Ma W, Bisby MA. Partial sciatic nerve transection induced tyrosine hydroxidase immunoreactive axon sprouting around both injured and spared dorsal root ganglion neurons which project to the gracile nucleus in middle-aged rats. Neurosci Lett. 1999;275:117–120. doi: 10.1016/s0304-3940(99)00746-6. [DOI] [PubMed] [Google Scholar]
- 33.Maruo K, Yamamoto H, Yamamoto S, Nagata T, Fujikawa H, Kanno T, Yaguchi T, Maruo S, Yoshiya S, Nishizaki T. Modulation of P2X receptors via adrenergic pathways in rat dorsal root ganglion neurons after sciatic nerve injury. Pain. 2006;120:106–112. doi: 10.1016/j.pain.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan EM, Jang W, Devor M, Michaelis M. Peripheral nerve injury triggers noradrenergic sprouting within dorsal root ganglia. Nature. 1993;363:543–546. doi: 10.1038/363543a0. [DOI] [PubMed] [Google Scholar]
- 35.Meisner JG, Waldron JB, Sawynok J. Alpha1-adrenergic receptors augment P2X3 receptor-mediated nociceptive responses in the uninjured state. J Pain. 2007;8:556–562. doi: 10.1016/j.jpain.2007.02.434. [DOI] [PubMed] [Google Scholar]
- 36.Nagy I, Urban L, Woolf CJ. Morphological and membrane properties of young rat lumbar and thoracic dorsal root ganglion cells with unmyelinated axons. Brain Res. 1993;609:193–200. doi: 10.1016/0006-8993(93)90873-l. [DOI] [PubMed] [Google Scholar]
- 37.Park SK, Chung K, Chung JM. Effects of purinergic and adrenergic antagonists in a rat model of painful peripheral neuropathy. Pain. 2000;87:171–179. doi: 10.1016/S0304-3959(00)00277-3. [DOI] [PubMed] [Google Scholar]
- 38.Pertin M, Allchorne AJ, Beggah AT, Woolf CJ, Decosterd I. Delayed sympathetic dependence in the spared nerve injury (SNI) model of neuropathic pain. Mol Pain. 2007;3:21. doi: 10.1186/1744-8069-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersen PM, Zhang J, Zhang J-M, LaMotte RH. Abnormal spontaneous activity and responses to norepinephrine in dissociated dorsal root ganglion cells after chronic nerve constriction. Pain. 1996;67:391–397. doi: 10.1016/0304-3959(96)03146-6. [DOI] [PubMed] [Google Scholar]
- 40.Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price J, Mudge AW. A subpopulation of rat dorsal root ganglion neurones is catecholaminergic. Nature. 1983;301:241–243. doi: 10.1038/301241a0. [DOI] [PubMed] [Google Scholar]
- 42.Ramer MS, Bisby MA. Rapid sprouting of sympathetic axons in dorsal root ganglia of rats with a chronic constriction injury. Pain. 1997;70:237–244. doi: 10.1016/s0304-3959(97)03331-9. [DOI] [PubMed] [Google Scholar]
- 43.Ramer MS, Bisby MA. Differences in sympathetic innervation of mouse DRG following proximal or distal nerve lesions. Exp Neurol. 1998;152:197–207. doi: 10.1006/exnr.1998.6855. [DOI] [PubMed] [Google Scholar]
- 44.Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- 45.Roberts WJ. A hypothesis on the physiological basis for causalgia and related pains. Pain. 1986;24:297–311. doi: 10.1016/0304-3959(86)90116-8. [DOI] [PubMed] [Google Scholar]
- 46.Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic-sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/a:1007090105840. [DOI] [PubMed] [Google Scholar]
- 47.Sihvonen T, Lindgren KA, Airaksinen O, Leino E, Partanen J, Hanninen O. Dorsal ramus irritation associated with recurrent low back pain and its relief with local anesthetic or training therapy. J Spinal Disord. 1995;8:8–14. [PubMed] [Google Scholar]
- 48.Song XJ, Hu SJ, Greenquist KW, Zhang J-M, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. J Neurophysiol. 1999;82:3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 49.Stebbing MJ, Eschenfelder S, Habler HJ, Acosta MC, Janig W, McLachlan EM. Changes in the action potential in sensory neurones after peripheral axotomy in vivo. Neuroreport. 1999;10:201–206. doi: 10.1097/00001756-199902050-00001. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi Y, Chiba T, Kurokawa M, Aoki Y, Takahashi K, Yamagata M. Stereoscopic structure of sensory nerve fibers in the lumbar spine and related tissues. Spine (Phila Pa 1976) 2003;28:871–880. doi: 10.1097/01.BRS.0000058717.43888.B9. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi Y, Nakajima Y. Dermatomes in the rat limbs as determined by antidromic stimulation of sensory C-fibers in spinal nerves. Pain. 1996;67:197–202. doi: 10.1016/0304-3959(96)03116-8. [DOI] [PubMed] [Google Scholar]
- 52.Vega JA. Presence of catecholamine-related enzymes in a subpopulation of primary sensory neurons in dorsal root ganglia of the rat. Cellular & Molecular Biology. 1991;37:519–530. [PubMed] [Google Scholar]
- 53.Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. J Neurophysiol. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- 54.Wiesenfeld-Hallin Z. Partially overlapping territories of nerves to hindlimb foot skin demonstrated by plasma extravasation to antidromic C-fiber stimulation in the rat. Neurosci Lett. 1988;84:261–265. doi: 10.1016/0304-3940(88)90517-4. [DOI] [PubMed] [Google Scholar]
- 55.Woodbury CJ, Kullmann FA, McIlwrath SL, Koerber HR. Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J Comp Neurol. 2008;508:500–509. doi: 10.1002/cne.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie W, Strong JA, Li H, Zhang J-M. Sympathetic sprouting near sensory neurons after nerve injury occurs preferentially on spontaneously active cells and is reduced by early nerve block. J Neurophysiol. 2007;97:492–502. doi: 10.1152/jn.00899.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie W, Strong JA, Zhang J-M. Sympathetic nerve sprouting is closely related to the pattern of spontaneous activity in axotomized DRG neurons. Soc Neurosci Abstr. 2008;759:2. [Google Scholar]
- 58.Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang J-M. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142:809–822. doi: 10.1016/j.neuroscience.2006.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J-M, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol. 1999;82:3359–3366. doi: 10.1152/jn.1999.82.6.3359. [DOI] [PubMed] [Google Scholar]
- 60.Zhang JM, Strong JA. Recent evidence for activity-dependent initiation of sympathetic sprouting and neuropathic pain. Sheng Li Xue Bao. 2008;60:617–627. Long-lasting excitatory effects on sensory neurons of stimulating, using a new in-vitro method, sympathetic fibers that sprout into the DRG ( spinal nerve ligation model) [PubMed] [Google Scholar]